Abstract
Although microvascular decompression (MVD) is a reliable treatment for trigeminal neuralgia (TN), neurosurgeons sometimes encounter patients whose symptoms do not improve postoperatively or who experience good treatment efficacy but develop other sensory disturbances. This study aims to objectively identify changes in nerve fibers before and after surgery by MRI and to clarify the relationship between the changes and residual postoperative symptoms. We retrospectively analyzed data from 36 consecutive patients who underwent MVD for classical TN at our hospital between November 2019 and November 2020. Cases that fulfilled the diagnostic criteria for multiple sclerosis were excluded. We confirmed the changes on the brainstem side of the trigeminal nerve preoperatively and at seven days postoperatively using 3D T2-SPACE MRI, in which the patients were divided into three groups: preoperative T2 high intensity positive (A), postoperative T2 high intensity positive (B), and no T2 high-intensity region (C). The primary outcome measures were therapeutic efficacy and frequency of postoperative numbness. The results of MVD surgery were evaluated one year postoperatively. The percentage of cases in which treatment outcomes were rated as excellent or good at one year: group A: 0 (0%), group B: 6 (100%), and group C: 25 (96.2%) (p < 0.05); the frequency of numbness: 2 (50%) in group A, 3 (50%) in group B, and 1 (3.8%) in group C, indicating significant differences between the three groups (p < 0.05). 3D T2-SPACE MRI sequences can be used to identify changes in trigeminal nerve fibers before and after MVD, which might correlate with eventual residual symptoms.
Keywords: trigeminal neuralgia, postoperative pain, numbness, microvascular decompression, solitary pontine lesion
Introduction
Trigeminal neuralgia (TN) is one of the most common forms of craniofacial neuropathic pain. The International Association for the Study of Pain describes TN as “sudden, usually unilateral, severe, brief, stabbing, recurrent episodes of pain in the area of distribution of one or more branches of the trigeminal nerve.”1) The main approach for patients with TN is to start with medications, and then, after careful follow-ups, if the patient does not respond to drug treatment or develops side effects to them, operative interventions, such as microvascular decompression (MVD), percutaneous balloon compression, percutaneous glycerol rhizotomy, percutaneous radiofrequency rhizotomy, and stereotactic radiosurgery, including gamma knife radiosurgery, are available.2)
MVD is based on the neurovascular compression hypothesis first described by Dandy.3,4) MVD was first performed by Gardner in 1959 and then popularized by Janetta. It has been considered the gold standard for TN treatment for nearly 60 years.5) MVD provides an approximately 80% chance of being pain-free, with a recurrence rate of approximately 10% over 10-20 years,6,7) However, neurosurgeons sometimes encounter patients whose symptoms do not improve postoperatively despite the removal of all the causative the vessels, or patients who experience excellent results in terms of treatment efficacy, but instead develop other sensory disturbances that were not present before surgery. Although trigeminal neuropathy associated with intraoperative manipulation might be a contributing factor, only one previous report has examined the relationship between eventual residual symptoms and objective evaluations using imaging studies.8)
This study examined pre- and postoperative three-dimensional T2 sampling perfection with application-optimized contrast using different flip angle evolution (3D T2‒SPACE) magnetic resonance imaging (MRI) sequences. We found a correlation between the appearance of signal changes on the brainstem side of trigeminal fibers and postoperative symptoms. This study aimed to objectively capture the changes in trigeminal nerve fibers before and after surgery by MRI and to clarify the correlation between the changes and residual postoperative symptoms.
Materials and Methods
Study design
The Ethics Committee of Nakamura Memorial Hospital approved this study protocol (Approval No. 2022032902), which was performed following the principles of the Declaration of Helsinki. This observational, non-randomized study identified participants via a retrospective electronic chart review of TN patients treated between November 2019 and November 2020 at the Nakamura Memorial Hospital. A skilled senior neurosurgeon doctor (S.N) with 22 years of experience performed all the surgical procedures.
Patients
We retrospectively collected the data of patients who underwent MVD surgery to treat classical TN (diagnosed using the International Headache Society diagnostic criteria)9) between November 2019 and November 2020. Cases that fulfilled the diagnostic criteria for multiple sclerosis were excluded.
