Abstract
Essential tremor (ET) is a common movement disorder affecting millions of people. Studies of ET patients and perturbations in animal models have provided a foundation for the neural networks involved in its pathophysiology. However, ET encompasses a wide variability of phenotypic expression, and this may be the consequence of dysfunction in distinct subcircuits in the brain. The cerebello-thalamo-cortical circuit is a common substrate for the multiple subtypes of action tremor. Within the cerebellum, three sets of cerebellar cortex-deep cerebellar nuclei connections are important for tremor. The lateral hemispheres and dentate nuclei may be involved in intention, postural and isometric tremor. The intermediate zone and interposed nuclei could be involved in intention tremor. The vermis and fastigial nuclei could be involved in head and proximal upper extremity tremor. Studying distinct cerebellar circuitry will provide important framework for understanding the clinical heterogeneity of ET.
Keywords: Cerebellum, Essential tremor, Action tremor, Intention tremor, Postural tremor, Kinetic tremor, Pathophysiology
Introduction
Essential tremor (ET) is one of the most common movement disorders and can be highly disabling. ET is typically characterized by action tremor of the upper extremities; however, the spectrum of phenotypes can be variable among individuals [1–4]. For example, tremor may be focal, with some individuals only having tremor in single digits while others can have tremor at the wrist or elbow [2]. Voice and head tremor also occur in a subset of ~10–15% and ~35% of ET patients, respectively [5–8]. Tremor can also be segmental, occurring in contiguous body parts [9–12]. Therefore, tremor can happen in different, spatially restricted parts of the body in different patients. In addition, the maneuvers that induce tremor are highly individualized in ET patients.
Action tremor is a broad term that encompasses different tremor subtypes. Some patients have tremor while performing voluntary movements, termed kinetic tremor, whereas others have tremor during extension of the arms fixed in place called postural tremor [2, 7, 13, 14]. Kinetic tremor can also take the form of task-specific manifestations, which involve skilled movements like writing, typing, and playing an instrument [15–17]. A subgroup of ET patients (~40–45%) have prominent tremor when reaching closer to a target, a unique type of tremor called intention tremor [7, 8, 13, 14]. These different types of tremor can co-occur in a single ET patient. It has been difficult to elucidate detailed pathophysiology of ET, in part, because of the heterogeneity of tremor expression. Research has not been focusing on separating ET patients with distinct tremor phenotypes to further investigate the pathophysiology.
Despite these challenges and diverse clinical presentations, our understanding of ET pathogenesis in recent decades has gained significant momentum in identifying the cerebellum as having a central role [18–21]. In addition, there has been significant progress in our basic neuroscience knowledge of how the cerebellum contributes to movements, and this knowledge can be applied to studying how the dysfunctional cerebellum creates distinct types of action tremor. In this article, we will review evidence from basic and clinical science fields to understand the pathophysiology of tremor subtypes and to point to future directions of research on ET heterogeneity.
Distinctive Tremor Subtypes in ET
Clinical diagnosis of ET is based on neurological examination, demonstrating action tremor in the bilateral upper extremities [12]. Since action tremor includes both postural tremor and kinetic tremor, these tremor subtypes are examined in separate neurological tests. Postural tremor is assessed with patients holding their arms outstretched in front of the body [2, 22]. Tremor during this positional hold can occur at more proximal sites like the shoulder or elbow, or more distally in the wrist or fingers and can be present in one or multiple joints [2, 23, 24]. Kinetic tremor is assessed by a finger-nose-finger maneuver, spiral drawing tasks, handwriting, and pouring water from one cup to another [4]. If tremor occurs mainly in the hands when close to reaching the target during these tasks, this type of tremor is categorized as intention tremor. The dot approximation and finger-nose-finger tests are particularly helpful to assess intention tremor [25]. If tremor only occurs in one of these tasks but not the other, this type of tremor is labeled as task-specific tremor. In addition to hand tremor, head tremor is assessed by observing patients with different head positions and looking in different directions, which can induce subtle head tremor [25]. Voice tremor is assessed by listening to patients producing monotone sounds or in unscripted, voluntary speech [25]. A small number of ET patients may also have face, leg, or standing tremor, which should also be assessed in the neurological examination. Finally, isometric tremor is another form of action tremor induced by contraction of a muscle against an unmoving object. Clinical tests for isometric tremor include squeezing the finger of the practitioner [15, 22]. Each ET patient can have any one or a combination of these types of tremor (Fig. 1). The Essential Tremor Rating Assessment Scale performance subscale (TETRAS-PS) is a clinical rating scale for assessing all abovementioned ET tremor subtypes (except for isometric tremor), and TETRAS-PS has been widely used by recent clinical trials of ET [26, 27].
Fig. 1.
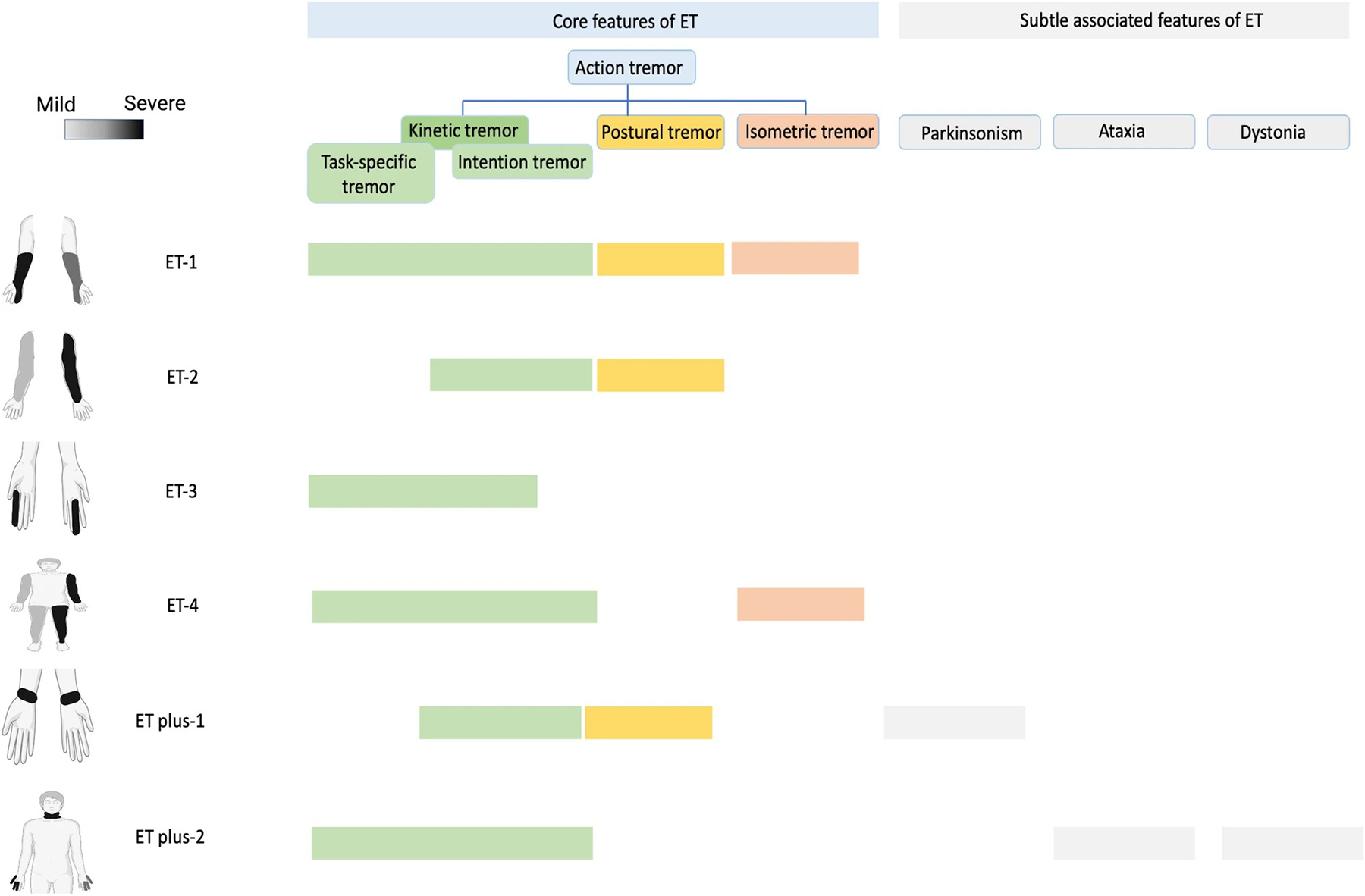
Heterogeneity of essential tremor. ET patients can exhibit action tremor (blue) in different parts of the body (leftmost images) with different severities (grayscale; lighter gray is mild and black is severe) and in different contexts (indicated by solid coloring; kinetic, postural, or isometric). In some ET cases (ET plus), patients may also express subtle features of parkinsonism, ataxia, or dystonia
The diagnosis of ET depends on the aforementioned assessment of action tremor and additional criteria of 1) at least 3-year duration and with or without head, voice, and lower limb tremor, and 2) the absence of other neurological signs, such as dystonia, ataxia, or parkinsonism [12]. However, there could be some “soft sign” features suggesting dystonia, ataxia, or parkinsonism in some ET patients, and this group is categorized by some experts using a controversial name: ET plus [12, 28] (Fig. 1), which is outside of the scope of this review.
It is important to keep in mind that none of the abovementioned action tremor subtypes are specific to ET, and a complete clinical context should be considered. For instance, about one third of Parkinson’s disease patients have action tremor, including postural tremor [29], while the predominant tremor in Parkinson’s disease is rest tremor [9, 30–34]. On the other hand, rest tremor occurs in approximately 10% of ET patients, particularly those with severe action tremor [7, 8]. Some neurologists classify these patients with both ET and Parkinson’s disease [35], particularly when bradykinesia and rigidity are also present. Similarly, isometric tremor can be observed in some ET patients [23, 36–38], but isometric tremor is often associated with enhanced physiological tremor [39].
It is likely that these different tremor subtypes in ET have distinct neural substrates. Diagnosis of patients to differentiate underlying causes of tremor typically includes an assessment of whether the tremor exhibits high or low frequency and amplitude, as different subtypes of tremor manifest in specific frequency and amplitude ranges [6, 19]. A recent study found Parkinson’s disease patients who have both rest and postural tremor often exhibit different tremor frequencies in these two subtypes of tremor [40]. ET patients who have both postural and kinetic tremor often have slightly different tremor frequencies in these two subtypes of action tremor [41]. Single neuron recordings in the thalamus of ET patients have shown different discharge frequencies in addition to differences in the proportion of sensory-responsive versus nonsensory-responsive cells that are active during kinetic and postural tremor [24], suggesting postural and kinetic tremor originate from different brain areas, or they are coming from the same origin with distinctive regulatory mechanisms involved. Detailed kinematic measurements will help us to have a higher resolution of tremor subtype characterization than clinical assessment, which will be a critical piece for understanding the heterogeneity of tremor disorders.
Cerebello-thalamo-cortical Loop in ET
Regardless of the heterogeneous expression of action tremor in ET, ample research studies conclude that the cerebello-thalamo-cortical loop is the key substrate involved in different tremor subtypes. These neuroimaging studies were conducted while ET patients performed different tremor-inducing tasks, such as posturing, writing, and force gripping.
