Abstract
Complementary to synaptophysin and chromogranin A, insulinoma‐associated protein 1 (INSM1) has emerged as a sensitive marker for the diagnosis of neuroendocrine neoplasms. Since there are no comparative data regarding INSM1 expression in conventional colorectal adenocarcinomas (CRCs) and colorectal mixed adenoneuroendocrine carcinomas/neuroendocrine carcinomas (MANECs/NECs), we examined INSM1 in a large cohort of conventional CRCs and MANECs/NECs. In conventional CRC, we put a special focus on conventional CRC with diffuse expression of synaptophysin, which carry the risk of being misinterpreted as a MANEC or a NEC. We investigated INSM1 according to the immunoreactive score in our main cohort of 1,033 conventional CRCs and 21 MANECs/NECs in comparison to the expression of synaptophysin and chromogranin A and correlated the results with clinicopathological parameters and patient survival. All MANECs/NECs expressed INSM1, usually showing high or moderate expression (57% high, 34% moderate, and 9% low), which distinguished them from conventional CRCs, which were usually INSM1 negative or low, even if they diffusely expressed synaptophysin. High expression of INSM1 was not observed in conventional CRCs. Chromogranin A was negative/low in most conventional CRCs (99%), but also in most MANECs/NECs (66%). Comparable results were observed in our independent validation cohorts of conventional CRC (n = 274) and MANEC/NEC (n = 19). Similar to synaptophysin, INSM1 expression had no prognostic relevance in conventional CRCs, while true MANEC/NEC showed a highly impaired survival in univariate and multivariate analyses (e.g. disease‐specific survival: p < 0.001). MANECs/NECs are a highly aggressive variant of colorectal cancer, which must be reliably identified. High expression of INSM1 distinguishes MANEC/NEC from conventional CRCs with diffuse expression of the standard neuroendocrine marker synaptophysin, which do not share the same dismal prognosis. Therefore, high INSM1 expression is a highly specific/sensitive marker that is supportive for the diagnosis of true colorectal MANEC/NEC.
Keywords: INSM1, colorectal carcinoma, neuroendocrine carcinoma, conventional adenocarcinoma, synaptophysin
Introduction
Colorectal mixed adenoneuroendocrine carcinomas (MANECs) and neuroendocrine carcinomas (NECs) are rare histological subtypes of colorectal carcinoma with distinctive morphology and prognosis [1, 2, 3]. Although genetically related [4], they behave more aggressively than conventional colorectal adenocarcinomas (CRCs) [5] and are treated differently [6]. A safe distinction of conventional CRCs from MANECs/NECs is therefore of great importance.
Despite their glandular, non‐neuroendocrine morphology, positivity for the neuroendocrine marker synaptophysin is often found in conventional CRCs, usually in the form of scattered positive cells [7]. However, in a small fraction of conventional CRCs, synaptophysin is diffusely expressed. Adenocarcinomas with a neuroendocrine component that accounts for more than 30% of the tumor theoretically fulfill the immunohistochemical criteria of a MANEC according to the current WHO classification. Therefore, conventional CRCs in which the number of synaptophysin positive cells reaches or even exceeds this 30% threshold may be highly problematic regarding their classification [3, 7], especially if they have an ambiguous morphology.
In a previous study, we were able to demonstrate that conventional CRCs with diffuse expression of synaptophysin do not have the same poor outcome as true MANECs/NECs, which reveal their inherent neuroendocrine nature by the presence of a large cell or small cell carcinoma component, which is already appreciable in hematoxylin–eosin (H&E) stained sections [3, 7, 8].
In addition to the traditional neuroendocrine markers synaptophysin and chromogranin A, insulinoma‐associated protein 1 (INSM1), a zinc‐finger transcription factor that regulates neuroendocrine cell differentiation [9], has been established as a complementary marker for the diagnosis of neuroendocrine neoplasms [10]. While high sensitivity and specificity [10, 11, 12, 13, 14, 15, 16, 17, 18, 19] of INSM1 expression have been reported for neuroendocrine neoplasms from various anatomic sites, a specific evaluation of INSM1 expression in colorectal MANECs/NECs is still lacking. Furthermore, there are no data regarding the frequency and prognostic relevance of INSM1 expression in conventional CRCs and it is also not known whether INSM1 expression in conventional CRCs and MANECs/NECs is fully comparable to the expression of the traditional neuroendocrine markers synaptophysin/chromogranin A.