Surgical procedure
Surgery was performed using a lateral suboccipital retrosigmoid approach with continuous intraoperative brain auditory evoked potentials monitoring. A C-shaped skin incision was made behind the ear within the hairline. A 4-cm bone flap was made in the supralateral portion of the suboccipital region to expose the transverse-sigmoid sinus junction. After the dural opening, a small amount of cerebrospinal fluid was drained from the supracerebellar cistern. Next, arachnoid membrane dissection was initiated from the supracerebellar cistern with gentle cerebellar retraction, ensuring that the supracerebellar bridging veins were not retracted. Following gentle inferomedial retraction of the cerebellar hemisphere to expose the superior petrosal vein, the arachnoid membrane inferior to the vein was sharply opened and additional cerebrospinal fluid was released. However, the arachnoid membrane over the petrosal vein and cranial nerves VII/VIII was left intact to protect these structures, if needed. Following exposure of the root entry zone of the trigeminal nerve, transposition of the offending vessels was performed using Teflon felt or fibrin glue. If a vein was intraoperatively suspected as being the causative vessel of TN, it was either carefully dissected out or a coagulation incision was performed after a comprehensive evaluation.
Evaluation of surgical results
We evaluated the results of MVD for TN one year after the surgery based on the classification proposed by the Japan Society for Microvascular Decompression Surgery (Table 1).10)
Table 1.
Assessment of results of microvascular decompression for trigeminal neuralgia
| Evaluation of postoperative pain (E) | |
| E-0 | Completely pain-free |
| E-1 | Occasional slight pain, self-controllable without medication |
| E-2 | Moderate pain, controllable with medication |
| E-3 | Persistent pain, not controllable with medication, not cured |
| Evaluation of complications (C) | |
| C-0 | No deficits, or only slight subjective complaints |
| C-1 | Slight cranial nerve or cerebellar dysfunction, does not interfere with daily life |
| C-2 | Both subjective and objective cranial nerve or cerebellar dysfunction, interferes with daily life |
| Total evaluation of results (T) | |
| T-0 | Excellent |
| T-1 | Good |
| T-2 | Fair |
| T-3 to T-5 | Poor |
Overall evaluation of results: If the results regarding complications are C1, the overall evaluation score is lowered by one rank; if it is C2, the overall evaluation score is lowered by two ranks compared to the results of E (example: E0 + C0 = T0, E0 + C1 = T1, E1 + C2 = T3).
Moreover, facial sensory disturbances were categorized according to the definition of Niwa et al.11) as follows: hypesthesia, decreased sensitivity to stimulation, excluding the special senses; dysesthesia, an unpleasant abnormal sensation that occurs spontaneously without external stimulation; and paresthesia, abnormal sensations caused by external stimuli.
The primary outcomes evaluated were the percentage of cases in each group in which the treatment was excellent or good and the frequency of numbness (including hypesthesia, dysesthesia, and paresthesia). All follow-ups in the patients in this study were conducted only by the senior doctor (S.N).
Clinical and statistical analysis
We confirmed the changes on the brainstem side of the trigeminal nerve preoperatively and at seven days postoperatively using the 3D T2-SPACE sequence, producing images with fewer cerebrospinal fluid flow artifacts and higher resolutions.12-14) Positive brainstem lesions were defined as those with significantly higher signal intensities than the healthy side. Subsequent imaging changes were examined in patients who underwent follow-up 3D T2-SPACE imaging in an outpatient setting. All patients underwent diffusion-weighted imaging (DWI) the day after surgery to check for the complication of brainstem infarction.
We classified the patients into three groups based on the preoperative and 7-days postoperative imaging results. Briefly, group A consisted of cases with preoperative signal changes in the brainstem region of the trigeminal nerve, group B consisted of cases with postoperative signal changes in the same region, and group C consisted of cases without any signal changes pre- and postoperatively in the same region. All images were analyzed by three experienced neurosurgeons blinded to the clinical data.
Along with assessing the primary outcome measures of therapeutic efficacy and frequency of postoperative numbness, each group was also evaluated for baseline characteristics, causative vessels, intraoperative manipulation, residual symptoms, and imaging features along the affected trigeminal pontine pathway.
Categorical variables were analyzed using the Chi-square test. Continuous variables were analyzed using analysis of variance. Statistical significance was set at p < 0.05. All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA).
Results
Clinical information
We analyzed the data of 36 consecutive patients who underwent MVD for classical TN at our hospital during the study period. Table 2 shows the general characteristics of the study group. Age, sex, affected side, preoperative duration of symptoms, baseline clinical risk factors, and offending vessels did not differ between the three groups.
Table 2.
