Abstract
Hematopoietic stem cell (HSC) gene therapy is currently performed on CD34+ hematopoietic stem and progenitor cells containing less than 1% true HSCs and requiring a highly specialized infrastructure for cell manufacturing and transplantation. We have previously identified the CD34+CD90+ subset to be exclusively responsible for short- and long-term engraftment. However, purification and enrichment of this subset is laborious and expensive. HSC-specific delivery agents for the direct modification of rare HSCs are currently lacking. Here, we developed novel targeted viral vectors to specifically transduce CD90-expressing HSCs. Anti-CD90 single chain variable fragments (scFvs) were engineered onto measles- and VSV-G-pseudotyped lentiviral vectors that were knocked out for native targeting. We further developed a custom hydrodynamic titration methodology to assess the loading of surface-engineered capsids, measure antigen recognition of the scFv, and predict the performance on cells. Engineered vectors formed with minimal impairment in the functional titer, maintained their ability to fuse with the target cells, and showed highly specific recognition of CD90 on cells ex vivo. Most important, targeted vectors selectively transduced human HSCs with secondary colony-forming potential. Our novel HSC-targeted viral vectors have the potential to significantly enhance the feasibility of ex vivo gene therapy and pave the way for future in vivo applications.
Keywords: hematopoietic stem cells, HSCs, CD90, gene therapy, surface-engineered viral vectors, single chain variable fragments, scFv, HSC targeting, measles, VSVG
Graphical abstract

Kiem et al. developed novel CD90-targeted viral vectors to selectively transduce HSCs with enhanced on-target efficiency to improve the feasibility and safety of ex vivo gene therapy. Direct modification of CD34+CD90+ HSCs with targeted vectors further paves the way for future in vivo HSC gene therapy applications.
Introduction
Currently existing gene therapy approaches modify CD34+ hematopoietic stem and progenitor cells (HSPCs), a heterogeneous population that contains less than 1% HSCs with long-term multi-lineage engraftment potential.1 Consequently, massive use of expensive reagents, potential off-target effects in non-HSCs, and low on-target efficiency currently impact the feasibility of HSC gene therapy. In addition, current gene therapy approaches rely on the modification of HSCs outside the patient’s body (ex vivo) and require highly specialized facilities similar to bone marrow transplantation, severely limiting the accessibility of this treatment option. Injection of gene therapy agents and modification of HSCs directly in the patient (in vivo) would overcome all these limitations. However, a major obstacle to perform gene therapy in vivo is the lack of HSC-targeted gene therapy agents to highly specifically deliver the therapeutic cargo and avoid off-target effects.
This targeted delivery depends on the uptake of the vector after attaching to the cell/antigen, which has not been achieved with CD34-targeted HSC gene therapy vectors.2 As a result, alternative markers of HSCs such as CD117 and CD133 have been considered.3,4 However, CD117+/CD133+ cells contain various erythro-myeloid and lympho-myeloid progenitors5,6 that act as sinks for targeted viral particles and decrease the chance of successfully transducing HSCs.
Previous work in the laboratory has shown that the CD90+ subset of CD34+ HSPCs is exclusively responsible for rapid recovery onset, robust long-term multi-lineage engraftment, and an entire reconstitution of the bone marrow stem cell compartment.3,7,8 Furthermore, CD34+CD90+ cells are almost entirely depleted for phenotypically, transcriptionally, and functionally committed progenitor cells.3,9,10,11 Isolation and direct targeting of this HSC-enriched subset from mobilized human apheresis products ex vivo significantly increased lentivirus-mediated gene transfer without impacting the cells long-term multi-lineage engraftment potential.2
To enable highly specific targeting of CD90+ HSCs, we designed CD90-targeted viral vectors engineering an anti-CD90 single chain variable fragment (scFv) onto measles- and VSV-G-pseudotyped lentiviral vectors. CD90-targeted viral vectors were further knocked out for their native targeting to optimize on-target specificity. Last, we developed a hydrodynamics-based methodology to easily and reliably titrate and validate our surface-engineered viral vectors.
Results
CD90 scFv design, modification, and validation
To generate HSC-targeted viral vectors for gene therapy, we initially designed and computationally validated an scFv recognizing CD90. Sequences for the scFv design were extracted from the hybridoma cell line producing the CD90 antibody clone 5E10.12 Briefly, mRNA was extracted from the cell line, reverse transcribed, and primers designed to sequence the cDNA fragments encoding for the variant light and heavy regions (Figure 1A). To determine the framework and complementary defining regions of the antibody, sequences were annotated using IMGT.13,14 Next, scFvs were designed linking the light- and heavy-chain sequences using the commonly used 3×(GGGGS) hinge (Figure 1B). Finally, to predict the binding ability of our anti-CD90 scFv with the extracellular domain of CD90, interactions were modeled using SwissDock15 (Figure 1C). Although bioinformatics predicted successful binding, expression of the anti-CD90 scFv could neither be achieved in the Daedalus protein expression system16 nor on viral envelopes (data not shown). Because of these challenges, we carried out a comprehensive comparison of our anti-CD90 scFv sequence in the database for previously reported functional scFvs. This comparison revealed two cysteine residues (positions 31 and 59) in the anti-CD90 scFv sequence that were not found in any other reference and were not predicted to form disulfate bonds running SAbPred modeling.17 Cysteines were, therefore, exchanged (position 31 tyrosine [Y], position 59 arginine [R]) to match the reference sequences. In addition, the linker was exchanged to an alternate linker sequence (GSDSN AGHAS AGNTS)18 to enable easier production and sequencing of variants.
Figure 1.
Design and computational validation of an anti-CD90 scFv
(A) Workflow to determine the variant heavy (VH) and variant light (VL) sequence from the hybridoma cell line producing the CD90 antibody clone 5E10. (B) Amino acid sequence of the initial scFv design connecting the VL and VH chain with a 3×(GGGGS) linker (yellow) sequence. Complementarity-determining regions (CDRs; color coded) for the VH and VL sequence were determined using IMGT. (C) Predicted interaction of CD90 (red) with the anti-CD90 scFv (green). CDRs of the scFv are highlighted in magenta. (D) Representative database crystal structure of 3S62 and 1RVF highlighting the regions of putative mismatched cysteines from our anti-CD90 scFv. (E) Sequence alignment of our anti-CD90 scFv with the reference database for highly homologous crystal structures. Mismatched cysteines in position 31 and 59 are highlighted in magenta.
Design, production, and hydrodynamic titration of CD90-targeted measles-pseudotyped viral vectors
Measles-pseudotyped lentiviral vectors efficiently transduce a wide variety of cell types that recognize the broadly expressed cell surface antigens CD46 and CD150.19,20 Here, we stepwise developed the measles envelope to remove the native targeting and instead exclusively recognize CD90, as previously described for other target cells.18 In addition to the wildtype (WT) envelope (variant H1 [WT]) (Figure S1), three variants of the measles envelope were generated (Figure 2A). To remove its ability to target CD46/150 and thus create a virus that should not be able to elicit fusion and transduce cells,21 four modifications were introduced to the amino acid sequence of the measles glycoprotein (variant H2: knockout [KO]) (Figure S2). We next created a variant that is capable of detecting CD46, CD150, and CD90, inserting the CD90 scFv at the end of the glycoprotein (variant H3: WT/CD90) (Figure S3). Finally, the CD46/CD150 KO and CD90 recognition was combined to lead to a variant that should only bind and fuse on CD90-expressing cells (variant H4: KO/CD90) (Figure S4).