To answer these questions, we investigated the frequency and the extent of INSM1 expression according to the immunoreactive score (IRS) [20] in our main cohort of 1,033 conventional colorectal carcinomas and 21 colorectal MANECs/NECs, and in two separate validation collectives (validation cohort 1: conventional CRC, validation cohort 2: MANEC/NEC). In the large main cohort of 1,054 cancers, we then correlated the results with clinicopathological parameters, expression of synaptophysin/chromogranin A, and patient survival. We put special emphasis on the expression of INSM1 in conventional CRCs with diffuse expression of synaptophysin, in order to find out whether INSM1 could be diagnostically useful for the distinction of these neoplasms from MANEC/NEC.
Materials and methods
Main cohort
The main cohort included 1,054 colorectal carcinomas from the University Hospital rechts der Isar of the Technical University Munich (n = 1,044, conventional CRC and MANEC/NEC) and the University Hospital Marburg (n = 10, MANEC/NEC), which were surgically resected from 1997 to 2022. Patients with other neoplasms of the colorectal system (e.g. well‐differentiated neuroendocrine tumors, nonepithelial tumors, etc.), appendiceal neoplasms, incomplete clinicopathological/survival data, or insufficient tissue on the constructed tissue microarray (TMA) were excluded. Survival data as well as clinicopathological characteristics from all patients were extracted from the Munich Cancer Registry or from hospital records. All recorded patient deaths were noted for overall survival (OS), and only tumor‐associated deaths were recorded as events for disease‐specific survival (DSS). An event for disease‐free survival (DFS) was defined as either locoregional or distant recurrence. For all survival comparisons, the endpoints were either events or a loss of follow‐up. In this case, the patients were censored at the time of the last available entry regarding the specific patient or after a maximum follow‐up of 120 months. The treatment concepts of included patients followed internal policies, which were based on the given German guidelines at the time of diagnosis, generally meaning that all patients were intended to receive stage‐adapted treatment.
Our cohort comprised 1,033 conventional CRC, which we defined as colorectal adenocarcinomas [adenocarcinomas, not otherwise specified (NOS) and variants according to the WHO classification] [3] without histological features suggestive of a NEC on H&E staining. In addition, we investigated 21 colorectal tumors which were diagnosed as MANECs/NECs, in which more than 30% of the tumor cell population showed the typical histology of a large or small cell NEC on H&E staining, which was immunohistochemically confirmed by the expression of synaptophysin (and/or chromogranin A). In accordance with the criteria given by the WHO classification, small cell NECs were composed of small to medium sized cells with scant basophilic cytoplasm, elongated hyperchromatic nuclei without distinctive nucleoli, and high mitotic activity. Large cell NECs were diagnosed if the neoplasms were composed of solid sheets of medium to large sized tumor cells with rounded vesicular nuclei harboring prominent nucleoli [3, 7, 21, 22]. Colorectal MANECs were also diagnosed according to the WHO classification guidelines; specifically, if one of the described, clearly morphologically recognizable NEC components were admixed with an adenocarcinoma component, either in a mosaic pattern or closely intermingled in a composite pattern.
A TMA from all cases comprising cores from the tumor center and the invasive front was constructed using the TMA grand master (Sysmex/3DHistech, Budapest, Hungary) as previously described [7]. The majority of tumors were already investigated in previous studies from our group [5, 7, 21, 22] and were characterized using morphological parameters such as grade and histological subtype according to the current WHO classification [3], expression of synaptophysin, or microsatellite instability (MSI) status. All tumors that had not been included in our previous studies were reclassified using the above parameters.
Our study was approved by the local ethics committees of the Technical University of Munich (reference numbers: 252/16 s and 2022/565‐S‐KK), the University Hospital Mainz (reference number: 837.075.16), and of the University Hospital Marburg (reference number: AZ 43/21).