Baseline characteristics of the study groups
| Baseline characteristics | group A (n = 4) |
group B (n = 6) |
group C (n = 26) |
P value |
|---|---|---|---|---|
| Age (mean ± SD), years | 68.5 ± 7.6 | 56.7 ± 13.0 | 61.3 ± 14.7 | 0.54 |
| Male, n (%) | 1 (25.0) | 3 (50.0) | 14 (53.8) | 0.8 |
| Side (left), n (%) | 3 (75.0) | 3 (50.0) | 7 (26.9) | 0.39 |
| Duration of symptoms (mean ± SD), months | 96.8 ± 93.7 | 56.0 ± 40.2 | 73.0 ± 78.2 | 0.82 |
| Risk factors, n (%) | ||||
| Hypertension | 0 (0) | 0 (0) | 9 (34.6) | 0.36 |
| Dyslipidemia | 0 (0) | 0 (0) | 2 (7.7) | 0.8 |
| Cerebrovascular disease | 1 (25.0) | 0 (0) | 2 (7.7) | 0.93 |
| Nerve block | 1 (25.0) | 1 (12.5) | 3 (11.5) | 0.92 |
| Gamma knife | 1 (25.0) | 0 (3.8) | 1 (3.8) | 0.8 |
| Offending vessels, n (%) | ||||
| SCA | 1 (25.0) | 5 (83.3) | 22 (84.6) | 0.13 |
| TCA | 2 (50.0) | 0 (0) | 7 (26.9) | 0.54 |
| AICA | 3 (75.0) | 2 (33.3) | 8 (30.8) | 0.49 |
| VA, BA | 0 (0) | 0 (0) | 3 (11.5) | 0.93 |
| Vein | 1 (25.0) | 4 (66.7) | 14 (53.8) | 0.8 |
Baseline data did not differ between the three groups. SD, standard deviation; SCA, superior cerebellar artery; TCA, trigeminal cerebellar artery; AICA, anterior inferior cerebellar artery; VA, vertebral artery; BA, basilar artery
Primary outcome measures
The percentage of cases in each group where the treatment outcome was excellent or good: group A: 0 (0%), group B: 6 (100%), and group C: 25 (96.2%), indicating significantly different results between the three groups (p < 0.05). The frequency of numbness was 2 (50%) in group A, 3 (50%) in group B, and 1 (3.8%) in group C, indicating significantly different frequencies between the three groups (p < 0.05), as assessed using the Chi-square test (Table 3).
Table 3.
Postoperative outcomes at one year in each group
| Assessment | Group A (n = 4) |
Group B (n = 6) |
Group C (n = 26) |
|---|---|---|---|
| Excellent (E0C0) | 0 | 0 | 25 |
| Good (E1C0/E0C1) | 0 | 6 | 0 |
| Fair (E2C0) | 0 | 0 | 1 |
| Poor (E3C0/E2C1) | 4 | 0 | 0 |
The treatment outcome rates evaluated as excellent or good were: group A: 0%, group B: 100%, and group C: 96.2%, indicating significant differences between the three groups (p < 0.05), as assessed using m*n Chi-square test.
Treatment of causative vessels
All cases showed distortion of the trigeminal nerve due to vascular compression, although there was no observable thickening of the arachnoid membrane or adhesion to the surrounding tissues. The causative artery was transposed in all cases using Teflon or fibrin glue. In group A, coagulation and dissection of the transverse pontine vein (TPV) branch was performed in one case, and it was attached to the petrous bone with fibrin glue. In group B, arachnoidectomy around the causative vein was performed in four cases. One of them underwent coagulation and dissection of the TPV. Among the other cases, Teflon was used in one patient, and fibrin glue was used in two patients to move the causative vein away from the trigeminal nerve (Table 4). In group C, arachnoidectomy around the causative vein was performed in eight cases, and coagulation and dissection of the causative vein was performed in six cases.
Table 4.
Summary of cases in groups A and B with preoperative and postoperative signal changes in the brainstem region of the trigeminal nerve
| Case | Age | Sex | Preoperative treatment history | Preoperative symptoms | Intraoperative manipulation | Postoperative symptoms | Postoperative imaging type | Follow up MRI at 6 months |
|---|---|---|---|---|---|---|---|---|
| A-1 | 66 | M | Medication | Stabbing pain inside the mouth | Transposition of the SCA, TCA with teflon | Stabbing pain inside the mouth | a | a |
| A-2 | 58 | F | Medication | Stabbing pain in the forehead and upper eyelid | Coagulation and dissection of the branch of the TPV Transposition of the TPV with fibrin glue | Stabbing pain relieved, but residual pain in the forehead and upper eyelid | a | a |
| A-3 | 79 | F | Medication Percutaneous nerve block | Stabbing pain in the cheek and sublingual zone | Transposition of the SCA, AICA, TCA with teflon | Stabbing pain relieved, but residual pain in the sublingual zone Hypesthesia of the sublingual zone | a | a |
| A-4 | 71 | F | Medication Gamma knife treatment | Stabbing pain in the cheek and sublingual zone | Transposition of the SCA, AICA with teflon | Stabbing pain relieved, but residual pain in the sublingual zone Hypesthesia of the entire face on the surgical side | c | c |