The original study employing functional magnetic resonance imaging (fMRI) of ET patients showed increased activation of cerebellar cortical hemispheres, dentate nuclei, red nuclei, thalamus, and primary motor cortex during postural tremor [42]. Subsequent studies recording fMRI and electromyographic (EMG) activity simultaneously during postural and kinetic tremor confirmed increased activation in motor cortex, cerebellum, thalamus, and pons [43, 44]. Other fMRI studies corroborate the involvement of the cerebello-thalamo-cortical loop in distinctive subtypes of action tremor in ET: postural tremor [37, 45–47], isometric tremor (forced grip) [37, 38], and kinetic tremor while writing [48] or performing finger tapping [49]. These studies demonstrated that fMRI could detect abnormalities in the cerebello-thalamo-cortical loop in ET patients having distinct tremor subtypes.
Furthermore, positron emission tomography (PET) studies of ET patients during postural tremor detected an increase in blood flow in the cerebellum, red nuclei, and thalamus [50–54] and in the cerebellum during a writing task [52]. These data also suggest the involvement of the cerebellum and its downstream target brain regions in action tremor. Motor cortex, however, did not appear to be clearly involved in these PET studies.
Perhaps the strongest evidence that the cerebello-thalamo-cortical loop is involved in ET comes from lesion evidence. Some ET patients have long-standing tremor and later developed discrete lesions either by small stroke or discrete hemorrhage, which surprisingly dampen tremor. These lesions are postulated to disrupt the brain circuits of tremor. The localization of these lesions are found to be in the cerebellum [55–57], thalamus [58–60], and pontine nuclei [57, 61]. For the cerebellum, specifically, two patients showed a hematoma involving all the deep cerebellar nuclei and alleviation of postural [55] and isometric tremor [57], while another patient showed a lesion largely of the dentate and superior cerebellar peduncle with cessation of postural tremor [56]. The part of the thalamus involved in the upper extremity-related brain circuit of tremor is the ventral intermediate nucleus (VIM), which receives dense projections from forelimb-related neurons in the deep cerebellar nuclei (DCN) of the cerebellum [62, 63]. Using a novel imaging technique, lesion-based mapping, investigators further confirmed the role of the cerebello-thalamo-cortical loop in ET [64]. Of note, none of these studies separate distinct tremor subtypes. It is likely that most subtypes of action tremor in ET are dampened in the lesions of the cerebello-thalamo-cortical loop.
Originally, thalamotomies were performed in ET patients and were very effective in reducing tremor [37, 65, 66]. More recently, magnetic resonance-guided focused ultrasound lesioning of VIM [67–70] has been approved by the Food and Drug Administration (FDA) as a novel therapy for ET. While no specific subtype of action tremor in ET has been described as being reduced with focused ultrasound lesioning of VIM, such a procedure improved many domains of activity of daily living, such as eating, drinking, writing, and working [68], suggesting at least kinetic tremor can be effectively dampened.
In addition to lesion-based studies supporting the role of cerebello-thalamo-cortical loop in ET, deep brain stimulation (DBS) in VIM can also robustly dampen postural and kinetic tremor in ET [71–79]. During the surgery for DBS implantation, intra-operative, single neuron activity recordings in ET patients showed that thalamic neurons exhibited distinct bursting activity and an inter-burst frequency that matches action tremor frequency [23, 80–82]. Therefore, VIM DBS may exert tremor suppressing effects by altering such unique neuronal activity in the thalamus.
The last stop of the cerebello-thalamo-cortical loop is the motor cortex, which is responsible for motor command execution. Therefore, lesions in the motor cortex are likely to cause widespread weakness, rather than merely tremor suppression in ET. The evidence of motor cortex involvement in ET comes from stimulation studies. Transcortical magnetic stimulation over motor cortex in ET patients can suppress postural tremor, observed by EMG recording [83] or by clinical observation [83–85]. A PET imaging study demonstrated VIM DBS can increase the activation of motor cortex along with tremor suppression [74], suggesting that VIM DBS can alter the patterns of activity underlying tremor that pass-through motor cortex.
The major hub in the cerebello-thalamo-cortical loop is the cerebellum. In the last two decades, cerebellar pathology has been studied in detail in the post-mortem brain of ET patients, and an array of circuit re-organization has been found [20]. Specifically, these pathological changes occur in or around Purkinje cells (PCs), the principal neurons in the cerebellar cortex. In the ET cerebellar cortex, the degenerative changes in PCs are evident, including PC loss [86, 87], PC axonal swelling (i.e., torpedoes) [86, 88], PC axon collateral formation [89], and PC dendritic spine loss [90]. These findings indicate that PCs may be the key neuronal type affected in ET. In addition to PC alterations, inhibitory input to PCs from basket cells, which form dense connections with the PC axon initial segment to control PC output, show morphological changes. In ET, basket cell axons around the PC axon initial segment become dense [91] and elongated [92], which may affect PC to DCN transmission fidelity. The PC-innervating excitatory synaptic organization in ET is also abnormal, in that climbing fiber synapses are distally extended to the parallel fiber synaptic territory near the pial surface [93–97] and climbing fibers abnormally extend laterally over multiple PCs [98]. These findings suggest abnormal climbing fiber to PC synaptic transmission in ET.
Aside from neuronal abnormalities, astrocytic alterations were also recently studied in ET because a genomewide association study suggested SLC1A2, which encodes excitatory amino acid transporter type 2 (EAAT2), is associated with ET [99]. EAAT2 is a transporter expressed by astrocytes to uptake excessive glutamate from excitatory synaptic transmission to prevent hyper-excitability. Consistently, studies have found a selective reduction in the levels of EAAT2, but not EAAT1, in the postmortem ET cerebellar cortex [17, 100], suggesting that hyper-excitability may contribute to PC degenerative changes in ET. Moreover, Bergmann glia, the main type of astrocyte in cerebellar cortex, shows gliosis in a subset of ET cases [101]. This may cause improper clearance of glutamate, which leads to neurotoxic effects of PCs, relieving the DCN of inhibitory tone and enhancing output that contributes to tremor. However, in a larger case-control study, no alterations of glial fibrillary acidic protein levels or distributions have been found [102]. More detailed morphological analysis of astrocytic processes are required to further explore the role of astrocytes in ET.
Many pathological features of ET fall within the neurodegenerative changes in the cerebellum. In a large study comparing ET with other prototypical cerebellar degenerative disorders, such as spinocerebellar ataxias and multiple system atrophy cerebellar type (i.e., ataxic disorders), ET has a milder degree of cerebellar degenerative changes compared to ataxic disorders [101]. Interestingly, distal and lateral extension of climbing fibers into parallel fiber synaptic territory appears to be one of the more specific findings in ET [98, 101]. This suggests that dendritic integration and synchrony are altered, which may cause mistimed cerebellar output and contribute to tremor generation. Pan et al. established a mouse model with ET-like distal and lateral extension of climbing fibers and this mouse model develops ET-like tremor, demonstrating the importance of climbing fiber and PC synaptic connections in tremor [103].
Other evidence that the cerebellum is a key player in ET comes from a direct physiological recording of the cerebellum using cerebellar electroencephalogram (EEG), which showed oscillatory activity at the tremor frequency and the strength of cerebellar oscillatory activity correlated with tremor severity [103]. This cerebellar oscillatory activity appears to occur in both familial and sporadic ET patients [104]. Finally, transcranial alternating stimulation applied to the cerebellum of ET patients can effectively dampen tremor [105]. Collectively, these studies demonstrate a crucial role of the cerebellum within the cerebello-thalamo-cortical loop in ET (Fig. 2).
Fig. 2.
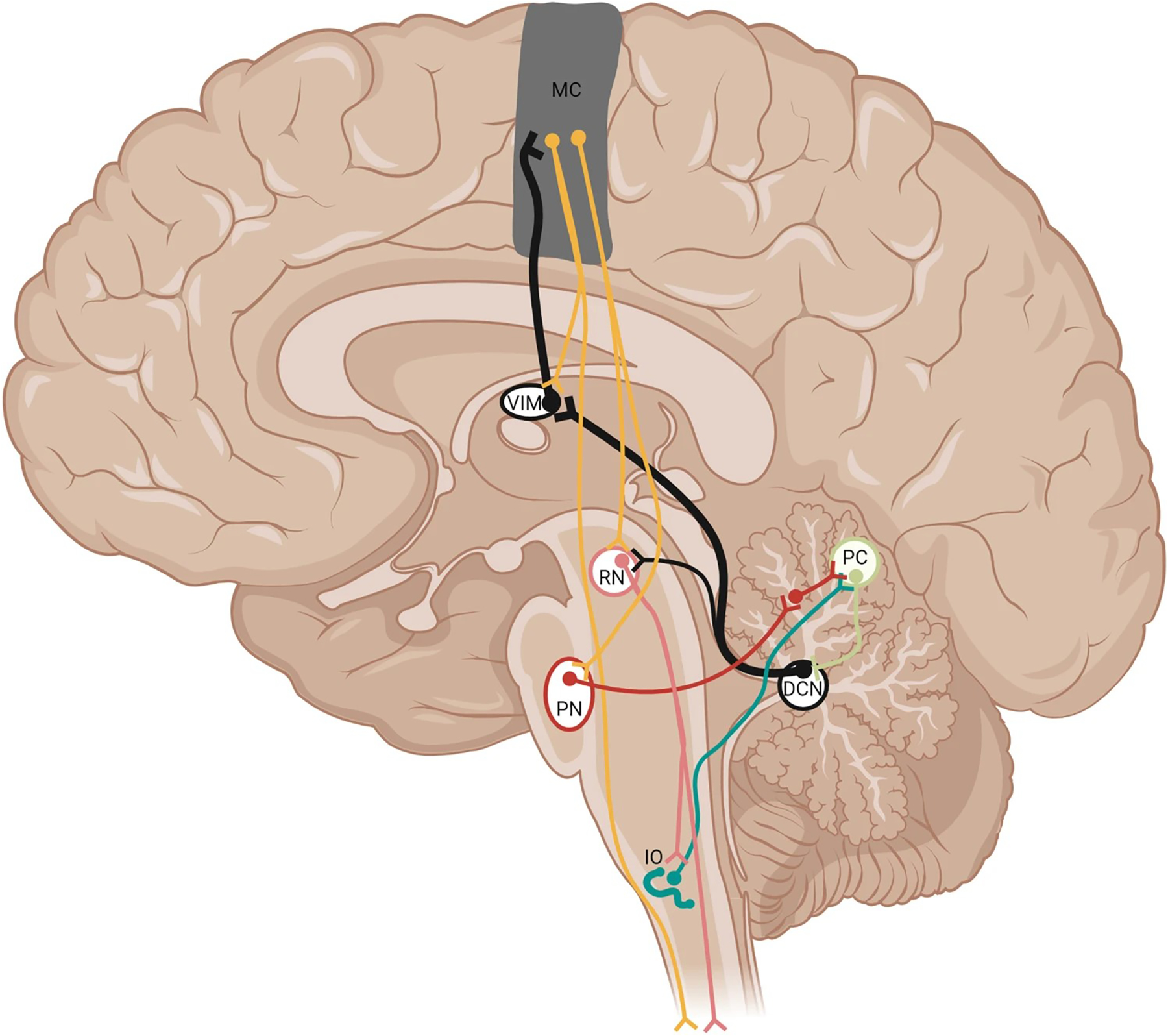
Pathways involved in action tremor. Purkinje cells send output to the deep cerebellar nuclei (DCN). The DCN, in turn, project to the red nucleus (RN; pink) and collateralize to innervate the ventral intermediate nucleus of the thalamus (VIM), recruiting ensembles of motor cortex neurons (MC; orange), forming the cerebello-thalamo-cortical loop (black, thickened projections). There are two different types of motor cortex projection neurons: one set projects to the pontine nuclei (PN; brown) and RN, which projects to the spinal cord, and another set that projects to the spinal cord with collaterals fed back to the thalamus. The PN project as mossy fibers onto granule cells, which form parallel fibers that innervate Purkinje cells (PC; beige). The inferior olive (IO; turquoise) sends climbing fiber axons to innervate PCs. Not all connections are shown
Cerebellar Organization and Function Relating to Action Tremor
To gain a deeper sense of the theoretical framework of how different types of action tremors are generated by the cerebellum, we will next review the organization of the cerebellum to provide context. The cerebellum consists of the cerebellar cortex and the DCN. The cerebellar cortex receives input from much of the forebrain, midbrain, and hindbrain primarily via pontine mossy fibers [106–110], while spinal cord and a subset of forebrain, midbrain, and hindbrain inputs are conveyed by the inferior olive via climbing fibers [111–117]. PCs integrate many inputs, including excitatory inputs from these two distinctive pathways, and provide the sole output to DCN neurons [118]. DCN neurons provide output to brainstem, midbrain, and thalamus, ultimately influencing movements and/or cognition [63, 110, 118, 119]. The anatomical organization of cerebellar cortex has a highly regular structure, such that the arrangement and connections of neurons is consistent across different lobules of the cerebellum [120]. This arrangement implies similar computations across different areas of cerebellar cortex. Despite similar anatomical organization of the cerebellar neurons across different lobules, the cerebellum is known to be divided into three parasagittal divisions because of the different connectivity of each DCN with the rest of the brain [62, 110]. These three divisions of the cerebellar cortex are the vermis, intermediate zone, and lateral hemisphere [121–123]. Compared to the larger surface area of cerebellar cortex, the DCN are compact structures with a PC to DCN convergence ratio of ~40:1 [124]. Each parasagittal division of cerebellar cortex projects to specific DCN. PCs in the vermis project to fastigial neurons, PCs in the intermediate zone (also referred to as the paravermis) project to interposed neurons, and PCs in the lateral hemisphere project to dentate neurons [62, 123, 125, 126]. Therefore, there are three distinct pairs of cerebellar cortex to DCN connections: 1) lateral hemispheres and dentate nuclei, 2) intermediate zone and interposed nuclei 3) vermis and fastigial nuclei (Fig. 3). Note that in humans, interposed nuclei is further divided into globose and emboliform nucleus, which are likely analogous to the anterior and posterior interposed nuclei in animals. In the remaining part of the article, we will discuss evidence of each subdivision of the cerebellum and their implications in subtypes of action tremor based on animal model evidence, most notably non-human primates.