Immunohistochemical analyses of the main cohort
A TMA containing two separate cores from 1,054 colorectal carcinomas from the main cohort was stained with an INSM1 antibody (clone BSB‐123, dilution: 1:50, Medac GmbH, Wedel, Germany). INSM1 expression was evaluated manually by an experienced gastrointestinal pathologist (MJ). Only nuclear staining of INSM1 was considered specific. The staining in normal pancreatic islets served as control. The number of positive carcinoma cells was assessed for each individual patient, counting a minimum of 500 tumor cells, resulting in a cumulative percentage score for both cores that was assigned for each CRC (range: 0–100%). The expression intensity was graded as strong (comparable to normal islet cells), moderate (clearly visible staining but notably weaker than in normal islet cells), weak (barely perceptible and only notable in high magnifications), and negative (no staining reaction). Afterwards, all carcinomas were assigned to different INSM1 expression groups according to their IRS [20], which is derived from a sum score of the percentage of expressing cells (score 0–4) and the maximum staining intensity (score 0–3), which are then multiplied by each other. According to the IRS, four INSM1 expression groups were defined (INSM1 negative, IRS 0–1; INSM1 low, IRS 2–3; INSM1 moderate, IRS 4–8; INSM1 high, IRS 9–12) [23, 24]. The algorithm to determine the IRS as well as the resulting INSM1 expression groups is shown in detail in Table 1.
Table 1.
Algorithm to determine the IRS and respective INSM1 expression groups
| Immunoreactive score (IRS) | ||
|---|---|---|
| Score | Staining intensity | Percentage of positive cells |
| 0 | No staining reaction | 0 |
| 1 | Weak staining reaction | <10 |
| 2 | Moderate staining reaction | 10–50 |
| 3 | Strong staining reaction | 51–80 |
| 4 | >80 | |
| IRS = score (staining intensity) × score (percentage of positive cells) | ||
| INSM1 expression groups | ||
| IRS 0–1 | INSM1 negative | |
| IRS 2–3 | INSM1 low | |
| IRS 4–8 | INSM1 moderate | |
| IRS 9–12 | INSM1 high | |
To correlate the results obtained from the analysis on the TMA with whole tissue sections, INSM1 expression was investigated on whole slides of 35 carcinomas from the main cohort (including all 21 MANECs/NECs).
Synaptophysin expression was evaluated as previously described [7]. Conventional CRCs were assigned to four synaptophysin expression groups: synaptophysin negative, synaptophysin low (>0–9% positive tumor cells), and synaptophysin moderate (10–29% positive tumor cells). Conventional CRCs that showed expression of synaptophysin in 30–100% of the tumor cells were classified as synaptophysin high/diffuse, as they reached or exceeded the 30% threshold of neuroendocrine differentiation given by the WHO classification for the diagnosis of a MANEC [3].
Cytoplasmic expression of chromogranin A (clone SP‐12, dilution: 1:200, ZytoMed, Berlin, Germany) was also evaluated for all tumors on the TMA. The expression groups were also allocated in accordance with the IRS described above for INSM1.
Validation cohort of conventional CRC
Our validation cohort of conventional CRC consisted of 274 resected cases collected from the archives of the University Hospital Mainz as well as from the University Hospital Marburg. Mean age at diagnosis was 69 years. Fifty‐seven percent of patients were male. After resection, the histopathological workup revealed 51 UICC stage I (19%), 81 stage UICC II (29%), 82 stage UICC III (3%), and 60 stage UICC IV patients (22%). All of these cases were investigated for INSM1/synaptophysin/chromogranin A as described above on a TMA carrying a minimum of two and up to three cores per case.
Validation cohort for colorectal MANEC/NEC
To further validate the expression of INSM1/synaptophysin/chromogranin A in colorectal MANEC and NEC, we also included a separate MANEC/NEC validation cohort consisting of 19 additional cases. These cases were diagnosed at the Institute of Pathology ÜGP/MVZ Mittelhessen between 2016 and 2021 and were reclassified before inclusion in our study as described above. Fifteen cases were classified as NEC and four were classified as MANEC. The cohort consisted of 10 male and 9 female patients, with a median age at diagnosis of 75 years. No clinical follow‐up data were available for these tumors. Because of the rarity of these tumors, our validation cohort included resection specimens and biopsies. From all cases of the MANEC/NEC validation cohort, whole tissue sections were examined for their immunohistochemical expression of INSM1, synaptophysin, and chromogranin A using the same methodology as described earlier.
Statistics
Statistical analyses were performed with IMB SPSS Statistics, version 28 (IBM Corp, Armonk, NY, USA). Hypothesis tests of associations were performed by χ 2 test and Fisher's exact test (two‐sided). Univariable survival probabilities were estimated with the Kaplan–Meier method and log‐rank tests were used to probe their statistical significance, p values ≤0.05 were considered statistically significant. Multivariable analyses were performed with the Cox proportional hazards model.