Figure 2.
Design and quality control of CD90-targeted measles-pseudotyped lentiviral vectors
(A) Summary of designed measles envelopes and the expected antigen recognition. (B) Viral variants were tested on HT1080 cells demonstrating inability of the KO versions to fuse and transduce. (C) Workflow for the hydrodynamic titration of engineered viral vectors using NTA. (D) Concentration of viral particles per ml culture medium harvested. (E) Concentration of RNA-loaded viral particles per ml culture medium measured with SYBR II. (F) RNA loading efficiency calculated based on values from (D) and (E). Statistics are mean ± SD and t test, with significance as indicated.
Because of the inability of the KO variants to be titrated on standard cell lines such as HT1080 (Figure 2B), we further developed a methodology using hydrodynamics combined with fluorescence to determine the number of total as well as RNA-filled viral particles. The size and number of viral particles were determined using the ZetaView, a Nanoparticle Tracking Analysis (NTA) instrument for measuring particle size, zeta potential, concentration, and fluorescence (Figure 2C). Regardless of the modifications and addition of the CD90 scFv, all four envelope variants equally produced viral particles (Figure 2D). Successful loading of viral capsids and loading efficiency was determined staining the particles with SYBR II. The number of RNA-loaded viral particles was highly variable across batches (Figure 2E). However, the loading efficiency of viral particles (frequency of RNA-loaded particles within total particles) was very consistent, averaging at 10%–20% with no obvious differences caused by the modification made (Figure 2F).
CD90 scFv on viral vectors bind CD90 protein
To ensure the ability of CD90-targeted viral vectors to recognize CD90 antigen, we designed a custom assay using NTA. The extracellular domain of CD90 (CD90ex) was fused with the fluorochrome mCherry (mCh) and the fusionprotein produced with the Daedalus system.16 The quality of the CD90-mCh fusion protein was confirmed via its interaction with anti-CD90 Fabs from the hybridoma cell line measured through size exclusion chromatography (SEC) using an A200 column run in PBS buffer. Each protein exhibited a single peak at the expected molecular weight (MW) (Figure 3A). Fab binding to CD90ex was confirmed by the shift of the elution peak to a larger MW species and by SDS-PAGE of the peak showing presence of both proteins.
Figure 3.
Assessment of antigen recognition by CD90-targeted lentiviral vectors
(A) Quality control of custom produced CD90ex + mCh fusion protein. CD90 Fab (1, green), CD90-mCh fusionprotein (2, blue), and a mix of CD90 Fab with CD90-mCh were analyzed by size exclusion chromatograph (line graph). Fractions/peaks indicated with numbers were collected and content validated by SDA page (left top). (B) Workflow for the assessment of CD90ex interaction with CD90-targeted viral vectors using NTA. (C) Size of viral particles measuring scattered light (top row). Size distribution of RNA-loaded particles stained with SYBR II and measuring the fluorescence in the 500-nm filter (middle row). SYBR II signal of RNA-loaded viral particles co-incubated with CD90ex measuring the fluorescence in the 500-nm filter (bottom row). Numbers in each plot indicate the average size of numbered peaks.
Next, we measured the size of our vector variants in the presence and absence of the CD90ex protein (Figure 3B). All four vectors were very similar in size (range, 110–130 nm) regardless of the modifications made measuring the scattered light (Figure 3C, top row). Adding SYBR II to stain only RNA-loaded particles and observing the fluorescence in the 500-nm filter, particles seemed to be slightly larger across all four variants and a broader size range because of variability in RNA loading (Figure 3C, middle row). Finally, vectors incubated with CD90ex protein were measured (Figure 3C, bottom row). Because of the background noise of unbound protein, measurements were performed in the 500-nm filter focusing only on RNA-loaded particles. As expected, CD90-decorated viral variants, H3 and H4 with CD90ex, showed new peaks at 300 nm, indicating the formation of a complex. Only a minor change in size was seen for variants H1 and H2, which lacked the CD90 scFv, because of additional background noise signal from the CD90ex protein.
In summary, we were able to successfully engineer an anti-CD90 scFv onto the measles envelope without impairing the vector production and maintaining viral titers. Most important, anti-CD90 scFvs on the viral capsid are fully functional and able to recognize CD90 antigen in our custom hydrodynamic readout, confirming CD90 specificity of the scFv.
Targeted viral vectors transduce CD90-expressing Jurkat cells and human HSCs
The measles envelope has been extensively modified and previous attempts to use similar systems to specifically transduce CD34+ cells failed because of the lack of fusion with the target cells.2 Consequently, we next tested our CD90-targeted viral vectors for fusion and transduction ability. For the initial tests, CD90-expressing Jurkat cells were used and incubated with all four viral variants. Jurkat cells were exposed to equal volume of virus from different batches overnight for 10–12 h, cells washed to remove virus, and transduced cells kept in culture for 5–7 days to guarantee stable integration of the transgene and wash out transiently delivered fluorescent protein sticking on the viral particles.
Determined by flow cytometry, we observed transduction of Jurkat cells with variant H1 (Figure 4A). Relative to variant H1, knock-out of CD46 and CD150 recognition reduced the ability of variant H2 to transduce Jurkat cells by 81.6% ± 13.4% (Figure 4A). Addition of the CD90 scFv on the H WT virus (variant H3) had no impact on the transduction ability in comparison to the WT variant H1. Finally, variant H4 (KO/CD90) regained transduction capability over variant H2 (KO), reaching on average 44.8% ± 17.9% transduction efficiency. We further evaluated the reliability of hydrodynamic titers determined with NTA for variant H1 as well as KO version H2 (Figure 4B). A very high correlation (H1, R2 = 0.9315; H2, R2 = 0.8689; H3, R2 = 0.9306; and H4, R2 = 0.7570) was found in between the number of RNA-loaded particles and the number of transduced Jurkat cells independent of the capsid modification.
Figure 4.
Selective transduction of cell lines and HSCs with CD90-targeted viral vectors
(A) Flow-cytometric assessment of the transduction efficiency of Jurkat cells using all four vector variants. Efficiencies are visualized relative to the efficiency of variant H1. (B) Correlation of the number of transduced cells with the number of RNA-loaded viral particles using the variants H1, H2, H3, and H4. R2 is Spearman’s rank correlation coefficient. (C) Functional assessment of transduced human CD34+ cells in CFC assays. mScarlet-expressing CD34+ cells were FACS-purified and introduced into primary (first) CFC assays (first graph). After 14 days, colonies were counted, all cells harvested, and 5% of all cells replated into secondary (second) CFC assays (second graph). Secondary CFCs were counted after 14 days, all cells harvested, the total number of white blood cells counted (third graph), and the mScarlet expression measured by flow cytometry (fourth graph). Statistics are mean ± SD and t test, with significance as indicated.