Fig. 3.
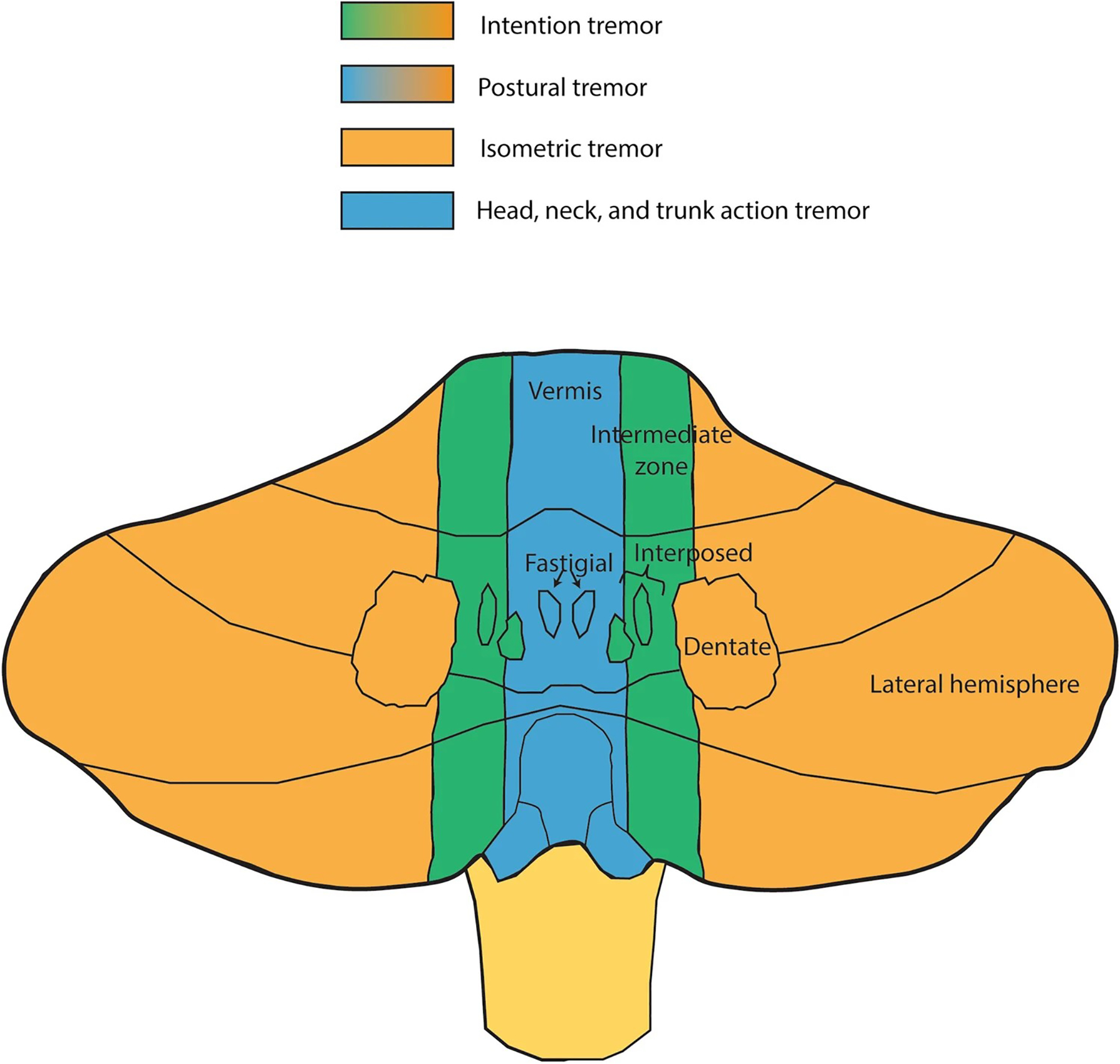
Cerebellar organization and functional relation to action tremor. Purkinje cells in vermis project to fastigial nuclei (blue) and are likely involved in action tremor of the head, trunk and postural tremor. Purkinje cells in the intermediate zone project to interposed nuclei (green) and are likely involved in intention tremor. The lateral hemispheres connect with dentate nuclei (orange) and appear to be involved in kinetic, isometric, and postural tremor
The three pairs of cerebellar cortex-DCN connections have distinct expansion during evolution, which reflects on the size of the DCN. Specifically, interposed nuclei evolved after fastigial nuclei, and dentate nuclei were the most recent evolutionary expansion [127]. In primates, the dentate nucleus appears to have expanded in conjunction with the expansion of frontal cortex [127–130], and frontal cortex and dentate nuclei are di-synaptically and functionally connected [62, 110, 131, 132]. In addition to the parasagittal compartmentalization of the cerebellum, there is a somatotopic organization in the form of multiple, opposite-facing homunculi [122, 133, 134], further dividing the cerebellum into a set of modules, each designed to control the respective motor plant from which they receive sensory input from [112, 135]. In the following sections, we will dive into each of the cortex-DCN divisions and their implications for subtypes of action tremor.
Lateral Hemispheres and Dentate Nuclei
Dysfunction in the lateral hemispheres and corresponding dentate nuclei appear to promote kinetic tremor, postural tremor, and isometric tremor. Inactivation or lesions of the dentate nuclei in non-human primates caused tremor near movement termination in self-initiated, goal-directed tasks involving forelimb movements [1, 136–141], which shares similarity to intention tremor in ET patients [4, 13, 14]. Importantly, removing sensory feedback through peripheral deafferentation from lesion of the dorsolateral funiculus did not affect the intention tremor [136]. This suggests tremor does not originate in the spinal circuitry that regulates the stretch reflex, since sensory feedback is needed to produce oscillatory activity outside the central nervous system, in the spinal-mediated stretch reflex [19]. In addition to intention tremor, lesions specific to the dentate nucleus or to the parvocellular portion of the red nucleus, which only receives dentate nucleus projections [142, 143], can cause postural tremor of the hands in non-human primates [144]. These studies collectively demonstrated that dysfunction of dentate nuclei is sufficient to induce intention and postural tremor. However, the details of how these two different subtypes of action tremor can come from the dentate nucleus remains to be further explored.
Human evidence implicating lateral hemispheres of cerebellar cortex and dentate nuclei in postural tremor comes from imaging studies. PET studies demonstrated activation of the cerebellar lateral hemispheres during postural tremor in ET patients [50, 54]. In fMRI studies, ET patients, when having postural tremor, showed involvement of lobules IV-VI, Crus I, and VIII, representing the lateral hemisphere [45, 46].
Other studies showed that patients with cerebellar-specific strokes in the superior cerebellar artery territory, affecting the lateral hemispheres and dentate nuclei, developed tremor during grip force tasks, suggesting a link between lateral hemispheres and dentate nuclei with isometric tremor [145–149]. Lastly, lesions specific to the dentate nuclei in non-human primates can produce dysmetria and tremor of the arms and hands [150], which could account for some cases of action tremor that are comorbid with ataxia. All these lesion studies suggest that the dysfunction of lateral hemispheres and dentate nuclei can create kinetic, postural, and isometric tremor (Fig. 3).
The dentate nucleus has dense connectivity with association cerebral cortex; thus, it is postulated to be involved in generating motor plans [62, 110, 126, 128, 132, 151]. Action tremor could be a result of erroneous programming of voluntary movements coming from dysfunctional connectivity of lateral hemisphere-dentate nucleus-association cortex [152, 153]. Loss of proper dentate nucleus function can force the brain to utilize an alternative strategy, switching from a preprogrammed, continuous movement to a more sensory feedback-dependent strategy where the interposed and/or fastigial nucleus could impose a set of stepwise movements, as has been noted in non-human primates with lesions/inactivation of the dentate nuclei [136, 137, 144]. Therefore, this adaptive strategy switching from dentate nuclei to other DCN can create tremor-like movements in non-human primates. Whether this adaptive strategy model is relevant to action tremor in ET patients still needs to be carefully considered. First, this particular model could only explain kinetic tremor and does not fully explain intention tremor, which has very characteristic tremor only near the target. Second, this model does not explain postural tremor or isometric tremor because these two subtypes of tremor come from specific postures of the hands, rather than movements. Third, pathological examination of postmortem ET brains did not reveal any loss of dentate neurons [154]. Fourth, prototypical diseases of dentate nucleus degeneration are Friedreich’s ataxia and spinocerebellar ataxia type 3 [101] and these diseases are not prominently associated with kinetic, postural, or isometric tremor.