Results
Clinicopathological features and survival in the main cohort
The detailed characteristics of the main cohort, including age, sex, TNM, UICC stage, resection status, MSI status, WHO grade, and tumor localization are shown in Table 2. As expected, the pTNM categories, resulting UICC stages, WHO grade, resection status, and MSI status profoundly impacted patient survival.
Table 2.
Distribution and survival associations of clinicopathological characteristics of the main cohort
| Overall n (%) | Mean OS (SE) [months] | p value | Mean DSS (SE) [months] | p value | Mean DFS (SE) [months] | p value | |
|---|---|---|---|---|---|---|---|
| Age | <0.001 | 0.02 | 0.945 | ||||
| Below median | 511 (48.5%) | 86.10 (2.2) | 90.88 (2.1) | 82.03 (2.3) | |||
| Median and above | 543 (51.5%) | 71.61 (2.2) | 83.63 (2.3) | 82.13 (2.4) | |||
| Sex | 0.26 | 0.94 | 0.69 | ||||
| Male | 608 (57.7%) | 77.52 (2.1) | 87.33 (2.1) | 82.83 (2.2) | |||
| Female | 446 (42.3%) | 80.64 (2.4) | 87.24 (2.4) | 80.96 (2.6) | |||
| pT | <0.001 | <0.001 | <0.001 | ||||
| 1 | 80 (7.6%) | 96.57 (4.9) | 114.12 (3.2) | 108.49 (3.8) | |||
| 2 | 188 (17.8%) | 92.35 (3.2) | 103.32 (2.8) | 99.37 (3.2) | |||
| 3 | 588 (55.8%) | 79.44 (2.1) | 88.03 (2.1) | 81.83 (2.2) | |||
| 4 | 198 (18.8%) | 56.92 (3.7) | 60.15 (3.8) | 54.83 (4.0) | |||
| pN | <0.001 | <0.001 | <0.001 | ||||
| 0 | 581 (55.1%) | 89.00 (1.9) | 101.20 (1.7) | 99.08 (1.8) | |||
| 1 | 296 (28.1%) | 75.01 (3.0) | 80.23 (3.0) | 72.49 (3.2) | |||
| 2 | 177 (16.8%) | 51.65 (3.9) | 54.73 (4.1) | 43.21 (4.0) | |||
| pM | <0.001 | <0.001 | <0.001 | ||||
| 0 | 897 (85.1%) | 86.21 (1.6) | 95.98 (1.5) | 90.77 (1.7) | |||
| 1 | 157 (14.9%) | 39.60 (3.4) | 42.15 (3.7) | 33.72 (3.6) | |||
| UICC stage | <0.001 | <0.001 | <0.001 | ||||
| 1 | 213 (20.2%) | 96.64 (2.9) | 111.11 (2.1) | 107.84 (2.4) | |||
| 2 | 351 (33.3%) | 86.01 (2.6) | 97.00 (2.4) | 95.76 (2.6) | |||
| 3 | 327 (31.0%) | 80.60 (2.8) | 86.44 (2.8) | 76.13 (3.0) | |||
| 4 | 163 (15.5%) | 38.80 (3.3) | 41.27 (3.6) | 32.69 (3.4) | |||
| WHO grade | <0.001 | <0.001 | <0.001 | ||||
| Low grade | 708 (67.2%) | 86.00 (1.8) | 95.24 (1.7) | 89.62 (1.9) | |||
| High grade | 346 (32.8%) | 64.74 (2.8) | 71.72 (2.9) | 66.83 (3.0) | |||
| Resection margin | <0.001 | <0.001 | <0.001 | ||||
| R0 | 974 (92.4%) | 82.53 (1.6) | 91.81 (1.5) | 86.78 (1.7) | |||
| R1 | 50 (4.7%) | 41.00 (7.2) | 42.53 (7.5) | 29.47 (6.1) | |||
| R2 | 30 (2.8%) | 24.99 (4.5) | 25.00 (4.5) | 21.52 (3.7) | |||
| Tumor localization | 0.14 | 0.34 | 0.16 | ||||
| Cecum | 150 (14.2%) | 78.24 (4.1) | 88.30 (4.1) | 87.20 (4.2) | |||