Next, we transduced human granulocyte-colony stimulating factor (GCSF)-mobilized CD34+ cells with variant H1 and H4 vectors to analyze the targeting efficiency of the CD34+CD90+ subset (Figure 4C). Because of the expected partial internalization/blocking and resulting inability to detect CD90 surface expression after exposure to the variant 4 virus by flow cytometry, mScarlet expressing CD34+ cells from both conditions were FACS-purified and introduced into colony-forming cell (CFC) assays to functionally determine differences in the targeting of both viruses. No differences in the total colony formation were seen comparing the WT and CD90-targeted virus in primary CFCs, whereas more colonies were found in secondary CFCs when the targeted virus was used, indicating selective transduction of more primitive human HSPCs with enhanced proliferation potential with variant 4. Colonies in secondary CFC assays from variant H4-transduced cells contained more total cells as well as a greater number of stably mScarlet-expressing cells, confirming greater proliferation and expansion potential of cells transduced with the CD90-targeted virus.
These data confirm that CD90-targeted viral vectors are capable of recognizing CD90 antigen on the surface of Jurkat cells as well as human HSCs and effectively fuse with the cells. Furthermore, hydrodynamic titers were highly predictable for the transduction efficiency, confirming accuracy and validity of our new titration methodology for surface-engineered viral vectors.
VSV-G-based viral vectors can be targeted to CD90
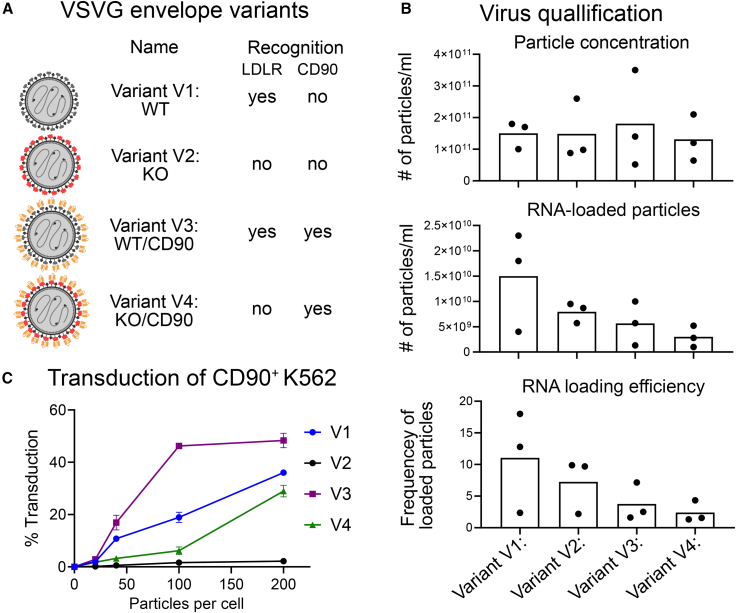
In addition to measles-pseudotyped viral vectors, we next evaluated the VSV-G-based system for surface engineering following the previously described strategy by Dobson et al.22 Native targeting of the VSV-G envelope for the low-density lipoprotein receptor (LDL-R) were further knocked out to introduce specific point mutations as described by Nikolic et al.23 (Figure S5). The CD90 scFv was introduced onto the capsid using a CD8 hinge and ICAM domain (Figure S6). Similar to the measles vectors, four VSV-G variants were generated with V1 (WT) recognizing LDL-R, V2 (KO) removing LDL-R recognition, V3 (WT/CD90) recognizing LDL-R and CD90, and V4 (KO/CD90) only binding to CD90 (Figure 5A).
Figure 5.
Design and quality control of CD90-targeted VSV-G-pseudotyped lentiviral vectors
(A) Summary of designed VSV-G envelopes and the expected antigen recognition. (B) Concentration of total and RNA-loaded viral particles per ml culture medium measured with NTA (top two graphs). Calculated RNA loading efficiency (bottom graph). (C) Transduction of 200,000 K562 cells with all four VSV-G variants at different doses as indicated below the graph. Transduction efficiency (mScarlet expression) measured by flow cytometry. Statistics are mean ± SD and t test, with significance as indicated.
The number of total viral as well as RNA-loaded particles was determined using the ZetaView instrument (Figure 5B). Regardless of the modifications and addition of the CD90 scFv, all four envelope variants equally produced viral particles. However, the loading efficiency of viral particles (the frequency of RNA-loaded particles within total particles) decreased as more engineering was done with the lowest loading efficiency seen in V4.
To test the functionality of the surface-engineered VSV-G vectors, K562 cells were exposed to increasing doses of all four viruses taking the number of RNA filled capsids into account determined by NTA (Figure 5C). Similar to the measles variants, the V2 (KO) variant failed to fuse with the cells, the V3 (WT/CD90) version demonstrated a gain of function relative to V1 (WT), and V4 (KO/CD90) regained the ability to fuse with cells comparable to V1 (WT). Transduction efficiency of K562 for variants V1, V3, and V4 was dose dependent in a linear fashion until reaching a plateau at approximately 50%, already at low particle number for the V3 variant, with no unwanted uptake seen for the KO variant V2, even in the highest dose.
On-target efficiency of CD90-targeted viral vectors
To test the on- and off-target activity of our CD90-targeted measles-as well as VSV-G-pseudotyped viral vectors, we performed co-culture experiments mixing CD90-lacking (off-target) and CD90-expressing (on-target) suspension cell lines. Raji cells, human B lymphoblastoid cells, do present very low levels of CD90 protein on the cell surface (off-target), whereas Jurkat cells, immortalized human T lymphocytes, express CD90 protein at high levels (on-target) (Figure 6A). GFP-transduced Jurkat cells and GFP– Raji cells were mixed and exposed to measles and VSV-G variants 1 and 4 in a serum-free, transduction enhancer-free, suspension culture system, and mScarlet expression flow cytometrically determined on day 3–5 post-transduction (Figure 6B). When the variant H1/V1 (WT) virus was used, mScarlet expression was seen for Raji as well as Jurkat cells by flow cytometry. However, when the cell mix was exposed to variant H4/V4 (KO/CD90), a strong preference of mScarlet signal was seen in the CD90+ Jurkat cells (on-target) and only minimal transduction in the CD90low/– Raji cells (off-target) (Figure 6B). To quantify the on-target specificity, we calculated the ratio of mScarlet+GFP– cells within total GFP– Raji to mScarlet+GFP+ cells within total GFP+ Jurkat cells (Figure 6C). For both virus types, the CD90-targeted variant 4 showed significantly higher on-target specificity in comparison with the variant 1 (WT) confirmed CD90-mediated target specificity. All targeting experiments were performed without the addition of any transduction enhancer, such as protamine sulfate; such additives led to expected improvement of transduction for WT virus (here variant H1) but an unspecific fusion of our KO variants (here variant H2) in a dose-dependent manner (Figure 6D). Finally, VSVG variants 1 and 4 were applied to human GCSF-mobilized CD34+ HSPCs to evaluate the targeting specificity. Similar to experiments on cell lines, our CD90-targeted variant 4 demonstrated significantly greater transduction of the CD34+CD90+ cell fraction in comparison with the WT variant V1 (Figure 6E). The on-target specificity of this CD90-targeted virus was entirely abrogated when the transduction enhancer protamine sulfate was used validating that CD90-specificity of our virus is mediate by the recognition of the antigen through the added scFv.
Figure 6.