Intermediate Zones and Interposed Nuclei
Several lines of evidence point to the forelimb-related neurons of the interposed nucleus being involved in reach-to-grasp movements [155–162]. More specifically, interposed nuclei appear to regulate proper deceleration commands in the upper limbs of cats and mice [163, 164]. This is consistent with abnormalities in the amplitude of deceleration during intention tremor, particularly during grasping, of ET patients [13]. Therefore, the dysfunction in the intermediate zone of cerebellar cortex and interposed nuclei may contribute to intention tremor involving reaching and grasping.
The connectivity of interposed nucleus is also particularly appealing to be a candidate for generating intention and/or other kinetic tremor, since the interposed nucleus receives feedback from forelimb muscle spindles in non-human primates [165]. In addition, the interposed nuclei also have a unique physiological attribute that could be relevant in tremor. Interposed neurons have bursts of neuronal firing that lags discharge coming from muscle spindle afferents by a few dozen milliseconds, which lags EMG bursts by about 12 ms [165]. In this loop, movements will send feedback signals from muscle spindles back to interposed neurons that will trigger activity to decelerate and stabilize the movement, thus acting as “brake.” Therefore, dysfunction of such a system can possibly set up oscillatory muscle activity (i.e., tremor). These basic science observations coincide with clinical studies that ET patients with intention tremor have abnormal EMG burst timing in forearm muscles and changes in deceleration amplitude during movement of a joystick, which indicates dysfunction in the “braking” component of muscle activation [14, 153, 165–168]. Consistently, studies have shown the development of intention tremor in the distal upper extremities following inactivation or lesioning of the interposed nuclei in the non-human primates [141, 169–172]. However, not all studies replicate such a finding [173].
Taken altogether, interposed nuclei and the associated intermediate zones of the cerebellar cortex are involved in stabilizing the upper extremities during reaching and grasping that could be an appealing candidate for producing intention tremor.
Vermis and Fastigial Nuclei
Dysfunction in the vermis and fastigial nuclei is likely responsible for action tremor of the head and trunk and may contribute to postural tremor of the upper extremities. The vermis receives input from the spinal cord and primary, somatosensory, and supplementary motor cortices via the pons that include sensory representations of predominantly proximal and some distal body parts [110, 174–177]. Neural recordings in animals, including cats, mice, and monkeys, have found the firing patterns of PCs in the vermis correlate with activity of the proximal body including the head, neck, limbs, trunk, and eyes [174, 178–182]. Vermis and fastigial nuclei are primarily involved in coordinating and stabilizing proximal musculature supporting posture, gait, and eye movements [161, 169, 170].
Lesions of the vermis or fastigial nuclei in non-human primates were found to produce “fine tremors” of the head and trunk upon trying to maintain an upright posture [150, 183, 184]. Human patients with selective loss of PCs in the vermis also exhibit whole-body postural tremor [184–186]. Moreover, lesions of the fastigial nucleus in cats produced a coarse tremor of the head and body, which was evoked by action of eating or sudden movement [187]. These studies of vermal or fastigial lesions may have more implications in understanding Holmes tremor, which is usually larger amplitude and lower frequency than ET and can involve the proximal truncal muscles.
Evidence for a role of the vermis in postural tremor of the upper extremities comes from ET patient imaging studies. Cerebellar vermis lobules IV-VI showed differences in activity compared to controls with fMRI during postural tremor in ET patients [46, 49]. Increases in blood flow in the vermis in ET patients during the same postural task was noted in a PET imaging study [54]. In a pathological study of ET, PC axonal pathology in the vermis is associated with neck, voice, and jaw tremor [88]. It seems that dysfunction in the vermis and fastigial pathway is involved in action tremor of proximal musculature and postural tremor of more distal musculature.
Further Research Considerations
While these studies over many decades have shed great insight into how the different areas of the cerebellum could generate diverse subtypes of action tremor, several major limitations need to be considered. First, only non-human primates exhibit distinct action tremor phenotypes mimicking human conditions, such as intention tremor, isometric tremor, or postural tremor. Behavioral assays of tremor in rodents or other animals are limited in their ability to mimic human action tremor [103, 188]. In addition, the techniques implemented to study non-human primates so far are mostly lesion-based or descriptive approaches. Lesion-based approaches cannot adequately model neuronal connectivity re-organization, which is likely to play an important role in ET. In addition, further exploration of cell-type specificity and the physiological correlates in animal models within the three sets of cerebellar cortex-DCN will be important to understand subtypes of action tremor in ET.
Second, the timing of tremor development in most of these studies is not well defined. In lesion-based studies, tremor may not develop immediately following the lesion [189]. Tremor can develop after local circuit re-organization and/or compensatory plastic changes. In other words, it is possible that tremor is due to the abnormal re-organization of the cerebellar circuit, rather than merely loss of normal function in a particular area. This point is further corroborated by observations in patients who suffer from traumatic head injury and later develop tremor after weeks, months, or even years following brain injury, suggesting plastic changes and circuit re-organization may play a role [190, 191].
Third, the extent to which multiple modules of the cerebellum are transmitting distinct activity that contributes to action tremor needs further study. Evidence in non-human primates and humans suggests that a somatotopic representation of the entire body exists in each of the three DCN and corresponding cerebellar cortex [63, 134, 169, 192–195]. However, the representations of each part of the body do not appear to be equal across the different parasagittal divisions. For instance, electrophysiological recordings during a forelimb-related task in non-human primates found large populations of neurons related to the movements in dentate and interposed nuclei, but to a much lesser extent in fastigial nuclei [169]. Similarly, stimulation of fastigial nuclei elicits proximal, but not upper extremity movements, whereas interposed and dentate nuclei act the opposite [196, 197]. Furthermore, inactivation of the dentate and interposed nuclei does not have much influence on proximal body posture [198], whereas disruption of fastigial nuclei appears to significantly alter posture [199, 200]. To add to the complexity, the projection patterns of dentate and interposed nuclei to the thalamus interdigitate and are similar in density [63, 201–203], suggesting a possible overlapping function. Recordings from both dentate and interposed nuclei suggest they are both involved and control different aspects of the same kinds of forelimb movements [197].
Conclusion
In summary, it seems the phenotypic expression of action tremor has specific neural correlates, where a cerebellar module or small set of modules may exhibit distinct patterns of activity. Future studies testing the idea that the somatotopic representations are not equal across the parasagittal divisions of the cerebellum will be useful in localizing where subtypes of action tremor are occurring and will help guide transcranial stimulation therapy to target one or multiple specific regions of the cerebellum in individual ET patients with distinct subtypes of action tremor.
Funding Information
This work was supported by National Institutes of Health R01NS104423, R01NS118179, R01NS124854.
Footnotes
Competing Interests The authors declare no competing interests.
References
- 1.Elble R, Deuschl G. Milestones in tremor research. Mov Disord: Off J Mov Disord Soc 2011;26(6):1096–1105. [DOI] [PubMed] [Google Scholar]
- 2.Sternberg EJ, Alcalay RN, Levy OA, Louis ED. Postural and Intention Tremors: A Detailed Clinical Study of Essential Tremor vs. Parkinson’s Dis Front Neurol. 2013;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuhmayer N, Weber C, Kieler M, Voller B, Pirker W, Auff E, Haubenberger D. Task-dependent Variability of Essential Tremor. Parkinsonism Relat Disord. 2017;41:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED. Essential tremor: A nuanced approach to the clinical features. Pract Neurol. 2019;19(5):389–98. [DOI] [PubMed] [Google Scholar]
- 5.Schrag A, Münchau A, Bhatia KP, Quinn NP, Marsden CD. Essential tremor: An overdiagnosed condition? J Neurol. 2000;247(12):955–9. [DOI] [PubMed] [Google Scholar]
- 6.Bhidayasiri R. Differential diagnosis of common tremor syndromes. Postgrad Med J. 2005;81(962):756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Louis ED, Frucht SJ, Rios E. Intention Tremor in Essential Tremor: Prevalence and Association with Disease Duration. Mov Disord: Off J Mov Disord Soc. 2009;24(4):626–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis ED. Twelve clinical pearls to help distinguish essential tremor from other tremors. Expert Rev Neurother. 2014;14(9):1057–65. [DOI] [PubMed] [Google Scholar]
- 9.Critchley M. Observations on essential (heredofamial) tremor. Brain: A. J Neurol. 1949;72(Pt. 2):113–39. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: How are we misdiagnosing essential tremor? Arch Neurol. 2006;63(8):1100–4. [DOI] [PubMed] [Google Scholar]
- 11.Phibbs F, Fang JY, Cooper MK, Charles DP, Davis TL, Hedera P. Prevalence of unilateral tremor in autosomal dominant essential tremor. Mov Disord: Off J Mov Disord Soc. 2009;24(1):108–11. [DOI] [PubMed] [Google Scholar]