| B-1 | 53 | F | Medication | Stabbing pain inside the mouth | Transposition of the SCA with teflon Coagulation and dissection of the TPV | Dysethesia inside the mouth | a | Not performed |
| B-2 | 61 | M | Medication | Stabbing pain in the cheek | Transposition of the SCA with teflon | Hypesthesia in the cheek | a | Not performed |
| B-3 | 73 | F | Medication | Stabbing pain in the cheek | Transposition of the SCA, AICA, TPV with teflon | Stabbing pain relieved, but residual pain in the cheek | a, b | a, b |
| B-4 | 70 | F | Medication Percutaneous nerve block | Stabbing pain in the sublingual zone | Transposition of the AICA with teflon Transposition of the TPV with fibrin glue | Dysethesia of sublingual zone | a, c | Not performed |
| B-5 | 67 | M | Medication | Stabbing pain in the cheek | Transposition of the SCA, TPV with fibrin glue | Disappearance of symptoms | a | a |
| B-6 | 54 | M | Medication | Stabbing pain in the cheek | Transposition of the SCA with teflon | Disappearance of symptoms | a | Diminished the signal |
In all cases, the causative artery was transposed using Teflon or fibrin glue. In group A, coagulation and dissection of the transverse pontine vein (TPV) branch was performed in one case and attached to the petrous bone with fibrin glue. In group B, arachnoidectomy around the causative vein was performed in four cases. One of them underwent coagulation and dissection of the TPV, while Teflon was used in one patient, and fibrin glue was used in two patients for the transposition of the causative vein.
In group B, three of the six patients underwent 3D T2–SPACE MRI after six months. In one of them (case B-6), the T2 high-intensity signals disappeared, with a resolved symptom of numbness. The remaining two patients had residual T2 high-intensity signals: case B-3 had residual stabbing pain, and case B-5 had resolution of symptoms. SCA, superior cerebellar artery; TCA, trigeminocerebellar artery; TPV, transverse pontine vein; AICA, anterior inferior cerebellar artery
Residual symptoms
In group A, four patients had positive brainstem lesions on preoperative imaging, all of whom had residual postoperative symptoms. All these patients could not withdraw from oral medications and remained in pain. One patient had no change in the preoperative pain condition. The remaining three patients had residual pain that became intrathecally regulated. Among these three patients, one of them had hypesthesia consistent with the preoperative nerve block injection area (case A-3), and another, which was a patient with a history of gamma knife therapy, developed hypesthesia (case A-4) (Table 4).
In group B, there were six patients with postoperative brainstem changes, four of whom had postoperative symptoms; one patient had residual pain that became intrathecally regulated (case B-3), and three patients experienced relief of the stabbing pain, although with the appearance of numbness that was absent before the surgery (case B-1, 2, 4). Among them, one patient had dysesthesia at the site of preoperative nerve block injection (case B-4). The other two patients had new subjective numbness, one with dysesthesia (case B-1) and the other with hypesthesia (case 2) (Table 4).
In group C, one patient had residual pain that became intrathecally regulated. Another experienced relief of the stabbing pain, while one had dysesthesia consistent with the preoperative nerve block injection area.
Imaging findings
There were no cases of high-intensity lesion positivity on DWI the day after surgery. Four patients presented with preoperative T2 high-intensity lesion positivity along the affected trigeminal pontine pathway (group A). Six patients presented with postoperative T2 high-intensity lesion positivity along the affected trigeminal pontine pathway on 3D T2-SPACE MRI sequences (group B). All these ten patients had T2 hyperintense signals uniformly from the brainstem transition area of the trigeminal nerve to the central side of the brainstem (Fig. 1-a). One of them showed high signal intensity in the main sensory nucleus of the trigeminal nerve (Fig. 1-b). This patient complained of postoperative numbness as a symptom. Two of the ten patients had high-intensity signals in the spinal trigeminal tract nucleus, both of whom had residual pain along the area of the third branch of the trigeminal nerve (Fig. 1-c). In another case, in addition to T2 signal changes along the trigeminal nerve fibers, there were also signal changes in the surrounding brainstem area, suggesting venous infarction on imaging (Fig. 1-d). However, brainstem infarction was unobservable in any of the other cases.
Fig. 1.