On-target specificity of CD90-targeted vectors
(A) Flow-cytometric assessment of CD90 expression on Raji and Jurkat cells. (B) Transduction efficiency of mixed cultures containing GFP− Raji and GFP+ Jurkat cells with either variant V1 or V4. (C) On target specificity of Measles- and VSV-G-based vector variants 1 and 4 determined by the ratio of mScarlet+GFP– cells within total GFP– Raji to mScarlet+GFP+ cells within total GFP+ Jurkat cells. (D) Dose-dependent impact of the transduction enhancer protamine sulfate on the transduction of Jurkat cells with engineered measles-based vector variants 1 and 2. (E) On-target specificity of VSV-G-based vector variants 1 and 4 determined by the ratio of mScarlet+CD90– cells within total CD34+ cells to mScarlet+CD90+ cells within total CD34+ cells in the absence and presence of protamine sulfate. Statistics are mean ± SD and t test, with significance as indicated.
Discussion
Here we demonstrate the successful design of measles and VSV-G pseudotyped lentiviral vectors with a novel anti-CD90 scFv to target HSCs. Surface engineering impacted neither the binding nor fusion of viral vectors to CD90-expressing cell lines and primary human HSCs. Most important, targeted vectors demonstrated enhanced on-target specificity in mixed cultures of cell lines, as well as for human HSCs within bulk CD34+ HSPCs ex vivo. We further describe novel NTA-based methods to reliably validate surface engineered viral vectors measuring their size, loading efficiency, and targeting functionality. Hydrodynamic titration of engineered vectors is highly reproducible and predictive of the transduction efficiency, providing a reliable surrogate to functional readouts on cell lines, which are not applicable to surface engineered vectors.
The development of targeted gene therapy agents has been very successful for non-hematological diseases.24,25,26,27,28 Previous work has shown that by conjugating antibodies or scFvs to the surface of nanoparticles (NPs) and viruses, one can reliably target cells of interest.2,4,20,24,27,29,30,31,32,33,34 Targeted delivery, however, depends on the uptake of the vector after attaching to the cell/antigen, which could not be achieved with CD34-targeted HSC gene therapy vectors.2 While this previous study aimed to integrate the anti-CD34 scFv in between the fusion protein and signaling peptide of the murine leukemia virus envelope protein, similar to our measles-based modification of the hemagglutinin, we here used a more recent strategy reported by Funke et al.21 independently co-expressing the anti-CD90 scFv together with either a WT or mutated version of the fusion protein on the VSV-G envelope. Adapting this strategy for VSV-based envelopes, we successfully designed CD90-targeted viral vectors that fused and transduced CD90-expressing cell lines as well as CD34+CD90+ HSCs with secondary colony-forming potential. Although the exact mechanism of CD90-mediated cellular uptake is not known, cytomegalovirus (CMV) and HIV use CD90 as a crucial part of their cellular entry process.35,36,37 CMV entry was interrupted when CD90 was either blocked with an antibody or downregulated using a small interfering RNA approach.37 In addition, liposomes conjugated with anti-CD90 antibodies have been shown to be capable of binding and internalizing through the CD90 antigen on liver cancer cells, demonstrating efficient uptake of CD90-targeted LNPs also in vivo.38 Our viral vectors have been genetically modified to lose their native binding (LDL-R for VSV-G; CD46 and CD150 for measles), but their ability to fuse with the target cell remains intact. Thus, the CD90 scFv enables cell-specific binding, while viral entry remains dependent on the viral envelope proteins. More in-depth studies are required to determine whether CD90 is actively involved in the uptake, which pathways are triggered, and whether this system can also be used completely independent from viral fusion proteins. Regardless of the exact pathways involved, CD90 protein was targeted on primary human HSCs without any obvious impact on the cells’ proliferation and differentiation potential. Follow-up work will be needed to evaluate the recycling and re-expression of CD90 protein on the cell surface after CD90-mediated viral entry to ensure that the long-term multi-lineage engraftment of HSCs is warranted.
A major bottleneck for the development of surface engineered viral vectors for HSC gene therapy is the inability to quickly and reliably verify the vectors before use. Genetically engineered viral vectors lose their native binding and functional titration methods on HEK293T or HT1080 cell lines are not possible. While the lack of cellular uptake can be overcome using quantitative PCR (qPCR)-based technologies to quantify RNA-loaded viral particles, functional qualification of the surface modification cannot be achieved with genomics. Validation of antigen/target recognition is needed to ensure that surface-engineered viral vectors are functional and targeting ligands have been successfully integrated into the capsid and correctly folded. While protein-protein interaction is traditionally demonstrated using SEC or biolayer interferometry, heterogeneity of viral particles and contamination with other proteins during vector preparation do not allow reliable testing using common technologies. To close this gap, we here developed a novel methodology to titrate and validate viral vectors using NTA.
NTA is commonly used to measure the size, charge, concentration, and heterogeneity/polydispersity of NPs such as LNPs and polymer NPs (PNPs), as well as exosomes39,40,41 isolated from human specimens and cell culture supernatant. Here we show that NTA also allows for the efficient and reliable quantification of viral vector concentration, size, and RNA loading. Most important, hydrodynamic viral titers correlated with the transduction efficiency on cell lines for WT variants, providing a surrogate method for the traditional titration on cell lines or other qPCR-based strategies. NTA was further used to demonstrate successful antigen recognition of targeted vectors. Custom-designed fluorochrome-conjugated CD90 protein was bound by the scFv-decorated VSV-G- and measles-pseudotyped viral vectors, resulting in a size increase measured by NTA and confirming fully functional antigen recognition when the targeting ligand was presented on the virus similar to SEC demonstrating the interaction of proteins resulting in a size increase and enhanced retention time. The measurement of these parameters is nearly instantaneous and provides a new alternative to traditional titration methods of WT, novel surface-engineered viral vectors, as well as virus-like particles. Viral titers can even be evaluated from the supernatant before purification and concentrating to combat the observed technical variability in the number of total particles and mRNA loading. The assessment of supernatants will allow the discontinuation of suboptimal preparations and high-throughput optimization of conditions to gain greater yields and better loading efficiencies. As viral vectors often undergo freezing and thawing, quality control and quantification of viral particles can even be performed right before applying them, providing an opportunity to adjust and carefully control viral titers in real time, removing a factor of variability in virus-mediated cell therapy applications.
Surface engineering of viral capsid bears the risk of decreased titers and RNA loading efficiency as previously reported by Friedel et al.42 While no to minimal impact was seen for CD90-targeted measles-pseudotyped viral vectors, we observed a decrease in the number of RNA-loaded viral particles for VSV-G-based variants adding the anti-CD90 scFv on the capsid and/or introducing the mutations to abolish LDL-R recognition. Although the impact of surface engineering on VSV-G-based vectors was mild, further evolution of the scFv and removing potentially problematic amino acid residues that can either interfere with folding of the targeting ligand, impact the integrity of the capsid, or lower the RNA-loading efficiency will be needed. Alternatively, other viral capsids in the VSV family such as Cocal43,44,45 should be evaluated for surface engineering and consequently the impact on viral titers. Finally, additional optimization is needed to increase the loading efficiency of our surface-modified viral particles. Although we significantly improved the target specificity of viral transduction, we observed a slight decrease in the transduction efficiency of the target cells because of the occupancy of CD90 antigen with empty particles, a phenomenon particularly important for the targeting of low-abundance antigens.