- 12.Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G, Tremor Task Force of the International Parkinson and Movement Disorder Society. Consensus Statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord: Off J Mov Disord Soc. 2018;33(1):75–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deuschl G, Wenzelburger R, Löffler K, Raethjen J, Stolze H. Essential tremor and cerebellar dysfunction clinical and kinematic analysis of intention tremor. Brain: A. J Neurol. 2000;123(Pt 8):1568–80. [DOI] [PubMed] [Google Scholar]
- 14.Köster B, Deuschl G, Lauk M, Timmer J, Guschlbauer B, Lücking CH. Essential tremor and cerebellar dysfunction: Abnormal ballistic movements. J Neurol Neurosurg Psychiatry. 2002;73(4):400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Habib-ur-Rehman MRCP. Diagnosis and management of tremor. Arch Intern Med. 2000;160(16):2438–44. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic J, Ashoori A. Movement disorders in musicians. Mov Disord: Off J Mov Disord Soc. 2008;23(14) [DOI] [PubMed] [Google Scholar]
- 17.Lee A, Furuya S, Altenmüller E. Epidemiology and treatment of 23 musicians with task specific tremor. J Clin Mov Disord. 2014;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Findley LJ, Capildeo R. Movement Disorders: Tremor. New York: Oxford University Press; 1984. [Google Scholar]
- 19.McAuley JH, Marsden CD. Physiological and pathological tremors and rhythmic central motor control. Brain: A. J Neurol. 2000;123(Pt 8):1545–67. [DOI] [PubMed] [Google Scholar]
- 20.Louis ED, Faust PL. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat Rev Neurol. 2020;16(2):69–83. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim MF, Beevis JC, Empson RM. Essential Tremor—A Cerebellar Driven Disorder? Neuroscience. 2021;462:262–73. [DOI] [PubMed] [Google Scholar]
- 22.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord: Off J Mov Disord Soc. 1998;13(Suppl 3):2–23. [DOI] [PubMed] [Google Scholar]
- 23.Hua SE, Lenz FA. Posture-related oscillations in human cerebellar thalamus in essential tremor are enabled by voluntary motor circuits. J Neurophysiol. 2005;93(1):117–27. [DOI] [PubMed] [Google Scholar]
- 24.Zakaria R, Lenz F, Hua S, Avin B, Liu C, Mari Z. Thalamic physiology of intentional essential tremor is more like cerebellar tremor than postural essential tremor. Brain Res. 2013;1529:188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elble RJ. The Essential Tremor Rating Assessment Scale. J Neurol Neuromed. 2016;1(4) [Google Scholar]
- 26.Isaacson SH, Peckham E, Tse W, Waln O, Way C, Petrossian MT, Dahodwala N, Soileau MJ, Lew M, Dietiker C, Luthra N, Agarwal P, Dhall R, Morgan J, Calakos N, Zesiewicz TA, Shamim EA, Kumar R, LeWitt P, et al. Prospective Home-use Study on Non-invasive Neuromodulation Therapy for Essential Tremor. Tremor Other Hyperkinetic Mov. 2020;10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papapetropoulos S, Lee MS, Versavel S, Newbold E, Jinnah HA, Pahwa R, Lyons KE, Elble R, Ondo W, Zesiewicz T, Hedera P, Handforth A, Elder J, Versavel M. A Phase 2 Proof-of-Concept, Randomized, Placebo-Controlled Trial of CX-8998 in Essential Tremor. Mov Disord: Off J Mov Disord Soc. 2021;36(8):1944–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis ED, Bares M, Benito-Leon J, Fahn S, Frucht SJ, Jankovic J, Ondo WG, Pal PK, Tan E-K. Essential tremor-plus: A controversial new concept. Lancet Neurol. 2020;19(3):266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta DK, Marano M, Zweber C, Boyd JT, Kuo S-H. Prevalence and Relationship of Rest Tremor and Action Tremor in Parkinson’s Disease. Tremor Other Hyperkinetic Mov. 2020;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koller WC, Rubino FA. Combined Resting-Postural Tremors. Arch Neurol. 1985;42(7):683–4. [DOI] [PubMed] [Google Scholar]
- 31.Rapoport A, Braun H, Aviv A, Sarova I. Combined resting-postural tremor of the head with a changing axis. Mov Disord: Off J Mov Disord Soc. 1991;6(3):261–2. [DOI] [PubMed] [Google Scholar]
- 32.Rajput AH, Rozdilsky B, Ang L, Rajput A. Significance of parkinsonian manifestations in essential tremor. Can J Neurol Sci Le J Can Des Sci Neurol. 1993;20(2):114–7. [DOI] [PubMed] [Google Scholar]
- 33.Cohen O, Pullman S, Jurewicz E, Watner D, Louis ED. Rest tremor in patients with essential tremor: Prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60(3):405–10. [DOI] [PubMed] [Google Scholar]
- 34.Thenganatt MA, Louis ED. Distinguishing essential tremor from Parkinson’s disease: Bedside tests and laboratory evaluations. Expert Rev Neurother. 2012;12(6):687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thenganatt MA, Jankovic J. The relationship between essential tremor and Parkinson’s disease. Parkinsonism Relat Disord. 2016;22(Suppl 1):S162–5. [DOI] [PubMed] [Google Scholar]
- 36.Lang AE, Jog M, Ashby P. “Weight-holding tremor”: An unusual task-specific form of essential tremor? Mov Disord: Off J Mov Disord Soc. 1995;10(2):228–9. [DOI] [PubMed] [Google Scholar]
- 37.Hesselmann V, Maarouf M, Hunsche S, Lasek K, Schaaf M, Krug B, Lackner K, Sturm V, Wedekind C. Functional MRI for immediate monitoring stereotactic thalamotomy in a patient with essential tremor. Eur Radiol. 2006;16(10):2229–33. [DOI] [PubMed] [Google Scholar]
- 38.Neely KA, Kurani AS, Shukla P, Planetta PJ, Wagle Shukla A, Goldman JG, Corcos DM, Okun MS, Vaillancourt DE. Functional Brain Activity Relates to 0–3 and 3–8 Hz Force Oscillations in Essential Tremor. Cereb Cortex. 2015;25(11):4191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Novak T, Newell KM. Physiological tremor (8–12Hz component) in isometric force control. Neurosci Lett. 2017;641:87–93. [DOI] [PubMed] [Google Scholar]
- 40.Dirkx MF, Zach H, Bloem BR, Hallett M, Helmich RC. The nature of postural tremor in Parkinson disease. Neurology. 2018;90(13):e1095–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cichaczewski E, Munhoz RP, Maia JM, Nohama P, Nóvak EM, Teive HA. Electrophysiologic characteristics of tremor in Parkinson’s disease and essential tremor. Arq Neuropsiquiatr. 2014;72(4):301–6. [DOI] [PubMed] [Google Scholar]
- 42.Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH. Activation mapping in essential tremor with functional magnetic resonance imaging. Ann Neurol. 1997;41(1):32–40. [DOI] [PubMed] [Google Scholar]
- 43.Contarino MF, Groot PFC, van der Meer JN, Bour LJ, Speelman JD, Nederveen AJ, van den Munckhof P, Tijssen MAJ, Schuurman PR, van Rootselaar A-F. Is There a Role for Combined EMG-fMRI in Exploring the Pathophysiology of Essential Tremor and Improving Functional Neurosurgery? PLoS One. 2012;7(10):e46234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broersma M, van der Stouwe AMM, Buijink AWG, de Jong BM, Groot PFC, Speelman JD, Tijssen MAJ, van Rootselaar A-F, Maurits NM. Bilateral cerebellar activation in unilaterally challenged essential tremor. NeuroImage: Clinical. 2015;11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buijink AWG, Madelein van der Stouwe AM, Broersma M, Sharifi S, Groot PFC, Speelman JD, Maurits NM, van Rootselaar AF. Motor network disruption in essential tremor: a functional and effective connectivity study. Brain J Neurol. 2015;138(Pt 10):2934–47. [DOI] [PubMed] [Google Scholar]
- 46.Fang W, Chen H, Wang H, Zhang H, Puneet M, Liu M, Lv F, Luo T, Cheng O, Wang X, Lu X. Essential tremor is associated with disruption of functional connectivity in the ventral intermediate Nucleus—Motor Cortex—Cerebellum circuit. Hum Brain Mapp. 2016;37(1):165–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boscolo Galazzo I, Magrinelli F, Pizzini FB, Storti SF, Agosta F, Filippi M, Marotta A, Mansueto G, Menegaz G, Tinazzi M. Voxel-based morphometry and task functional magnetic resonance imaging in essential tremor: Evidence for a disrupted brain network. Sci Rep. 2020;10(1):15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicoletti V, Cecchi P, Frosini D, Pesaresi I, Fabbri S, Diciotti S, Bonuccelli U, Cosottini M, Ceravolo R. Morphometric and functional MRI changes in essential tremor with and without resting tremor. J Neurol. 2015;262(3):719–28. [DOI] [PubMed] [Google Scholar]
- 49.Buijink AWG, Broersma M, van der Stouwe AMM, van Wingen GA, Groot PFC, Speelman JD, Maurits NM, van Rootselaar AF. Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor. Parkinsonism Relat Disord. 2015;21(4):383–8. [DOI] [PubMed] [Google Scholar]
- 50.Colebatch JG, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. Preliminary report: Activation of the cerebellum in essential tremor. Lancet (London, England). 1990;336(8722):1028–30. [DOI] [PubMed] [Google Scholar]
- 51.Jenkins IH, Bain PG, Colebatch JG, Thompson PD, Findley LJ, Frackowiak RS, Marsden CD, Brooks DJ. A positron emission tomography study of essential tremor: Evidence for overactivity of cerebellar connections. Ann Neurol. 1993;34(1):82–90. [DOI] [PubMed] [Google Scholar]
- 52.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. Red nuclear and cerebellar but no olivary activation associated with essential tremor: A positron emission tomographic study. Ann Neurol. 1994;36(4):636–42. [DOI] [PubMed] [Google Scholar]
- 53.Wills AJ, Jenkins IH, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of cerebral activation associated with essential and writing tremor. Arch Neurol. 1995;52(3):299–305. [DOI] [PubMed] [Google Scholar]
- 54.Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thompson PD, Findley LJ, Brooks DJ. The effect of ethanol on alcoholresponsive essential tremor: A positron emission tomography study. Ann Neurol. 1996;39(5):650–8. [DOI] [PubMed] [Google Scholar]
- 55.Rajput AH, Maxood K, Rajput A. Classic Essential Tremor Changes Following Cerebellar Hemorrhage. Neurology. 2008;71(21):1739–40. [DOI] [PubMed] [Google Scholar]
- 56.Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE. Homolateral disappearance of essential tremor after cerebellar stroke. Mov Disord: Off J Mov Disord Soc. 1989;4(2):183–7. [DOI] [PubMed] [Google Scholar]
- 57.Dupuis MJ-M, Evrard FLA, Jacquerye PG, Picard GR, Lermen OG. Disappearance of essential tremor after stroke. Mov Disord: Off J Mov Disord Soc. 2010;25(16):2884–7. [DOI] [PubMed] [Google Scholar]