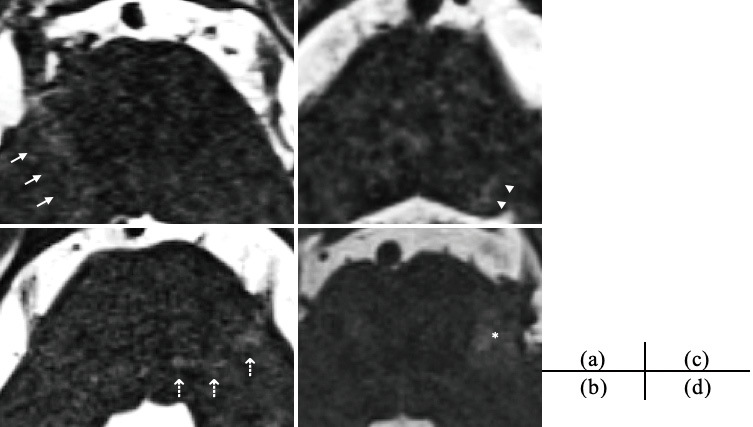
Positive findings of T2 high-intensity lesions along the affected trigeminal pontine pathway with MRI using 3D T2 sampling perfection with application-optimized contrast using different flip angle evolution (SPACE) sequences.
(a) Extensive hyperintense area along the affected trigeminal pontine pathway on the right side, (b) Extensive hyperintense area toward the principal sensory nucleus of the trigeminal nerve on the left side, (c) Extensive hyperintense area toward the trigeminal spinal subnucleus on the left side, (d) Extensive hyperintense area over the trigeminal nerve in the brainstem region on the left side. The white arrows show hyperintense areas along the affected trigeminal pontine pathway, the white dotted arrows show the principal sensory nucleus of the trigeminal nerve, the white arrowhead shows the trigeminal spinal subnucleus, and the asterisk shows the hyperintense area over the trigeminal nerve.
In group A, all patients who underwent 3D T2-SPACE MRI after six months showed no changes, neither improvement nor deterioration, in the findings for any of them. In group B, three of the six patients underwent 3D T2-SPACE MRI after six months. In one of them (case 6), the T2 high-intensity signals had disappeared, resolving the symptom of numbness. The remaining two patients had residual T2 high-intensity signals: case 3 had residual stabbing pain, and case 5 had resolution of symptoms (Table 4).
Discussion
TN is one of the most common forms of craniofacial neuropathic pain. Treatment with MVD provides an approximately 80% chance of becoming pain-free, with a recurrence rate of approximately 10% over 10-20 years.6,7) In Japan, evaluation of discharge outcomes using data in the Diagnosis Procedure Combination database from July 2010 to March 2013 indicated that cure and improvement after MVD for TN were achieved in 97.6% of cases.15) Yet, neurosurgeons occasionally still encounter patients whose symptoms do not improve postoperatively despite removing all the causative vessels, or those who experience excellent or good results in terms of treatment efficacy but develop some other sensory disturbance that was absent before surgery. This study showed a correlation between pre- and postoperative hyperintense lesions in the trigeminal nerve on 3D T2-SPACE MRI and residual postoperative pain and the appearance of numbness following MVD for TN. These results are useful because they provide an objective picture of the subjective symptoms of postoperative pain and numbness.
Preoperative T2 high-intensity lesion positivity along the affected trigeminal pontine pathway
TN associated with brainstem lesions is considered rare. To the best of our knowledge, only nine cases of TN patients with preoperative brainstem lesions have previously been reported before 2020.16-20) Chang et al.16) suggested an old pontine viral neuritis as a possible cause of the lesion in their two patients. Arrese et al.17) suggested demyelination or ischemia as the likely the lesion's pathology. There are also three single case reports of TN resulting from pontine infarction.18-20)
In 2020, Tohyama et al. defined the new clinical syndrome of TN associated with a solitary pontine lesion (SPL-TN), presenting 18 such cases.21) Patients with SPL-TN have clinical features identical to TN but are almost non-responders to surgical treatment (94.4%). They have a single pontine lesion distributed along the affected trigeminal pontine pathway of the trigeminal brainstem sensory nuclear complex. The lesions demonstrated an abnormal white-matter microstructure, characterized by lower fractional anisotropy and higher mean, radial, and axial diffusivities than the unaffected side.
In this study, four patients with preoperative brainstem lesions distributed along the affected trigeminal pontine pathway. The findings were considered similar to those of previously reported brainstem lesions. The cause of the lesions was thought to be viral neuritis, demyelination, ischemia, or Wallerian degeneration after gamma knife therapy, although further accumulation of cases is required to evaluate the significance of these observations. All four of these patients also had residual symptoms, suggesting that patients with preoperative brainstem lesions might resist MVD treatment, as previously reported. However, in three of the four cases, there was a reduction in the extent of pain or change in the nature of the pain after surgery to a dull type of pain, which reduced patient suffering. This suggests that careful consideration should be given to the indications for treatment in consultation with individual patients.
Postoperative T2 high-intensity lesion positivity along the affected trigeminal pontine pathway
Although there have been reports of brainstem infarction as a postoperative complication following MVD for TN,22,23) to the best of our knowledge, only one previous report has examined the relationship between eventual residual symptoms and objective evaluations using imaging studies.8)
In this study, we identified six patients with postoperative T2 high-intensity lesion positivity along the affected trigeminal pontine pathway, one of whom had residual pain, with three patients expressing newly developed numbness. These results are useful in providing an objective picture of postoperative pain and numbness symptoms that were previously only subjectively described.