| Ascending colon | 276 (26.1%) | 77.62 (3.2) | 86.24 (3.2) | 78.61 (3.4) | |||
| Transverse colon | 83 (7.9%) | 70.26 (6.0) | 83.40 (5.9) | 83.82 (6.1) | |||
| Descending colon | 94 (8.9%) | 74.69 (5.1) | 81.15 (5.1) | 77.02 (5.5) | |||
| Sigmoid colon | 332 (31.4%) | 84.60 (2.7) | 91.67 (2.6) | 86.41 (2.9) | |||
| Rectum | 119 (11.3%) | 75.80 (4.6) | 83.88 (5.9) | 74.48 (5.1) | |||
| Tumor histology | <0.001 | <0.001 | <0.001 | ||||
| Conventional adenocarcinoma | 1,033 (98.0%) | 79.44 (1.6) | 88.05 (1.6) | 82.81 (1.7) | |||
| MANEC/NEC | 21 (2.0%) | 26.51 (6.2) | 26.51 (6.2) | 22.99 (6.1) | |||
| MSI status | 0.014 | <0.001 | <0.001 | ||||
| Microsatellite stable | 889 (84.3%) | 77.21 (1.7) | 85.04 (1.7) | 79.46 (1.8) | |||
| Microsatellite unstable | 165 (15.7%) | 88.36 (3.8) | 100.88 (3.4) | 97.29 (3.7) |
INSM1 expression in conventional CRCs and MANECs/NECs in the main cohort
INSM1 expression was found in 145/1,033 (14%) conventional CRCs and in all MANECs/NECs (21/21, 100%). Most conventional CRCs were INSM1 negative (888/1,033, 86%). Conventional CRCs with expression of INSM1 mostly showed low (133/145, 92%) and rarely moderate (12/145, 8%) positivity. In contrast, most MANECs/NECs fell into the INSM1 high (12/21, 57%) or the INSM1 moderate subgroup (7/21, 34%), with only 2/21 (9%) of these neoplasms in the INSM1 low subgroup (Table 3, p < 0.001). INSM1 expression in conventional CRC did not correlate with pTNM, UICC‐stage, resection status, MSI status, age, or gender. In 75% of the MANEC, the adenocarcinoma component was completely INSM1 negative; 25% showed weak INSM1 expression.
Table 3.
Distribution of INSM1 expression subgroups among synaptophysin expression groups of conventional CRCs and colorectal MANEC/NEC in the main cohort
| INSM1 negative (IRS 0–1) | INSM1 weak (IRS 2–3) | INSM1 moderate (IRS 4–8) | INSM1 strong (IRS 9–12) | Total number | p value | |
|---|---|---|---|---|---|---|
| Adenocarcinoma, synaptophysin negative | 733 | 54 | 3 | 0 | 790 | |
| Adenocarcinoma, synaptophysin low (1–9%) | 132 | 67 | 2 | 0 | 201 | |
| Adenocarcinoma, synaptophysin moderate (10–29%) | 7 | 4 | 4 | 0 | 15 | |
| Adenocarcinoma, synaptophysin high (30–100%) | 16 | 8 | 3 | 0 | 27 | |
| MANEC/NEC | 0 | 2 | 7 | 12 | 21 | |
| Total number | 888 | 135 | 19 | 12 | 1,054 | <0.001 |
Comparison of the INSM1 scores from the TMA and whole slides of 35 tumors including all MANECs/NECs showed almost perfect concordance (κ = 0.96, p < 0.001). Figures 1 and 2 illustrate and compare INSM1 expression in conventional CRCs (Figure 1) and in MANECs/NECs (Figure 2).
Figure 1.