A major advantage of our CD90-targeted viral vectors is the ability to incorporate them into already existing ex vivo gene therapy pipelines focusing on the gene modification of CD34+ HSPCs. Further purification of the CD90+ subset for direct targeting and to enhance the transduction efficiency3 would potentially no longer be needed to transduce long-term engrafting HSCs and likely require even lower viral titers, reducing the overall costs. Even the purification of CD34+ cells from the leukapheresis products may no longer be necessary, providing a novel vector to realize closed-system vein-to-vein applications with the transduction of cells in bags and immediate infusion of the transduced cells back into the patient. Finally, direct intravenous or intraosseous injection of the HSC-targeted viral vectors would be warranted to further simplify HSC gene therapy, providing full portability, and consequently better accessibility. However, before such targeted vectors can enter the clinical routine, comprehensive ex vivo and in vivo studies in humanized mice as well as large animals such as the nonhuman primate will be needed to test the long-term impact of CD90-mediated transduction, confirm on-target specificity, determine the half-life and pharmacokinetics in circulation, evaluate serum susceptibility, and analyze potential off-target effects.
Especially for in vivo applications, various avenues need to be explored to provide the best targeting, highest efficiency, and least off-target effects. Adaptation of the targeting system using Cocal-pseudotyped lentiviral vectors, which are less susceptible to serum inactivation, may be favorable for in vivo administrations.43,44 Furthermore, the targeting of multiple HSC-associated cell surface markers using colocalization-dependent protein switches (Co-LOCKR) protein switches may help to increase the on-target specificity; most HSC markers are expressed to various degrees on cells in the lung, liver, brain, and reproductive organs (proteinatlas.org).46 Finally, adaptation of our targeting ligand for use with non-viral delivery platforms such as polymer or LNPs,47 as well as virus-like particles,48 may enable the transient delivery of nucleases or therapeutic mRNAs and proteins without the risk of insertional mutagenesis.
Our new CD90-targeted vectors have the potential to improve already existing ex vivo HSC gene therapies as well as overcome current hurdles to apply gene therapy in vivo. The development of targeted viral vectors has the potential to increase the on-target efficiency, safety, and accessibility of HSC gene transfer for a variety of different diseases and will be a crucial step in democratizing access to gene therapies.
Materials and methods
mRNA extraction, cDNA synthesis, and heavy/light chain sequencing of the hybridoma cells
mRNA was extracted from the hybridoma cells using an RNAeasy kit (Qiagen). Extracted mRNA was run on TapeStation (Agilent Technologies) for integrity. cDNA was generated from mRNA using an applied biosystem high capacity RNA-to-cDNA kit (Thermo Fisher Scientific). Primers for the variant regions of the antibody were acquired from the literature49 and ordered using IDT. CD90 ab internal reverse primer (TTC AGT CAC CAT GCT GTT GAC) forward primer (CCA GCA GAA GCC AGG ATC). Antibody variable regions were sequenced using on an Applied Biosystems 3730xl DNA Analyzers (Thermo Fisher Scientific). Raw sequencing data was analyzed using International ImMunoGeneTics V-QUEry and STandardization program to align and annotate the antibody sequence.
Modeling and visualization of proteins
The antibody structure was predicted with SabPred.17 Antibody-protein docking was predicted using Swissdock.15 Visualizations were generated with Pymol (The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC).
Cloning of virus plasmids
Measles plasmid were provided by Els Verhoeyen.19 Cloning was performed using restriction enzymes from New England Biolabs (NEB) and Gibson Assembly Master Mix (NEB). DNA tiles containing the desired sequences to be cloned into the plasmids were acquired from Codex DNA. Cloned products were confirmed via sequencing on an Applied Biosystems 3730xl DNA Analyzers (Thermo Fisher Scientific). The relevant sequences are in Figures S1–S6.
VSV-G KO and targeted binder plasmids were provided by the Baker Laboratory (Institute of Protein Design, University of Washington). Cloning of CD90 scFv into the targeted binder plasmid was performed by Eco721-FastDigest (Thermo Fisher Scientific, cat # FD0364) and Eco1471-FastDigest (Thermo Fisher Scientific, cat # FD0424) restriction enzyme digest of targeted binder plasmid followed by Gibson Assembly (NEB, cat #E2611S) with synthesized Gibson block sequence (Integrated Data Technologies) containing the CD90 scfv sequence (Figures S3, S4, and S6). Successful cloning was confirmed by EcoRI (NEB, cat #R3101S) restriction enzyme digest, followed by sequencing on Applied Biosystems 373xl DNA Analyzers (Thermo Fisher Scientific).
Virus production
HEK293T cells were expanded on gelatin coated plates and transfected with plasmids mixed with PEI (Polysciences) at a ¼ μg plasmid to μg PEI ratio. Measles virus plasmid ratios were 27 μg pLenti-EF1a-mScarlet-I-WPRE transfer plasmid, 17 μg second-generation Gag/Pol Helper plasmid pCMVdelta8.74, 10 μg measles hemagglutinin Delta 24H, and 10 μg measles fusion Delta F per 15-cm cell plate. VSV-G virus plasmid ratios were 27 μg pLenti-EF1a-mScarlet-I-WPRE transfer, 17 μg second-generation Gag/Pol Helper plasmid pCMVdelta8.74, 10 μg VSV-G fusion, and 10 μg relevant targeting ligand plasmid per 15-cm cell plate. Twenty-four hours after transfection, the media was changed to LV-MAX (Thermo Fisher Scientific) media with 20 mM HEPES (Thermo Fisher Scientific) and 1× l-glutamine (Thermo Fisher Scientific). Virus production media was allowed to sit for a further 24 h, then the supernatant was collected, spun down at 800×g to remove cellular debris, filtered with an 8-μm filter, and spun down for 24 h at 4,800×g. The concentrated supernatant was resuspended at a 1/100 volume.
Hydrodynamic titration
Hydrodynamic titration of viral particles was performed on a Zetaviewer machine (Particle Metrix, GmbH). The machine was calibrated with a 100-nm bead test sample prior to all measurements. Viruses were diluted 1 to 1,000 in molecular-grade water and analyzed for their size characteristics. Readouts from the machine were back calculated to account for dilution. Diluted virus samples were next incubated with 1× SYBR II Green RNA stain (Thermo Fisher Scientific) for 5 min before running on the Zetaviewer with a 488-nm filter in place to only identify virus particles loaded with RNA and stained with SYBR II. Additionally, viruses targeted to CD90 were incubated with SYBR II and CD90ex protein for 30 min. Virus-protein complexes were analyzed on the Zetaviewer with a 488- or 520-nm filter in place to capture particles loaded with RNA carrying CD90ex.
CD90 Fab production
Anti-CD90 IgGs were produced by the Antibody Technology Core at the Fred Hutchinson Cancer Center from the previously reported hybridoma cell lines producing the CD90 antibody clone 5E10.12 Fab fragments were generated from full-length CD90 antibodies with immobilized papain (Thermo Fisher Scientific cat. number 20341) per manufacture protocol. After verifying full digestion by SDSPAGE, the reaction mixture was buffer exchanged into PBS and the Fab separated from the Fc by anion exchange chromatography using a HiTrap Q column on an ÄKTA Pure.