In the three patients who underwent follow-up imaging after six months postoperatively, the T2 high-intensity lesion remained unchanged in one patient and disappeared in the other two, along with the disappearance or localization of the symptoms. This infers two types of postoperative T2 high-intensity findings: reversible and irreversible. The former might be due to transient neurogenic edema. The latter was considered a finding suggestive of eventual degeneration of the nerve. These results suggest that intraoperative traction on the trigeminal nerve might have caused stress on the central side from the less mobile brainstem transition region, leading to neurodegeneration. Imaging changes seen within a few days to a week of disease onset have been reported as early Wallerian degeneration.24-27) In those reports, the imaging changes might have been transient or irreversible, and the neurological prognosis of early Wallerian degeneration was poor, consistent with the present results. However, further accumulation of cases will be necessary to determine the traction threshold at which transient neurogenic edema and irreversible neurodegeneration appear.
In another case, alongside T2 signal changes along the trigeminal nerve fibers, there were also signal changes in the surrounding brainstem area, suggesting venous infarction on imaging. Among the veins that comprise the petrosal vein complex, some can be cut without any adverse effects,28,29) whereas in others, the cut may cause severe venous infarction and serious symptoms.30,31) Matsushima et al.32) classified veins around the trigeminal nerve into the following four groups: group 1: anterior pontomesencephalic draining; group 2: posterior mesencephalic draining; group 3: tentorial cerebellar surface draining; and group 4: petrosal fissure draining. Generally, the veins belonging to group 4 in Matsushima's classification should not be cut due to their wide perfusion range, but they can reportedly be cut when they are thinner than the petrosal veins.28,32) The risk of postoperative problems due to transection of the TPV, which has sufficient collateral outflow anastomosing with the Galenic draining system via the anterior pontomesencephalic vein, is considered to be low.33,34) By contrast, TPV congestion has also been reported to cause broad-ranging venous infarction at the dorsolateral midbrain and superior cerebellar peduncle,35) leading to serious symptoms. Although there are various opinions regarding managing the causative vein, it is important to carefully determine whether an incision is truly necessary. If transection of the vein is inevitable, it is helpful to temporarily clamp the causative vein and observe changes in the auditory brainstem response and venous congestion on indocyanine green (ICG) video angiography.36-39) There were no significant differences in the management of the responsible vein between the three groups in our study, suggesting that further case accumulation must clarify the appropriate treatment of the vein considered the causative vessel.
In this study, we observed imaging findings that might suggest signal changes along the main sensory nucleus of the trigeminal nerve and the spinal trigeminal tract nucleus. In particular, in the two cases with T2 high-intensity lesions in the spinal trigeminal tract nucleus, both had residual pain symptoms consistent with the three-branch region of the trigeminal nerve, similar to results previously reported in basic research in the field of oral surgery.40-42) To the best of our knowledge, no previous literature reports on clinical studies have captured imaging changes related to the neuronal nuclei, suggesting that accumulating further cases to evaluate the correlation between T2 high-intensity lesions and residual symptoms is necessary.
There are some limitations to this study. First, since this was a single-center, retrospective observational study with a small sample size, further studies with a larger sample size are required to confirm our results. Second, we could only evaluate follow-up 3D T2-SPACE images at one week postoperatively in all patients and capture subsequent imaging changes in only a subset of these cases. More cases are needed to prove the long-term results and imaging changes.
Conclusions
3D T2-SPACE MRI might capture the changes in trigeminal nerve fibers before and after MVD for classical TN, possibly relating to eventual residual symptoms.
Funding Sources
The authors received no funding for this work.
Conflicts of Interest Disclosure
No company had influence on or knowledge of the results of this study.