- 58.Duncan R, Bone I, Melville ID. Essential tremor cured by infarction adjacent to the thalamus. J Neurol Neurosurg Psychiatry. 1988;51(4):591–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Im JH, Kim JS, Lee MC. Disappearance of essential tremor after small thalamic hemorrhage. Clin Neurol Neurosurg. 1996;98(1):40–2. [DOI] [PubMed] [Google Scholar]
- 60.Barbaud A, Hadjout K, Blard JM, Pagès M. Improvement in Essential Tremor after Pure Sensory Stroke due to Thalamic Infarction. Eur Neurol. 2001;46(1):57–9. [DOI] [PubMed] [Google Scholar]
- 61.Nagaratnam N, Kalasabail G. Contralateral abolition of essential tremor following a pontine stroke. J Neurol Sci. 1997;149(2):195–6. [DOI] [PubMed] [Google Scholar]
- 62.Allen GI, Tsukahara N. Cerebrocerebellar communication systems. Physiol Rev. 1974;54(4):957–1006. [DOI] [PubMed] [Google Scholar]
- 63.Asanuma C, Thach WR, Jones EG. Anatomical evidence for segregated focal groupings of efferent cells and their terminal ramifications in the cerebellothalamic pathway of the monkey. Brain Res. 1983a;286(3):267–97. [DOI] [PubMed] [Google Scholar]
- 64.Joutsa J, Shih LC, Horn A, Reich MM, Wu O, Rost NS, Fox MD. Identifying therapeutic targets from spontaneous beneficial brain lesions. Ann Neurol. 2018;84(1):153–7. [DOI] [PubMed] [Google Scholar]
- 65.Hirai T, Miyazaki M, Nakajima H, Shibazaki T, Ohye C. The correlation between tremor characteristics and the predicted volume of effective lesions in stereotaxic nucleus ventralis intermedius thalamotomy. Brain: A. J Neurol. 1983;106(Pt 4):1001–18. [DOI] [PubMed] [Google Scholar]
- 66.Speelman JD, Schuurman PR, de Bie RM, Bosch DA. Thalamic surgery and tremor. Mov Disord: Off J Mov Disord Soc. 1998;13(Suppl 3):103–6. [DOI] [PubMed] [Google Scholar]
- 67.Elias WJ, Huss D, Voss T, Loomba J, Khaled M, Zadicario E, Frysinger RC, Sperling SA, Wylie S, Monteith SJ, Druzgal J, Shah BB, Harrison M, Wintermark M. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2013;369(7):640–8. [DOI] [PubMed] [Google Scholar]
- 68.Elias WJ, Lipsman N, Ondo WG, Ghanouni P, Kim YG, Lee W, Schwartz M, Hynynen K, Lozano AM, Shah BB, Huss D, Dallapiazza RF, Gwinn R, Witt J, Ro S, Eisenberg HM, Fishman PS, Gandhi D, Halpern CH, et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N Engl J Med. 2016;375(8):730–9. [DOI] [PubMed] [Google Scholar]
- 69.Lipsman N, Schwartz ML, Huang Y, Lee L, Sankar T, Chapman M, Hynynen K, Lozano AM. MR-guided focused ultrasound thalamotomy for essential tremor: A proof-of-concept study. Lancet Neurol. 2013;12(5):462–8. [DOI] [PubMed] [Google Scholar]
- 70.Wintermark M, Huss DS, Shah BB, Tustison N, Druzgal TJ, Kassell N, Elias WJ. Thalamic connectivity in patients with essential tremor treated with MR imaging-guided focused ultrasound: In vivo fiber tracking by using diffusion-tensor MR imaging. Radiology. 2014;272(1):202–9. [DOI] [PubMed] [Google Scholar]
- 71.Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet (London, England). 1991;337(8738):403–6. [DOI] [PubMed] [Google Scholar]
- 72.Koller WC, Pahwa PR, Lyons KE, Wilkinson SB. Deep brain stimulation of the Vim nucleus of the thalamus for the treatment of tremor. Neurology. 2000;55(12 Suppl 6):S29–33. [PubMed] [Google Scholar]
- 73.Koller WC, Lyons KE, Wilkinson SB, Troster AI, Pahwa R. Long-term safety and efficacy of unilateral deep brain stimulation of the thalamus in essential tremor. Mov Disord: Off J Mov Disord Soc. 2001;16(3):464–8. [DOI] [PubMed] [Google Scholar]
- 74.Ceballos-Baumann AO, Boecker H, Fogel W, Alesch F, Bartenstein P, Conrad B, Diederich N, von Falkenhayn I, Moringlane JR, Schwaiger M, Tronnier VM. Thalamic stimulation for essential tremor activates motor and deactivates vestibular cortex. Neurology. 2001;56(10):1347–54. [DOI] [PubMed] [Google Scholar]
- 75.Perlmutter JS, Mink JW, Bastian AJ, Zackowski K, Hershey T, Miyawaki E, Koller W, Videen TO. Blood flow responses to deep brain stimulation of thalamus. Neurology. 2002;58(9):1388–94. [DOI] [PubMed] [Google Scholar]
- 76.Zackowski KM, Bastian AJ, Hakimian S, Mink JW, Perlmutter JS, Koller WC, Thach WT. Thalamic stimulation reduces essential tremor but not the delayed antagonist muscle timing. Neurology. 2002;58(3):402–10. [DOI] [PubMed] [Google Scholar]
- 77.Vaillancourt DE, Sturman MM, Verhagen Metman L, Bakay R, a. E, & Corcos DM Deep brain stimulation of the VIM thalamic nucleus modifies several features of essential tremor. Neurology. 2003;61(7):919–25. [DOI] [PubMed] [Google Scholar]
- 78.Chen H, Hua SE, Smith MA, Lenz FA, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex. 2006;16(10):1462–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein JC, Barbe MT, Seifried C, Baudrexel S, Runge M, Maarouf M, Gasser T, Hattingen E, Liebig T, Deichmann R, Timmermann L, Weise L, Hilker R. The tremor network targeted by successful VIM deep brain stimulation in humans. Neurology. 2012;78(11):787–95. [DOI] [PubMed] [Google Scholar]
- 80.Hua SE, Lenz FA, Zirh TA, Reich SG, Dougherty PM. Thalamic neuronal activity correlated with essential tremor. J Neurol Neurosurg Psychiatry. 1998;64(2):273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Molnar GF, Pilliar A, Lozano AM, Dostrovsky JO. Differences in neuronal firing rates in pallidal and cerebellar receiving areas of thalamus in patients with Parkinson’s disease, essential tremor, and pain. J Neurophysiol. 2005;93(6):3094–101. [DOI] [PubMed] [Google Scholar]
- 82.Scherer M, Steiner LA, Kalia SK, Hodaie M, Kühn AA, Lozano AM, Hutchison WD, Milosevic L. Single-neuron bursts encode pathological oscillations in subcortical nuclei of patients with Parkinson’s disease and essential tremor. Proc Natl Acad Sci U S A. 2022;119(35):e2205881119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Modulation of postural wrist tremors by magnetic stimulation of the motor cortex in patients with Parkinson’s disease or essential tremor and in normal subjects mimicking tremor. Ann Neurol. 1993;33(5):473–9. [DOI] [PubMed] [Google Scholar]
- 84.Hellriegel H, Schulz EM, Siebner HR, Deuschl G, Raethjen JH. Continuous theta-burst stimulation of the primary motor cortex in essential tremor. Clin Neurophysiol. 2012;123(5):1010–5. [DOI] [PubMed] [Google Scholar]
- 85.Chuang W-L, Huang Y-Z, Lu C-S, Chen R-S. Reduced cortical plasticity and GABAergic modulation in essential tremor. Mov Disord: Off J Mov Disord Soc. 2014;29(4):501–7. [DOI] [PubMed] [Google Scholar]
- 86.Louis ED, Faust PL, Vonsattel J-PG, Honig LS, Rajput A, Robinson CA, Rajput A, Pahwa R, Lyons KE, Ross GW, Borden S, Moskowitz CB, Lawton A, Hernandez N. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain: A. J Neurol. 2007;130(Pt 12):3297–307. [DOI] [PubMed] [Google Scholar]
- 87.Choe M, Cortés E, Vonsattel J-PG, Kuo S-H, Faust PL, Louis ED. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord: Off J Mov Disord Soc. 2016;31(3):393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Louis ED, Faust PL, Ma KJ, Yu M, Cortes E, Vonsattel J-PG. Torpedoes in the cerebellar vermis in essential tremor cases vs. Controls. Cerebellum (London, England). 2011;10(4):812–9. [DOI] [PubMed] [Google Scholar]
- 89.Babij R, Lee M, Cortés E, Vonsattel J-PG, Faust PL, Louis ED. Purkinje cell axonal anatomy: Quantifying morphometric changes in essential tremor versus control brains. Brain: A. J Neurol. 2013;136(Pt 10):3051–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Louis ED, Lee M, Babij R, Ma K, Cortés E, Vonsattel J-PG, Faust PL. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain: A. J Neurol. 2014;137(Pt 12):3142–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Erickson-Davis CR, Faust PL, Vonsattel J-PG, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69(3):262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kuo S-H, Tang G, Louis ED, Ma K, Babji R, Balatbat M, Cortes E, Vonsattel J-PG, Yamamoto A, Sulzer D, Faust PL. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125(6):879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lin C-Y, Louis ED, Faust PL, Koeppen AH, Vonsattel J-PG, Kuo S-H. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain: A. J Neurol. 2014;137(Pt 12):3149–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Louis RJ, Lin C-Y, Faust PL, Koeppen AH, Kuo S-H. Climbing fiber synaptic changes correlate with clinical features in essential tremor. Neurology. 2015;84(22):2284–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuo S-H, Lin C-Y, Wang J, Liou J-Y, Pan M-K, Louis RJ, Wu W-P, Gutierrez J, Louis ED, Faust PL. Deep brain stimulation and climbing fiber synaptic pathology in essential tremor. Ann Neurol. 2016;80(3):461–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuo S-H, Lin C-Y, Wang J, Sims PA, Pan M-K, Liou J-Y, Lee D, Tate WJ, Kelly GC, Louis ED, Faust PL. Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases. Acta Neuropathol. 2017;133(1):121–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee D, Gan S-R, Faust PL, Louis ED, Kuo S-H. Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes. Parkinsonism Relat Disord. 2018;51:24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wu Y-C, Louis ED, Gionco J, Pan M-K, Faust PL, Kuo S-H. Increased Climbing Fiber Lateral Crossings on Purkinje Cell Dendrites in the Cerebellar Hemisphere in Essential Tremor. Mov Disord: Off J Mov Disord Soc. 2021;36(6):1440–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thier S, Lorenz D, Nothnagel M, Poremba C, Papengut F, Appenzeller S, Paschen S, Hofschulte F, Hussl A-C, Hering S, Poewe W, Asmus F, Gasser T, Schöls L, Christensen K, Nebel A, Schreiber S, Klebe S, Deuschl G, Kuhlenbäumer G. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology. 2012;79(3):243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang J, Kelly GC, Tate WJ, Li YS, Lee M, Gutierrez J, Louis ED, Faust PL, Kuo S-H. Excitatory Amino acid transporter expression in the essential tremor dentate nucleus and cerebellar cortex: A postmortem study. Parkinsonism Relat Disord. 2016;32:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Louis ED, Kerridge CA, Chatterjee D, Martuscello RT, Diaz DT, Koeppen AH, Kuo S-H, Vonsattel J-PG, Sims PA, Faust PL. Contextualizing the pathology in the essential tremor cerebellar cortex: A patholog-omics approach. Acta Neuropathol. 2019;138(5):859–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee M, Cheng MM, Lin C-Y, Louis ED, Faust PL, Kuo S-H. Decreased EAAT2 protein expression in the essential tremor cerebellar cortex. Acta Neuropathol Commun. 2014;2:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pan MK, Li YS, Wong SB, Ni CL, Wang YM, Liu WC, Lu LY, Lee JC, Cortes EP, Vonsattel JPG, Sun Q, Louis ED, Faust PL, Kuo SH. Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology. Sci Transl Med. 2020;12(526):eaay1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong S-B, Wang Y-M, Lin C-C, Geng SK, Vanegas-Arroyave N, Pullman SL, Kuo S-H, Pan M-K. Cerebellar Oscillations in Familial and Sporadic Essential Tremor. Cerebellum (London, England). 2022;21(3):425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schreglmann SR, Wang D, Peach RL, Li J, Zhang X, Latorre A, Rhodes E, Panella E, Cassara AM, Boyden ES, Barahona M, Santaniello S, Rothwell J, Bhatia KP, Grossman N. Non-invasive suppression of essential tremor via phase-locked disruption of its temporal coherence. Nat Commun. 2021;12(1):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brodal P The pontocerebellar projection in the rhesus monkey: An experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4(2):193–208. [DOI] [PubMed] [Google Scholar]