CD90 production
The CD90-mCh Fusion protein was expressed in Freestyle293F cells as the following amino acid sequence:
METDTLLLWVLLLWVPGSTGQKVTSLTACLVDQSLRLDCRHENTSSSPIQYEFSLTRETKKHVLFGTVGVPEHTYRSRTNFTSKYNMKVLYLSAFTSKDEGTYTCALHHSGHSPPISSQNVTVLRDKLVKCEGENLYFQGSHHHHHH∗.
The DNA was codon optimized for human expression, cloned into a modified lentivirus expression system, and expressed as described by Bandaranayake et al.16 The media containing the secreted CD90 was concentrated and purified using a P200 SEC column equilibrated in PBS buffer.
Cell lines
Jurkat (ATCC CLR-2899), Raji (ATCC CCL-86), and K-562 (ATCC CCL-243) cells were all cultured in RPMI with penicillin streptomycin at a final concentration of 50–100 I.U./mL (Thermo Fisher Scientific) and supplemented with 10% cosmic calf serum (Thermo Fisher Scientific).
Primary human HSCs
Primary human CD34+ cells were purchased from the Co-operative Center for Excellence in Hematology at the Fred Hutchinson Cancer Center. Collections were performed according to the Declaration of Helsinki and were approved by a local ethics committee/institutional review board of the Fred Hutchinson Cancer Center. All healthy adult donors were mobilized with GCSF. Human CD34+ cells were enriched as previously described on a CliniMACS Prodigy according to the manufacturer’s instructions (Miltenyi Biotec).
Human CD34+ cells were cultured in StemSpan (STEMCELL Technologies) medium supplemented with penicillin-streptomycin (100 U/mL) (Gibco by Life Technologies) and 100 ng/mL of each stem cell factor (PeproTech), thrombopoietin (PeproTech), and Fms-related tyrosine kinase 3 ligand (Miltenyi Biotec). Cells were cultured at 37°C, 85% relative humidity, and 5% CO2.
Virus transduction
Cells (Jurkat, Raji, and human HSCs) were resuspended in serum-free media at a density of 150,000 cells per 150 μL per well of a 96-well plate (Corning). Concentrated viral supernatant was added to the cells and incubated for 8–12 h. After transduction cells were washed and media replaced with relevant growth media specific to cell type (see above). Transgene expression was determined flow-cytometrically 72–120 h after transduction.
Viability assessment and counting of human CD34+ cells and cell lines
The cell viability was analyzed using the Countess II FL automated cell counter (Thermo Fisher Scientific). A 10-μL volume of trypan blue stain (0.4%) (Invitrogen) was mixed with 10 μL cell suspension, and 10 μL of the mixture was applied to a disposable cell counting chamber slide and inserted into the device. The percentage of cell viability of each sample was recorded in duplicate and reported as the mean ± SEM.
Flow cytometry analysis and cell sorting
Flow cytometric analysis and sorting of cell lines and human CD34+ cells were performed using the fluorochrome-conjugated antibodies listed in Table S1. Dead cells and debris were excluded via forward light scatter/side light scatter gating and DAPI staining. Flow cytometric analysis and cell sorting were performed on a FACSymphony A5, FACSCelesta, FACSAria IIu, and Symphony S6 (BD Biosciences). Data were acquired using FACSDiva version 6.1.3 and newer (BD Biosciences). Data analysis was performed using FlowJo version 8 and higher (BD Biosciences).
CFC assay
For CFC assays, 200 FACS-purified CD34+mScarlet+ cells were seeded on 30-mm plates in 1 mL methylcellulose (MethoCult H4435, STEMCELL Technologies). Colonies were counted and scored after 12–14 days according to morphology into colony-forming unit (CFU)-granulocyte, CFU-macrophage, granulocyte-macrophage, and burst-forming unit-erythrocyte. Colonies consisting of erythroid and myeloid cells were scored as CFU-mix. For secondary CFC assays, primary colonies were harvested, washed twice with PBS, and 5% of cell suspension replated into 1 mL methylcellulose. Secondary colonies were counted and scored after 12–14 days.
Data and code availability
The raw data required to reproduce the above findings are available upon request.
Acknowledgments
We thank Helen Crawford for help in preparing this manuscript and figures. This work was supported in part by grants to H.P.K. from the National Institutes of Health (R01 AI135953–01 and R01 HL136135). H.P.K. also received support as a Markey Molecular Medicine Investigator, as the inaugural recipient of the José Carreras/E. Donnall Thomas Endowed Chair for Cancer Research and as the Stephanus Family Endowed Chair for Cell and Gene Therapy.
Author contributions
S.R. and H.P.K. designed the study. K.B. generated the CD90 scFVs. K.B. and J.T. generate and tested the vectors. E.A.T., P.B.R., and R.S. generated protein. K.B., J.T., S.C., R.M., and G.K. performed in vitro experiments and analyzed data. S.R., K.B., and J.T. generated the figures. H.P.K. and R.S. funded the study. S.R., K.B., J.T., and H.P.K. wrote the manuscript. All authors reviewed and edited the final manuscript.
Declaration of interests
S.R. is consultant to Forty-Seven Inc. (Gilead Sciences) and Ensoma Inc. H.P.K is or was a consultant to and has or had ownership interests with Rocket Pharmaceuticals, Homology Medicines, VOR Biopharma, and Ensoma Inc. H.P.K. has also been a consultant to CSL Behring and Magenta Therapeutics.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.ymthe.2023.08.003.
Contributor Information
Hans-Peter Kiem, Email: hkiem@fredhutch.org.
Stefan Radtke, Email: sradtke@fredhutch.org.