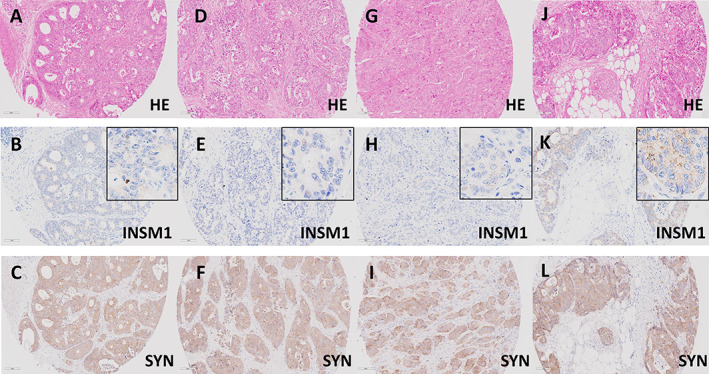
INSM1 expression groups in conventional CRCs with diffuse expression of synaptophysin. (A–C) Conventional CRC (A, H&E, ×20) with diffuse expression of synaptophysin (C, ×20). The adenocarcinoma shows only scattered INSM1 positive cells (<10%), some showing strong nuclear staining (B, ×20, inset ×40), resulting in an IRS of 3 (INSM1 low). (D–F) Conventional CRC (D, H&E, ×20) with diffuse expression of synaptophysin (F, ×20). The adenocarcinoma shows scattered INSM1 positive cells (<10%) with very weak, barely perceptible nuclear staining (E, ×20, inset ×40), resulting in an IRS of 1 (INSM1 negative). (G–L) Further examples of two conventional CRCs with diffuse expression of synaptophysin (G and J, H&E, ×20). Both adenocarcinomas show diffuse expression of synaptophysin in all tumor cells (I and L, ×20). Nuclear expression of INSM1 was not observed, resulting in an IRS 0 (INSM1 negative). Note the nonspecific cytoplasmic staining, that was not considered specific (H and K, ×20, inset ×40).
Figure 2.
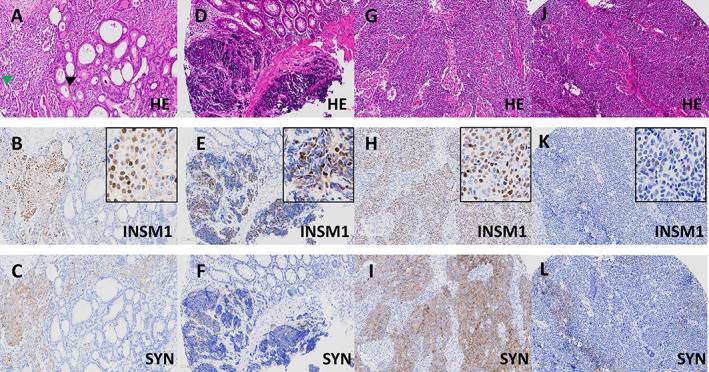
INSM1 expression groups in colorectal MANEC and NEC. (A–C) Colorectal MANEC (A, H&E, ×20) consisting of an adenocarcinoma NOS (black arrow) intermingled with a large cell NEC (green arrow), which shows diffuse expression of synaptophysin (C, ×20). The NEC shows a substantial number of positive cells (60%) with variable staining intensities with some showing strong nuclear staining (B, ×20, inset ×40), resulting in an IRS of 9 (INSM1 high). The adenocarcinoma component is INSM1 negative. (D–F) Colorectal small cell NEC (D, H&E, ×20) showing weak to moderate expression of synaptophysin (F, ×20). The NEC shows a substantial number of positive cells (55%) with variable staining intensities ranging from very weak to strong (E, ×20, inset ×40), resulting in an IRS of 9 (INSM1 high). (G–I) Colorectal large cell NEC (G, H&E, ×20) with diffuse expression of synaptophysin (I, ×20) and diffuse and strong (90%) expression of INSM1 (H, ×20, inset ×40), resulting in an IRS of 12 (INSM1 high). (J–L) Colorectal large cell NEC (J, H&E, ×20) with patchy expression of synaptophysin (I, ×20) and scattered (10%) expression of INSM1, with most positive nuclei showing weak but some showing moderate staining intensity (K, ×20, inset ×40), resulting in an IRS of 4 (INSM1 moderate).
Synaptophysin expression in conventional CRCs and MANECs/NECs in the main cohort
Synaptophysin expression was found in 243/1,033 conventional CRCs (24%). In 201/243 (83%) tumors, there were only scattered positive cells accounting for up to 9% of the tumor cell population, while in 15/243 (6%) tumors synaptophysin expression was moderate. Synaptophysin expression in 30–100% of the tumor cells was observed in 27/243 (11%) conventional CRCs with any expression of synaptophysin; this represents 3% of all conventional CRCs (27/1,033) in the main cohort. By definition, in MANECs and NECs of the main cohort, all neuroendocrine solid components suggestive of neuroendocrine differentiation expressed synaptophysin [7].