- 107.Thielert CD, Thier P. Patterns of projections from the pontine nuclei and the nucleus reticularis tegmenti pontis to the posterior vermis in the rhesus monkey: A study using retrograde tracers. J Comp Neurol. 1993;337(1):113–26. [DOI] [PubMed] [Google Scholar]
- 108.Cicirata F, Serapide MF, Parenti R, Pantò MR, Zappalà A, Nicotra A, Cicero D. The basilar pontine nuclei and the nucleus reticularis tegmenti pontis subserve distinct cerebrocerebellar pathways. Prog Brain Res. 2005;148:259–82. [DOI] [PubMed] [Google Scholar]
- 109.Tsukahara N, Korn H, Stone J. Pontine Relay from Cerebral Cortex to Cerebellar Cortex and Nucleus Interpositus. Brain Res. 1968;10(3):448–53. [DOI] [PubMed] [Google Scholar]
- 110.Evarts EV, Thach WT. Motor mechanisms of the CNS: Cerebrocerebellar interrelations. Annu Rev Physiol. 1969;31:451–98. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki L, Coulon P, Sabel-Goedknegt EH, Ruigrok TJH. Organization of cerebral projections to identified cerebellar zones in the posterior cerebellum of the rat. J Neurosci Off J Soc Neurosci. 2012;32(32):10854–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci. 2005;6(4):297–311. [DOI] [PubMed] [Google Scholar]
- 113.Ruigrok TJH. Ins and outs of cerebellar modules. Cerebellum (London, England). 2011;10(3):464–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Berkley KJ, Hand PJ. Projections to the Inferior Olive of the Cat. II. Comparisons of Input from the Gracile, Cuneate and the Spinal Trigeminal Nuclei. J Comp Neurol. 1978;180(2):253–64. 10.1002/cne.901800205. [DOI] [PubMed] [Google Scholar]
- 115.Hoffmann KP, Distler C, Erickson RG, Mader W. Physiological and Anatomical Identification of the Nucleus of the Optic Tract and Dorsal Terminal Nucleus of the Accessory Optic Tract in Monkeys. Exp Brain Res. 1988;69(3):635–44. 10.1007/BF00247315. [DOI] [PubMed] [Google Scholar]
- 116.Onodera S Olivary Projections from the Mesodiencephalic Structures in the Cat Studied by Means of Axonal Transport of Horseradish Peroxidase and Tritiated Amino Acids. J Comp Neurol. 1984;227(1):37–49. 10.1002/cne.902270106. [DOI] [PubMed] [Google Scholar]
- 117.Saint-Cyr JA. The Projection from the Motor Cortex to the Inferior Olive in the Cat. An Experimental Study Using Axonal Transport Techniques. Neuroscience. 1983;10(3):667–84. 10.1016/03064522(83)90209-9. [DOI] [PubMed] [Google Scholar]
- 118.Palay SL, Chan-Palay V. Cerebellar Cortex Berlin. Heidelberg: Heidelberg: Springer Berlin; 1974. [Google Scholar]
- 119.Chan-Palay V. Cerebellar Dentate Nucleus; Organization, Cytology and Transmitters. Berlin: Springer-Verlag; 1977. [Google Scholar]
- 120.Eccles JC. Circuits in the cerebellar control of movement. Proc Natl Acad Sci U S A. 1967;58(1):336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jansen J, Brodal A. Experimental studies on the intrinsic fibers of the cerebellum. II. The cortico-nuclear projection. J Comp Neurol. 1940;73(2):267–321. [PubMed] [Google Scholar]
- 122.Chambers WW, Sprague JM. Functional localization in the cerebellum. II. Somatotopic organization in cortex and nuclei. A.M.A. Arch Neurol Psychiatr. 1955b;74(6):653–80. [DOI] [PubMed] [Google Scholar]
- 123.Gould BB. The organization of afferents to the cerebellar cortex in the cat: Projections from the deep cerebellar nuclei. J Comp Neurol. 1979;184(1):27–42. [DOI] [PubMed] [Google Scholar]
- 124.Person AL, Raman IM. Purkinje neuron synchrony elicits time-locked spiking in the cerebellar nuclei. Nature. 2011;481(7382):502–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Päällysaho J, Sugita S, Noda H. Cerebellar corticonuclear and nucleocortical projections in the vermis of posterior lobe of the rat as studied with anterograde and retrograde transport of WGAHRP. Neurosci Res. 1990;8(3):158–78. [DOI] [PubMed] [Google Scholar]
- 126.Kebschull JM, Richman EB, Ringach N, Friedmann D, Albarran E, Kolluru SS, Jones RC, Allen WE, Wang Y, Cho SW, Zhou H, Ding JB, Chang HY, Deisseroth K, Quake SR, Luo L. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science (New York, N.Y.) 2020;370(6523):eabd5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yopak KE, Pakan JMP, Wylie D. In: Kaas JH, editor. Evolution of Nervous Systems, vol. 1–4. 2nd ed. Academic Press; 2016. p. 373–85. [Google Scholar]
- 128.Sasaki K, Jinnai K, Gemba H, Hashimoto S, Mizuno N. Projection of the cerebellar dentate nucleus onto the frontal association cortex in monkeys. Exp Brain Res. 1979;37(1):193–8. [DOI] [PubMed] [Google Scholar]
- 129.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17(5):241–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tellmann S, Bludau S, Eickhoff S, Mohlberg H, Minnerop M, Amunts K. Cytoarchitectonic mapping of the human brain cerebellar nuclei in stereotaxic space and delineation of their co-activation patterns. Front Neuroanat. 2015;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Allen GI, Gilbert PF, Yin TC. Convergence of cerebral inputs onto dentate neurons in monkey. Exp Brain Res. 1978;32(2):151–70. [DOI] [PubMed] [Google Scholar]
- 132.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89(1):634–9. [DOI] [PubMed] [Google Scholar]
- 133.Gibson AR, Robinson FR, Alam J, Houk JC. Somatotopic alignment between climbing fiber input and nuclear output of the cat intermediate cerebellum. J Comp Neurol. 1987;260(3):362–77. [DOI] [PubMed] [Google Scholar]
- 134.Boillat Y, Bazin P-L, van der Zwaag W. Whole-body somatotopic maps in the cerebellum revealed with 7T fMRI. NeuroImage. 2020;211:116624. [DOI] [PubMed] [Google Scholar]
- 135.Ito M and Bases and implications of learning in the cerebellum—Adaptive control and internal model mechanism. Prog Brain Res. 2005;148:95–109. [DOI] [PubMed] [Google Scholar]
- 136.Growdon JH, Chambers WW, Liu CN. An experimental study of cerebellar dyskinesia in the rhesus monkey. Brain: A. J Neurol. 1967;90(3):603–32. [DOI] [PubMed] [Google Scholar]
- 137.Brooks VB, Kozlovskaya IB, Atkin A, Horvath FE, Uno M. Effects of cooling dentate nucleus on tracking-task performance in monkeys. J Neurophysiol. 1973;36(6):974–95. [DOI] [PubMed] [Google Scholar]
- 138.Cooke JD, Thomas JS. Forearm oscillation during cooling of the dentate mucleus in the monkey. Can J Physiol Pharmacol. 1976;54(4):430–6. [DOI] [PubMed] [Google Scholar]
- 139.Vilis T, Hore J. Effects of changes in mechanical state of limb on cerebellar intention tremor. J Neurophysiol. 1977;40(5):1214–24. [DOI] [PubMed] [Google Scholar]
- 140.Flament D, Hore J. Movement and electromyographic disorders associated with cerebellar dysmetria. J Neurophysiol. 1986;55(6):1221–33. [DOI] [PubMed] [Google Scholar]
- 141.Monzée J, Drew T, Smith AM. Effects of muscimol inactivation of the cerebellar nuclei on precision grip. J Neurophysiol. 2004;91(3):1240–9. [DOI] [PubMed] [Google Scholar]
- 142.Flumerfelt BA, Otabe S, Courville J. Distinct projections to the red nucleus from the dentate and interposed nuclei in the monkey. Brain Res. 1973;50(2):408–14. [DOI] [PubMed] [Google Scholar]
- 143.Walberg F, Dietrichs E, Nordby T. The origin and termination of the dentatorubral fibres in the cat as studied with retrograde and anterograde transport of peroxidase labelled lectin. Exp Brain Res. 1986;63(2):294–300. [DOI] [PubMed] [Google Scholar]
- 144.Carrea RME, Mettler FA. Function of the primate brachium conjunctivum and related structures. J Comp Neurol. 1955;102(1):151–322. [DOI] [PubMed] [Google Scholar]
- 145.Mai N, Bolsinger P, Avarello M, Diener HC, Dichgans J. Control of isometric finger force in patients with cerebellar disease. Brain: A. J Neurol. 1988;111(Pt 5):973–98. [DOI] [PubMed] [Google Scholar]
- 146.Müller F, Dichgans J. Impairments of precision grip in two patients with acute unilateral cerebellar lesions: A simple parametric test for clinical use. Neuropsychologia. 1994;32(2):265–9. [DOI] [PubMed] [Google Scholar]
- 147.Serrien DJ, Wiesendanger M. Role of the cerebellum in tuning anticipatory and reactive grip force responses. J Cogn Neurosci. 1999;11(6):672–81. [DOI] [PubMed] [Google Scholar]
- 148.Fellows SJ, Ernst J, Schwarz M, Töpper R, Noth J. Precision grip deficits in cerebellar disorders in man. Clin Neurophysiol. 2001;112(10):1793–802. [DOI] [PubMed] [Google Scholar]
- 149.Anens E, Kristensen B, Häger-Ross C. Reactive grip force control in persons with cerebellar stroke: Effects on ipsilateral and contralateral hand. Exp Brain Res. 2010;203(1):21–30. [DOI] [PubMed] [Google Scholar]
- 150.Carrea RME, Mettler FA. Physiologic consequences following extensive removals of the cerebellar cortex and deep cerebellar nuclei and effect of secondary cerebral ablations in the primate. J Comp Neurol. 1947;87(3):169–288. [DOI] [PubMed] [Google Scholar]
- 151.Leiner HC, Leiner AL, Dow RS. Reappraising the cerebellum: What does the hindbrain contribute to the forebrain? Behav Neurosci. 1989;103(5):998–1008. [DOI] [PubMed] [Google Scholar]
- 152.Brooks VB. How are “move” and “hold” programs matched? In: Bloedel JR, Dichigans J, Precht W, editors. Cerebellar Functions. Berlin: Springer-Verlag; 1984. p. 1–23. [Google Scholar]
- 153.Hore J, Vilis T. A Cerebellar-dependent efference copy mechanism for generating appropriate muscle responses to limb perturbations. In: Bloedel JR, Dichigans J, Precht W, editors. Cerebellar Functions. Berlin: Springer-Verlag; 1984. p. 24–35. [Google Scholar]