Supplemental information
References
- 1.Majeti R., Park C.Y., Weissman I.L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 2007;1:635–645. doi: 10.1016/j.stem.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedict C.A., Tun R.Y., Rubinstein D.B., Guillaume T., Cannon P.M., Anderson W.F. Targeting retroviral vectors to CD34-expressing cells: binding to CD34 does not catalyze virus-cell fusion. Hum. Gene Ther. 1999;10:545–557. doi: 10.1089/10430349950018625. [DOI] [PubMed] [Google Scholar]
- 3.Radtke S., Pande D., Cui M., Perez A.M., Chan Y.Y., Enstrom M., Schmuck S., Berger A., Eunson T., Adair J.E., Kiem H.P. Purification of human CD34(+)CD90(+) HSCs reduces target cell population and improves lentiviral transduction for gene therapy. Mol. Ther. Methods Clin. Dev. 2020;18:679–691. doi: 10.1016/j.omtm.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brendel C., Goebel B., Daniela A., Brugman M., Kneissl S., Schwäble J., Kaufmann K.B., Müller-Kuller U., Kunkel H., Chen-Wichmann L., et al. CD133-targeted gene transfer into long-term repopulating hematopoietic stem cells. Mol. Ther. 2015;23:63–70. doi: 10.1038/mt.2014.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Görgens A., Radtke S., Möllmann M., Cross M., Dürig J., Horn P.A., Giebel B. Revision of the human hematopoietic tree: granulocyte subtypes derive from distinct hematopoietic lineages. Cell Rep. 2013;3:1539–1552. doi: 10.1016/j.celrep.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Radtke S., Görgens A., Kordelas L., Schmidt M., Kimmig K.R., Köninger A., Horn P.A., Giebel B. CD133 allows elaborated discrimination and quantification of haematopoietic progenitor subsets in human haematopoietic stem cell transplants. Br. J. Haematol. 2015;169:868–878. doi: 10.1111/bjh.13362. [DOI] [PubMed] [Google Scholar]
- 7.Radtke S., Adair J.E., Giese M.A., Chan Y.Y., Norgaard Z.K., Enstrom M., Haworth K.G., Schefter L.E., Kiem H.P. A distinct hematopoietic stem cell population for rapid multilineage engraftment in nonhuman primates. Sci. Transl. Med. 2017;9:eaan1145. doi: 10.1126/scitranslmed.aan1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Humbert O., Radtke S., Samuelson C., Carrillo R.R., Perez A.M., Reddy S.S., Lux C., Pattabhi S., Schefter L.E., Negre O., et al. Therapeutically relevant engraftment of a CRISPR-Cas9-edited HSC-enriched population with HbF reactivation in nonhuman primates. Sci. Transl. Med. 2019;11:eaaw3768. doi: 10.1126/scitranslmed.aaw3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masiuk K.E., Brown D., Laborada J., Hollis R.P., Urbinati F., Kohn D.B. Improving gene therapy efficiency through the enrichment of human hematopoietic stem cells. Mol. Ther. 2017;25:2163–2175. doi: 10.1016/j.ymthe.2017.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zonari E., Desantis G., Petrillo C., Boccalatte F.E., Lidonnici M.R., Kajaste-Rudnitski A., Aiuti A., Ferrari G., Naldini L., Gentner B. Efficient ex vivo engineering and expansion of highly purified human hematopoietic stem and progenitor cell populations for gene therapy. Stem Cell Rep. 2017;8:977–990. doi: 10.1016/j.stemcr.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon P.R., Leimig T., Babarin-Dorner A., Houston J., Holladay M., Mueller I., Geiger T., Handgretinger R. Large-scale isolation of CD133+ progenitor cells from G-CSF mobilized peripheral blood stem cells. Bone Marrow Transpl. 2003;31:17–22. doi: 10.1038/sj.bmt.1703792. [DOI] [PubMed] [Google Scholar]
- 12.Craig W., Kay R., Cutler R.L., Lansdorp P.M. Expression of Thy-1 on human hematopoietic progenitor cells. J. Exp. Med. 1993;177:1331–1342. doi: 10.1084/jem.177.5.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lefranc M.P., Giudicelli V., Duroux P., Jabado-Michaloud J., Folch G., Aouinti S., Carillon E., Duvergey H., Houles A., Paysan-Lafosse T., et al. IMGT(R), the international ImMunoGeneTics information system(R) 25 years on. Nucleic Acids Res. 2015;43:D413–D422. doi: 10.1093/nar/gku1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefranc M.P. Immunoglobulin and T Cell Receptor Genes: IMGT((R)) and the Birth and Rise of Immunoinformatics. Front. Immunol. 2014;5:22. doi: 10.3389/fimmu.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grosdidier A., Zoete V., Michielin O. SwissDock, a protein-small molecule docking web service based on EADock DSS. Nucleic Acids Res. 2011;39:W270–W277. doi: 10.1093/nar/gkr366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bandaranayake A.D., Correnti C., Ryu B.Y., Brault M., Strong R.K., Rawlings D.J. Daedalus: a robust, turnkey platform for rapid production of decigram quantities of active recombinant proteins in human cell lines using novel lentiviral vectors. Nucleic Acids Res. 2011;39:e143. doi: 10.1093/nar/gkr706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunbar J., Krawczyk K., Leem J., Marks C., Nowak J., Regep C., Georges G., Kelm S., Popovic B., Deane C.M. SAbPred: a structure-based antibody prediction server. Nucleic Acids Res. 2016;44:W474–W478. doi: 10.1093/nar/gkw361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anliker B., Abel T., Kneissl S., Hlavaty J., Caputi A., Brynza J., Schneider I.C., Münch R.C., Petznek H., Kontermann R.E., et al. Specific gene transfer to neurons, endothelial cells and hematopoietic progenitors with lentiviral vectors. Nat. Methods. 2010;7:929–935. doi: 10.1038/nmeth.1514. [DOI] [PubMed] [Google Scholar]
- 19.Lévy C., Amirache F., Girard-Gagnepain A., Frecha C., Roman-Rodríguez F.J., Bernadin O., Costa C., Nègre D., Gutierrez-Guerrero A., Vranckx L.S., et al. Measles virus envelope pseudotyped lentiviral vectors transduce quiescent human HSCs at an efficiency without precedent. Blood Adv. 2017;1:2088–2104. doi: 10.1182/bloodadvances.2017007773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frecha C., Costa C., Nègre D., Gauthier E., Russell S.J., Cosset F.L., Verhoeyen E. Stable transduction of quiescent T cells without induction of cycle progression by a novel lentiviral vector pseudotyped with measles virus glycoproteins. Blood. 2008;112:4843–4852. doi: 10.1182/blood-2008-05-155945. [DOI] [PubMed] [Google Scholar]
- 21.Funke S., Maisner A., Mühlebach M.D., Koehl U., Grez M., Cattaneo R., Cichutek K., Buchholz C.J. Targeted cell entry of lentiviral vectors. Mol. Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobson C.S., Reich A.N., Gaglione S., Smith B.E., Kim E.J., Dong J., Ronsard L., Okonkwo V., Lingwood D., Dougan M., et al. Antigen identification and high-throughput interaction mapping by reprogramming viral entry. Nat. Methods. 2022;19:449–460. doi: 10.1038/s41592-022-01436-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolic J., Belot L., Raux H., Legrand P., Gaudin Y., A Albertini A. Structural basis for the recognition of LDL-receptor family members by VSV glycoprotein. Nat. Commun. 2018;9:1029. doi: 10.1038/s41467-018-03432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kedmi R., Veiga N., Ramishetti S., Goldsmith M., Rosenblum D., Dammes N., Hazan-Halevy I., Nahary L., Leviatan-Ben-Arye S., Harlev M., et al. A modular platform for targeted RNAi therapeutics. Nat. Nanotechnol. 2018;13:214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 25.De B.P., Chen A., Salami C.O., Van de Graaf B., Rosenberg J.B., Pagovich O.E., Sondhi D., Crystal R.G., Kaminsky S.M. In vivo potency assay for adeno-associated virus-based gene therapy vectors using AAVrh.10 as an example. Hum. Gene Ther. Methods. 2018;29:146–155. doi: 10.1089/hgtb.2017.246. [DOI] [PubMed] [Google Scholar]