References
- 1). Cruccu G, Gronseth G, Alksne J, et al. : American Academy of Neurology Society; European Federation of Neurological Society: AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol 15: 1013-1028, 2008 [DOI] [PubMed] [Google Scholar]
- 2). Bick SKB, Eskandar EN: Surgical treatment of trigeminal neuralgia. Neurosurg Clin N Am 28: 429-438, 2017 [DOI] [PubMed] [Google Scholar]
- 3). Dandy WE: The treatment of trigeminal neuralgia by the cerebellar route. Ann Surg 96: 787-795, 1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4). Dandy WE: Concerning the cause of trigeminal neuralgia. Am J Surg 24: 447-455, 1934 [Google Scholar]
- 5). Adams CB: Microvascular compression: an alternative view and hypothesis. J Neurosurg 70: 1-12, 1989 [DOI] [PubMed] [Google Scholar]
- 6). Zhang H, Lei D, You C, et al. : The long-term outcome predictors of pure microvascular decompression for primary trigeminal neuralgia. World Neurosurg 79: 756-762, 2013 [DOI] [PubMed] [Google Scholar]
- 7). Sarsam Z, Garcia-Fiñana M, Nurmikko TJ, et al. : The long-term outcome of microvascular decompression for trigeminal neuralgia. Br J Neurosurg 24: 18-25, 2010 [DOI] [PubMed] [Google Scholar]
- 8). Jawahar A, Kondziolka D, Kanal E, Bissonette DJ, Lunsford LD: Imaging the trigeminal nerve and pons before and after surgical intervention for trigeminal neuralgia. Neurosurgery 48: 101-107, 2001 [DOI] [PubMed] [Google Scholar]
- 9). Headache Classification Subcommittee of the International Headache Society : The international classification of headache disorders. Cephalalgia 24: 9-160, 2004 [DOI] [PubMed] [Google Scholar]
- 10). Kondo A, Date I, Endo S, et al. : A proposal for standardized analysis of the results of microvascular decompression for trigeminal neuralgia and hemifacial spasm. Acta Neurochir (Wien) 154: 773-778, 2012 [DOI] [PubMed] [Google Scholar]
- 11). Niwa J, Takaya S, Otaki M, Morimoto S, Tanabe S, Hashi K: Residual symptoms after microvascular decompression for trigeminal neuralgia previously treated by percutaneous nerve blocking. Neurol Med Chir (Tokyo) 28: 1103-1106, 1988 [DOI] [PubMed] [Google Scholar]
- 12). Chokshi FH, Sadigh G, Carpenter W, Allen JW: Diagnostic quality of 3D T2-SPACE compared with T2-FSE in the evaluation of cervical spine MRI anatomy. A.J.N.R Am J Neurorad 38: 846-850, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Okamura N, Otake Y, Anzai K, et al. : Evaluation of cervical spine lesions by the 3D T2―SPACE compared with the 2D T2WI. Spinal Surg 34: 59-65, 2020 [Google Scholar]
- 14). Otake Y, Okamura N, Fukuda M, et al. : Efficacies of 3D T2 sampling perfection with application-optimized contrast with the use of different flip-angle evolution(3D T2―SPACE)sequences and 3D true FISP sequences. Spinal Surg 35: 218-221, 2021 [Google Scholar]
- 15). Mizobuchi Y, Ohtani M, Satomi J, Fushimi K, Matsuda S, Nagahiro S: The current status of microvascular decompression for the treatment of trigeminal neuralgia in Japan: An analysis of 1619 patients using the Japanese Diagnosis Procedure Combination Database. Neurol Med Chir (Tokyo) 58: 10-16, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Chang JW, Choi JY, Yoon YS, Park YG, Chung SS: Unusual causes of trigeminal neuralgia treated by gamma knife radiosurgery: report of two cases. J Neurosurg 97: 533-535, 2002 [DOI] [PubMed] [Google Scholar]
- 17). Arrese I, Lagares A, Alday R, Ramos A, Rivas JJ, Lobato RD: Typical trigeminal neuralgia associated with brainstem white matter lesions on MRI in patients without criteria of multiple sclerosis. Acta Neurochir 150: 1157-1161, 2008 [DOI] [PubMed] [Google Scholar]
- 18). Katsuno M, Teramoto A: Secondary trigeminal neuropathy and neuralgia resulting from pontine infarction. J Stroke Cerebrovasc Dis 19: 251-252, 2010 [DOI] [PubMed] [Google Scholar]
- 19). Kim JS, Kang JH, Lee MC: Trigeminal neuralgia after pontine infarction. Neurology 51: 1511-1512, 1998 [DOI] [PubMed] [Google Scholar]
- 20). Peker S, Akansel G, Sun I, Pamir NM: Trigeminal neuralgia due to pontine infarction. Headache 44: 1043-1045, 2004 [DOI] [PubMed] [Google Scholar]
- 21). Tohyama S, Hung PSP, Cheng JC, et al. : Trigeminal neuralgia associated with a solitary pontine lesion: clinical and neuroimaging definition of a new syndrome. Pain 161: 916, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Hanakita J, Kondo A: Serious complications of microvascular decompression operations for trigeminal neuralgia and hemifacial spasm. Neurosurgery 22: 248-352, 1988 [DOI] [PubMed] [Google Scholar]