Chromogranin A expression in conventional CRCs and MANECs/NECs in the main cohort
Expression of chromogranin A was found in 116/1033 conventional CRCs (11%). Most of these conventional CRCs (106/116, 91%) had only few chromogranin A positive cells (IRS 2–3, chromogranin A low). Only 10/116 (9%) conventional CRCs showed moderate chromogranin A expression, equaling 1% of all conventional CRCs (10/1,033) in the main cohort. Colorectal MANECs/NECs expressed chromogranin A in 13/21 cases (62%); 8 MANECs/NECs (38%) were completely chromogranin A negative. Of the 13 chromogranin A positive MANECs/NECs, 5 showed high chromogranin A expression, 2 cases showed moderate, and 6 neoplasms showed weak expression.
Correlation of INSM1 expression in conventional CRCs and in MANECs/NECs with expression of synaptophysin in the main cohort
Table 3 shows that the majority of conventional CRCs with diffuse synaptophysin positivity were INSM1 negative (16/27, 59%). The remaining conventional CRCs with diffuse expression of synaptophysin mostly belonged to the subgroups with low (8/27, 29.6%) or rarely moderate INSM1 (3/27, 11%) expression.
In contrast, all MANECs/NECs were INSM1 positive and mostly showed high or at least moderate INSM1 expression (19/21, 91%), thus differing significantly (p < 0.001) from conventional CRCs with diffuse synaptophysin positivity. Very few synaptophysin negative conventional CRCs showed low (54/790, 7%) or moderate (3/790, <1%) INSM1 expression.
Correlation of INSM1 expression with chromogranin A expression in conventional CRCs and in MANECs/NECs in the main cohort
Conventional CRCs with chromogranin A expression were mostly INSM1 negative (72/116, 62%) or weakly positive (41/116, 35%). Among the 918 chromogranin A negative conventional CRCs, weak or moderate expression of INSM1 was noted in 92/917 (10%) and 9/917 (1%) cases, respectively. The remaining chromogranin A negative conventional CRCs were also INSM1 negative (p < 0.001, supplementary material, Table S1).
In MANECs/NECs, there was no significant correlation between chromogranin A and INSM1 expression (p = 0.70). Seven of the 12 MANECs/NECs with strong INSM1 expression (58%) were either chromogranin A negative or only weakly positive, while in 7/8 (88%) chromogranin A negative NECs INSM1 expression was either moderate or strong (supplementary material, Table S2).
Patient survival in INSM1‐expressing conventional CRCs compared with MANECs/NECs in the main cohort
We observed no survival differences between conventional CRCs regardless of their expression of INSM1 (OS: p = 0.849; DSS: p = 0.273; DFS: p = 0.884, Figure 3). In contrast, MANECs/NECs showed significantly poorer survival for OS, DSS, or DFS (p < 0.001, respectively) compared with all INSM1 expression subgroups of conventional CRC. For example, mean DSS for conventional CRCs without INSM1 expression was 87.1 months, compared with 95.0 and 89.0 months for conventional CRCs with low or moderate INSM1 expression, while MANEC/NEC showed a mean DSS of 26.5 months. Figure 3 depicts the respective Kaplan–Meier curves. The detailed mean survival times of the different groups for all survival comparisons are shown in supplementary material, Tables S3 and S4.
Figure 3.
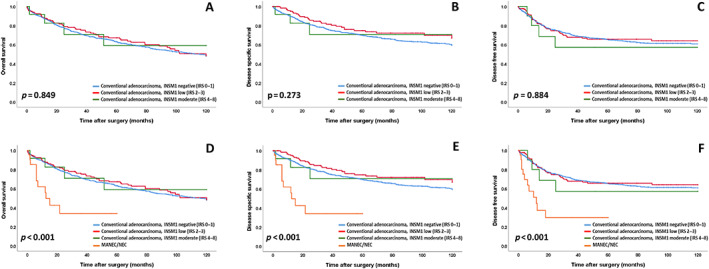
OS, DSS, and DFS for (A–C) INSM1 expression groups in conventional adenocarcinomas with a non‐neuroendocrine morphology and for (D–F) INSM1 expression groups in conventional adenocarcinomas compared with MANEC/NEC.
The stage‐independent impact on patient survival of true MANEC/NEC but not of conventional CRC with INSM1 expression was confirmed by multivariate analyses including UICC stage, resection status, MSI, gender, sex, and WHO grade (DSS: p = 0.005, hazard ratio: 2.435, Table 4; OS: p = 0.014; hazard ratio: 2.167, supplementary material, Table S5; DFS: p = 0.03, hazard ratio: 1.936, supplementary material, Table S6).