- 26.van Haasteren J., Hyde S.C., Gill D.R. Lessons learned from lung and liver in-vivo gene therapy: implications for the future. Expert Opin. Biol. Ther. 2018;18:959–972. doi: 10.1080/14712598.2018.1506761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinlützum D., Hanauer J.D.S., Muik A., Hanschmann K.M., Kays S.K., Ayala-Breton C., Peng K.W., Mühlebach M.D., Abel T., Buchholz C.J. Enhancing the oncolytic activity of CD133-targeted measles virus: Receptor extension or chimerism with vesicular stomatitis virus are most effective. Front. Oncol. 2017;7:127. doi: 10.3389/fonc.2017.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei T., Cheng Q., Min Y.L., Olson E.N., Siegwart D.J. Systemic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020;11:3232. doi: 10.1038/s41467-020-17029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niu F., Yan J., Ma B., Li S., Shao Y., He P., Zhang W., He W., Ma P.X., Lu W. Lanthanide-doped nanoparticles conjugated with an anti-CD33 antibody and a p53-activating peptide for acute myeloid leukemia therapy. Biomaterials. 2018;167:132–142. doi: 10.1016/j.biomaterials.2018.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblum D., Gutkin A., Kedmi R., Ramishetti S., Veiga N., Jacobi A.M., Schubert M.S., Friedmann-Morvinski D., Cohen Z.R., Behlke M.A., et al. CRISPR-Cas9 genome editing using targeted lipid nanoparticles for cancer therapy. Sci. Adv. 2020;6:eabc9450. doi: 10.1126/sciadv.abc9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zheng Y., Stephan M.T., Gai S.A., Abraham W., Shearer A., Irvine D.J. In vivo targeting of adoptively transferred T-cells with antibody- and cytokine-conjugated liposomes. J. Control Release. 2013;172:426–435. doi: 10.1016/j.jconrel.2013.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kneissl S., Abel T., Rasbach A., Brynza J., Schneider-Schaulies J., Buchholz C.J. Measles virus glycoprotein-based lentiviral targeting vectors that avoid neutralizing antibodies. PLoS One. 2012;7:e46667. doi: 10.1371/journal.pone.0046667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moffett H.F., Coon M.E., Radtke S., Stephan S.B., McKnight L., Lambert A., Stoddard B.L., Kiem H.P., Stephan M.T. Hit-and-run programming of therapeutic cytoreagents using mRNA nanocarriers. Nat. Commun. 2017;8:389. doi: 10.1038/s41467-017-00505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Veiga N., Goldsmith M., Granot Y., Rosenblum D., Dammes N., Kedmi R., Ramishetti S., Peer D. Cell specific delivery of modified mRNA expressing therapeutic proteins to leukocytes. Nat. Commun. 2018;9:4493. doi: 10.1038/s41467-018-06936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rudnicka D., Feldmann J., Porrot F., Wietgrefe S., Guadagnini S., Prévost M.C., Estaquier J., Haase A.T., Sol-Foulon N., Schwartz O. Simultaneous cell-to-cell transmission of human immunodeficiency virus to multiple targets through polysynapses. J. Virol. 2009;83:6234–6246. doi: 10.1128/JVI.00282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q., Fischer E., Cohen J.I. Cell surface THY-1 contributes to human cytomegalovirus entry via a macropinocytosis-like process. J. Virol. 2016;90:9766–9781. doi: 10.1128/JVI.01092-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q., Wilkie A.R., Weller M., Liu X., Cohen J.I. THY-1 cell surface antigen (CD90) has an important role in the initial stage of human cytomegalovirus infection. Plos Pathog. 2015;11:e1004999. doi: 10.1371/journal.ppat.1004999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang R., An L.Y., Miao Q.F., Li F.M., Han Y., Wang H.X., Liu D.P., Chen R., Tang S.Q. Effective elimination of liver cancer stem-like cells by CD90 antibody targeted thermosensitive magnetoliposomes. Oncotarget. 2016;7:35894–35916. doi: 10.18632/oncotarget.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giebel B., Helmbrecht C. Methods to analyze EVs. Methods Mol. Biol. 2017;1545:1–20. doi: 10.1007/978-1-4939-6728-5_1. [DOI] [PubMed] [Google Scholar]
- 40.Rozo A.J., Cox M.H., Devitt A., Rothnie A.J., Goddard A.D. Biophysical analysis of lipidic nanoparticles. Methods. 2020;180:45–55. doi: 10.1016/j.ymeth.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Gross J., Sayle S., Karow A.R., Bakowsky U., Garidel P. Nanoparticle tracking analysis of particle size and concentration detection in suspensions of polymer and protein samples: Influence of experimental and data evaluation parameters. Eur. J. Pharm. Biopharm. 2016;104:30–41. doi: 10.1016/j.ejpb.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Friedel T., Hanisch L.J., Muth A., Honegger A., Abken H., Plückthun A., Buchholz C.J., Schneider I.C. Receptor-targeted lentiviral vectors are exceptionally sensitive toward the biophysical properties of the displayed single-chain Fv. Protein Eng. Des. Sel. 2015;28:93–106. doi: 10.1093/protein/gzv005. [DOI] [PubMed] [Google Scholar]
- 43.Trobridge G.D., Wu R.A., Hansen M., Ironside C., Watts K.L., Olsen P., Beard B.C., Kiem H.P. Cocal-pseudotyped lentiviral vectors resist inactivation by human serum and efficiently transduce primate hematopoietic repopulating cells. Mol. Ther. 2010;18:725–733. doi: 10.1038/mt.2009.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajawat Y.S., Humbert O., Cook S.M., Radtke S., Pande D., Enstrom M., Wohlfahrt M.E., Kiem H.P. In vivo gene therapy for canine SCID-X1 using cocal-pseudotyped lentiviral vector. Hum. Gene Ther. 2021;32:113–127. doi: 10.1089/hum.2020.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Humbert O., Gisch D.W., Wohlfahrt M.E., Adams A.B., Greenberg P.D., Schmitt T.M., Trobridge G.D., Kiem H.P. Development of third-generation cocal envelope producer cell lines for robust lentiviral gene transfer into hematopoietic stem cells and T-cells. Mol. Ther. 2016;24:1237–1246. doi: 10.1038/mt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lajoie M.J., Boyken S.E., Salter A.I., Bruffey J., Rajan A., Langan R.A., Olshefsky A., Muhunthan V., Bick M.J., Gewe M., et al. Designed protein logic to target cells with precise combinations of surface antigens. Science. 2020;369:1637–1643. doi: 10.1126/science.aba6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Kharrag R., Berckmueller K.E., Madhu R., Cui M., Campoy G., Mack H.M., Wolf C.B., Perez A.M., Humbert O., Kiem H.P., Radtke S. Efficient polymer nanoparticle-mediated delivery of gene editing reagents into human hematopoietic stem and progenitor cells. Mol. Ther. 2022;30:2186–2198. doi: 10.1016/j.ymthe.2022.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Banskota S., Raguram A., Suh S., Du S.W., Davis J.R., Choi E.H., Wang X., Nielsen S.C., Newby G.A., Randolph P.B., et al. Engineered virus-like particles for efficient in vivo delivery of therapeutic proteins. Cell. 2022;185:250–265.e16. doi: 10.1016/j.cell.2021.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y., Liu H.Y., Mu L., Luo E.J. Degenerate primer design to clone the human repertoire of immunoglobulin heavy chain variable regions. World J. Microbiol. Biotechnol. 2012;28:381–386. doi: 10.1007/s11274-011-0830-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data required to reproduce the above findings are available upon request.