- 23). Singh D, Jagetia A, Sinha S: Brain stem infarction: a complication of microvascular decompression for trigeminal neuralgia. Neurol India 54: 325, 2006 [DOI] [PubMed] [Google Scholar]
- 24). DeVetten GD, Coutts SB, Hill MD, et al. : Acute corticospinal tract Wallerian degeneration is associated with stroke outcome. Stroke 41: 751-756, 2010 [DOI] [PubMed] [Google Scholar]
- 25). Uchino A, Sawada A, Takase A, et al. : Transient detection of early wallerian degeneration on diffusion-weighted MRI after an acute cerebrovascular accident. Neuroradiology 46: 183-188, 2004 [DOI] [PubMed] [Google Scholar]
- 26). Yamada K, Kizu O, Nakamura H, et al. : Wallaerian degeneration of the inferior cerebellar peduncle depicted by diffusion weighted imaging. J Neurol Neurosurg Psychiatry 74: 977-978, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27). Mazumdar A, Mukherjee P, Miller JH, Malde H, McKinstry RC: Diffusion-weighted imaging of acute corticospinal tract injury preceding Wallerian degeneration in the maturing human brain. A.J.N.R Am J Neuroradiol 24: 1057-1066, 2003 [PMC free article] [PubMed] [Google Scholar]
- 28). Inoue T, Hirai H, Shima A, Suzuki F, Fukushima T, Matsuda M: Diagnosis and management for trigeminal neuralgia caused solely by venous compression. Acta Neurochir (Wien) 159: 681-688, 2017 [DOI] [PubMed] [Google Scholar]
- 29). Zheng X, Feng B, Hong W, et al. : Management of intraneural vessels during microvascular decompression surgery for trigeminal neuralgia. World Neurosurg 77: 771-774, 2012 [DOI] [PubMed] [Google Scholar]
- 30). Helbig GM, Callahan JD, Cohen-Gadol AA: Variant intraneural vein-trigeminal nerve relationships: an observation during microvascular decompression surgery for trigeminal neuralgia. Neurosurgery 65: 958-961, discussion 961, 2009 [DOI] [PubMed] [Google Scholar]
- 31). Liebelt BD, Barber SM, Desai VR, et al. : Superior petrosal vein sacrifice during microvascular decompression: perioperative complication rates and comparison with venous preservation. World Neurosurg 104: 788-794, 2017 [DOI] [PubMed] [Google Scholar]
- 32). Matsushima K, Matsushima T, Kuga Y, et al. : Classification of the superior petrosal veins and sinus based on drainage pattern. Neurosurgery 10: 357-367, 2014 [DOI] [PubMed] [Google Scholar]
- 33). Cai M, Zhang XF, Qiao HH, et al. : Susceptibility-weighted imaging of the venous networks around the brain stem. Neuroradiology 57: 163-169, 2015 [DOI] [PubMed] [Google Scholar]
- 34). Matsushima T, Rhoton AL, de Oliveira E, Peace D: Microsurgical anatomy of the veins of the posterior fossa. J Neurosurg 59: 63-105, 1983 [DOI] [PubMed] [Google Scholar]
- 35). Liebelt BD, Barber SM, Desai VR, et al. : Superior petrosal vein sacrifice during microvascular decompression: perioperative complication rates and comparison with venous preservation. World Neurosurg 104: 788-794, 2017 [DOI] [PubMed] [Google Scholar]
- 36). Ferroli P, Nakaji P, Acerbi F, Albanese E, Broggi G: Indocyanine green (ICG) temporary clipping test to assess collateral circulation before venous sacrifice. World Neurosurg 75: 122-125, 2011 [DOI] [PubMed] [Google Scholar]
- 37). Strauss C, Neu M, Bischoff B, Romstöck J: Clinical and neurophysiological observations after superior petrosal vein obstruction during surgery of the cerebellopontine angle: case report. Neurosurgery 48: 1157-1161, 2001 [DOI] [PubMed] [Google Scholar]
- 38). Tsunoda S, Inoue T, Segawa M, Akabane A: Vein-related trigeminal neuralgia: how to determine the treatment method of the causative vein: A technical note. Neurol Med Chir (Tokyo) 62: 105-109, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Zhong J, Li ST, Xu SQ, Wan L, Wang X: Management of petrosal veins during microvascular decompression for trigeminal neuralgia. Neurol Res 30: 697-700, 2008 [DOI] [PubMed] [Google Scholar]
- 40). Yokota T: Somatotopic organization of trigeminal neurons within caudal medulla oblongata. Pain Trigeminal Reg 1977 [Google Scholar]
- 41). Yokota T: Trigeminal nociceptive neurons in the trigeminal subnucleus caudalis and bulbar lateral reticular formation. Adv Pain Res Ther 3: 211-217, 1979 [Google Scholar]
- 42). Yokota T: Neural mechanisms of trigeminal pain. Advances in pain research and therapy 9: 211-232, 1985 [Google Scholar]