- 154.Hartstone WG, Brown MH, Kelly GC, Tate WJ, Kuo S-H, Dwork AJ, Louis ED, Faust PL. Dentate Nucleus Neuronal Density: A Postmortem Study of Essential Tremor Versus Control Brains. Mov Disord: Off J Mov Disord Soc. 2021;36(4):995–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.van Kan PL, Gibson AR, Houk JC. Movement-related inputs to intermediate cerebellum of the monkey. J Neurophysiol. 1993;69(1):74–94. [DOI] [PubMed] [Google Scholar]
- 156.van Kan PL, Horn KM, Gibson AR. The importance of hand use to discharge of interpositus neurones of the monkey. J Physiol. 1994;480(Pt 1):171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gibson AR, Horn KM, Van Kan PLE. Chapter 5 Grasping Cerebellar Function. In: Bennett KMB, Castiello U, editors. Advances in Psychology, vol. 105. North-Holland; 1994. p. 85–108. [Google Scholar]
- 158.Gibson AR, Horn KM, Stein JF, Van Kan PL. Activity of interpositus neurons during a visually guided reach. Can J Physiol Pharmacol. 1996;74(4):499–512. [PubMed] [Google Scholar]
- 159.Mason CR, Miller LE, Baker JF, Houk JC. Organization of reaching and grasping movements in the primate cerebellar nuclei as revealed by focal muscimol inactivations. J Neurophysiol. 1998;79(2):537–54. [DOI] [PubMed] [Google Scholar]
- 160.Cooper SE, Martin JH, Ghez C. Effects of inactivation of the anterior interpositus nucleus on the kinematic and dynamic control of multijoint movement. J Neurophysiol. 2000;84(4):1988–2000. [DOI] [PubMed] [Google Scholar]
- 161.Martin JH, Cooper SE, Hacking A, Ghez C. Differential effects of deep cerebellar nuclei inactivation on reaching and adaptive control. J Neurophysiol. 2000;83(4):1886–99. [DOI] [PubMed] [Google Scholar]
- 162.Low AYT, Thanawalla AR, Yip AKK, Kim J, Wong KLL, Tantra M, Augustine GJ, Chen AI. Precision of Discrete and Rhythmic Forelimb Movements Requires a Distinct Neuronal Subpopulation in the Interposed Anterior Nucleus. Cell Rep. 2018;22(9):2322–33. [DOI] [PubMed] [Google Scholar]
- 163.Ekerot CF, Garwicz M, Jörntell H. The control of forelimb movements by intermediate cerebellum. Prog Brain Res. 1997;114:423–9. [DOI] [PubMed] [Google Scholar]
- 164.Becker MI, Person AL. Cerebellar Control of Reach Kinematics for Endpoint Precision. Neuron. 2019;103(2):335–348.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Thach WT, Schieber SH, Elble RH. Motor Programs: Trajectory Versus Stability. In: Bloedel JR, Dichigans J, Precht W, editors. Cerebellar Functions. Berlin: Springer-Verlag; 1984. p. 36–51. [Google Scholar]
- 166.Hore J, Wild B, Diener HC. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol. 1991;65(3):563–71. [DOI] [PubMed] [Google Scholar]
- 167.Berardelli A, Hallett M, Rothwell JC, Agostino R, Manfredi M, Thompson PD, Marsden CD. Single-joint rapid arm movements in normal subjects and in patients with motor disorders. Brain: A. J Neurol. 1996;119(Pt 2):661–74. [DOI] [PubMed] [Google Scholar]
- 168.Britton TC, Thompson PD, Day BL, Rothwell JC, Findley LJ, Marsden CD. Rapid wrist movements in patients with essential tremor. The critical role of the second agonist burst. Brain: A. J Neurol. 1994;117(Pt 1):39–47. [DOI] [PubMed] [Google Scholar]
- 169.Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–42. [DOI] [PubMed] [Google Scholar]
- 170.Goodkin HP, Keating JG, Martin TA, Thach WT. Preserved simple and impaired compound movement after infarction in the territory of the superior cerebellar artery. Can J Neurol Sc Le J Can Des Sci Neurol. 1993;20(Suppl 3):S93–104. [DOI] [PubMed] [Google Scholar]
- 171.Milak MS, Shimansky Y, Bracha V, Bloedel JR. Effects of inactivating individual cerebellar nuclei on the performance and retention of an operantly conditioned forelimb movement. J Neurophysiol. 1997;78(2):939–59. [DOI] [PubMed] [Google Scholar]
- 172.Elble RJ, Schieber MH, Thach WT. Activity of muscle spindles, motor cortex and cerebellar nuclei during action tremor. Brain Research. 1984;323(2):330–4. [DOI] [PubMed] [Google Scholar]
- 173.Uno M, Kozlovskaya IB, Brooks VB. Effects of cooling interposed nuclei on tracking-task performance in monkeys. J Neurophysiol. 1973;36(6):996–1003. [DOI] [PubMed] [Google Scholar]
- 174.Oscarsson O, Sjölund B. The ventral spino-olivocerebellar system in the cat. I. Identification of five paths and their termination in the cerebellar anterior lobe. Exp Brain Res. 1977;28(5):469–86. [DOI] [PubMed] [Google Scholar]
- 175.Joseph JW, Shambes GM, Gibson JM, Welker W. Tactile projections to granule cells in caudal vermis of the rat’s cerebellum. Brain Behav Evol. 1978;15(2):141–9. [DOI] [PubMed] [Google Scholar]
- 176.Coffman KA, Dum RP, Strick PL. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc Natl Acad Sci U S A. 2011;108(38):16068–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sengul G, Fu Y, Yu Y, Paxinos G. Spinal cord projections to the cerebellum in the mouse. Brain Struct Funct. 2015;220(5):2997–3009. [DOI] [PubMed] [Google Scholar]
- 178.Pompeiano O, Andre P, Manzoni D. Spatiotemporal response properties of cerebellar Purkinje cells to animal displacement: A population analysis. Neuroscience. 1997;81(3):609–26. [DOI] [PubMed] [Google Scholar]
- 179.Manzoni D, Pompeiano O, Bruschini L, Andre P. Neck input modifies the reference frame for coding labyrinthine signals in the cerebellar vermis: A cellular analysis. Neuroscience. 1999;93(3):1095–107. [DOI] [PubMed] [Google Scholar]
- 180.Dash S, Catz N, Dicke PW, Thier P. Encoding of smooth-pursuit eye movement initiation by a population of vermal Purkinje cells. Cereb Cortex. 2012;22(4):877–91. [DOI] [PubMed] [Google Scholar]
- 181.Muzzu T, Mitolo S, Gava GP, Schultz SR. Encoding of locomotion kinematics in the mouse cerebellum. PLoS One. 2018;13(9):e0203900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zobeiri OA, Cullen KE. Distinct representations of body and head motion are dynamically encoded by Purkinje cell populations in the macaque cerebellum. ELife. 2022;11:e75018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ferrier D, Turner WA III. A record of experiments illustrative of the symptomatology and degenerations following lesions of the cerebellum and its peduncles and related structures in monkeys. Proc R Soc Lond. 1894;54(326–330):476–8. [Google Scholar]
- 184.Mauritz KH, Dichgans J, Hufschmidt A. Quantitative analysis of stance in late cortical cerebellar atrophy of the anterior lobe and other forms of cerebellar ataxia. Brain: A. J Neurol. 1979;102(3):461–82. [DOI] [PubMed] [Google Scholar]
- 185.Silfverskiöld BP. Cortical cerebellar degeneration associated with a specific disorder of standing and locomotion. Acta Neurol Scand. 1977;55(4):257–72. [DOI] [PubMed] [Google Scholar]
- 186.Mauritz KH, Schmitt C, Dichgans J. Delayed and enhanced long latency reflexes as the possible cause of postural tremor in late cerebellar atrophy. Brain: A. J Neurol. 1981;104(Pt 1):97–116. [DOI] [PubMed] [Google Scholar]
- 187.Chambers WW, Sprague JM. Functional localization in the cerebellum. I. Organization in longitudinal cortico-nuclear zones and their contribution to the control of posture, both extrapyramidal and pyramidal. J Comp Neurol. 1955a;103(1):105–29. [DOI] [PubMed] [Google Scholar]
- 188.Cheng MM, Tang G, Kuo S-H. Harmaline-induced tremor in mice: Videotape documentation and open questions about the model. Tremor Other Hyperkinetic Mov. 2013;3 tre-03-205-4668-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Goldberger ME, Growdon JH. Pattern of recovery following cerebellar deep nuclear lesions in monkeys. Exp Neurol. 1973;39(2):307–22. [DOI] [PubMed] [Google Scholar]
- 190.Gadot R, Shofty B, Najera RA, Anand A, Banks G, Khan AB, LoPresti MA, Vanegas Arroyave N, Sheth SA. Case Report: Dual Target Deep Brain Stimulation With Externalized Programming for Post-traumatic Complex Movement Disorder. Front Neurosci. 2021;15:774073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Moon D. Disorders of Movement due to Acquired and Traumatic Brain Injury. Curr Phys Med Rehabil Rep. 2022;10(4):311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Rispal-Padel L, Cicirata F, Pons C. Cerebellar nuclear topography of simple and synergistic movements in the alert baboon (Papio papio). Exp Brain Res. 1982;47(3):365–80. [DOI] [PubMed] [Google Scholar]
- 193.Rijntjes M, Buechel C, Kiebel S, Weiller C. Multiple somatotopic representations in the human cerebellum. Neuroreport. 1999;10(17):3653–8. [DOI] [PubMed] [Google Scholar]
- 194.Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saadon-Grosman N, Angeli PA, DiNicola LM, Buckner RL. A third somatomotor representation in the human cerebellum. J Neurophysiol. 2022;128(4):1051–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schultz W, Montgomery EB, Marini R. Proximal limb movements in response to microstimulation of primate dentate and interpositus nuclei mediated by brain-stem structures. Brain: A. J Neurol. 1979;102(1):127–46. [DOI] [PubMed] [Google Scholar]
- 197.MacKay WA. Cerebellar nuclear activity in relation to simple movements. Exp Brain Res. 1988;71(1):47–58. [DOI] [PubMed] [Google Scholar]
- 198.Goodkin HP, Thach WT. Cerebellar control of constrained and unconstrained movements I Nuclear Inactivation. J Neurophysiol. 2003;89(2):884–95. [DOI] [PubMed] [Google Scholar]
- 199.Botterell EH, Fulton JF. Functional localization in the cerebellum of primates III. Lesions of hemispheres (neocerebellum). J Comp Neurol. 1938;69(1):63–87. [Google Scholar]
- 200.Sprague JM, Chambers WW. Control of posture by reticular formation and cerebellum in the intract, anesthetized and unanesthetized and in the decerebrated cat. Am J Phys Anthropol. 1954;176(1):52–64. [DOI] [PubMed] [Google Scholar]
- 201.Asanuma C, Thach WT, Jones EG. Cytoarchitectonic delineation of the ventral lateral thalamic region in the monkey. Brain Res. 1983b;286(3):219–35. [DOI] [PubMed] [Google Scholar]
- 202.Asanuma C, Thach WT, Jones EG. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res. 1983c;286(3):237–65. [DOI] [PubMed] [Google Scholar]
- 203.Kalil K Projections of the cerebellar and dorsal column nuclei upon the thalamus of the rhesus monkey. J Comp Neurol. 1981;195(1):25–50. [DOI] [PubMed] [Google Scholar]