Table 4.
Multivariate analysis for DSS including INSM1 expression groups in conventional adenocarcinomas compared with MANECs including UICC stage, WHO grade, MSI, resection status, age, and gender in the main cohort
| Hazard ratio (DSS) | Lower CI (95%) | Upper CI (95%) | p value | |
|---|---|---|---|---|
| INSM1 expression groups in conventional adenocarcinoma versus MANEC/NEC | 0.005 | |||
| Conventional adenocarcinoma INSM1 negative (lRS 0–1) | 1 | |||
| Conventional adenocarcinoma INSM1 low (IRS 2–3) | 0.785 | 0.536 | 1.149 | |
| Conventional adenocarcinoma INSM1 moderate (IRS 4–8) | 0.588 | 0.187 | 1.849 | |
| MANEC/NEC | 2.435 | 1.303 | 4.549 | |
| UICC stage | <0.001 | |||
| I | 1 | |||
| II | 2.504 | 1.521 | 4.122 | |
| III | 3.457 | 2.128 | 5.616 | |
| IV | 9.430 | 5.671 | 15.681 | |
| Resection status | <0.001 | |||
| R0 | 1 | |||
| R1 | 2.243 | 1.517 | 3.316 | |
| R2 | 2.072 | 1.330 | 3.231 | |
| MSI | 0.014 | |||
| Microsatellite stable | 1 | |||
| Microsatellite unstable | 0.592 | 0.391 | 0.899 | |
| WHO grade | 0.001 | |||
| Low grade | 1 | |||
| High grade | 1.486 | 1.167 | 1.891 | |
| Gender | 0.173 | |||
| Male | 1 | |||
| Female | 0.850 | 0.673 | 1.074 | |
| Age | <0.001 | |||
| Below median | 1 | |||
| Median and above | 1.585 | 1.263 | 1.990 |
As described previously [7], synaptophysin expression in conventional CRCs did not impact OS (p = 0.67) or DSS (p = 0.80), but showed a prognostic impact on DFS (p = 0.011) in univariate analysis, which was not confirmed in multivariate analysis (p = 0.38). Expression of chromogranin A in conventional CRCs had no impact on survival (p = n.s., data not shown).
Expression of INSM1/synaptophysin/chromogranin A in the conventional CRC validation cohort
Of the 274 cases in the conventional CRC validation cohort, 20 (7%) showed weak expression of INSM1 and 27 (10%) showed a weak expression of chromogranin A. Synaptophysin was expressed in 38 conventional CRC (14%), of which 5 (2%) showed diffuse expression of synaptophysin exceeding 30%. Four of these diffuse synaptophysin expressing conventional CRC were INSM1 negative (4/5, 80%), while one tumor showed low INSM1 expression. The clinicopathological characteristics and the expression of INSM1/synaptophysin/chromogranin A in the conventional CRC validation cohort are summarized in supplementary material, Table S7.
Expression of INSM1/synaptophysin/chromogranin A in the MANEC/NEC validation cohort
In the MANEC/NEC validation cohort, all cases expressed synaptophysin. INSM1 was expressed in all tumors, with 9/19 tumors showing strong (47%) and 6/19 tumors showing moderate INSM1 positivity (32%), 4/19 tumors showed weak expression (21%). Chromogranin A was negative in 12/19 tumors (63%), one tumor showed weak expression (5%), while moderate positivity was observed in 5/19 cases (26%).
In the four MANECs, INSM1 expression was not detected in the adenocarcinoma components. The clinicopathological characteristics and the expression of INSM1/ synaptophysin/chromogranin A in the MANEC/NEC validation cohort are summarized in supplementary material, Table S8.
Discussion
INSM1 is a highly conserved transcription factor that regulates the development of neuroendocrine cells throughout the body [9, 25, 26, 27, 28]. INSM1 has emerged as a sensitive immunohistochemical marker for the diagnosis of neuroendocrine neoplasms, with several studies showing higher specificity and sensitivity compared with the traditional neuroendocrine markers synaptophysin and chromogranin A [10, 12, 13].
Our study investigated the nuclear immunoreactivity of INSM1 according to the IRS [20] in more than 1,050 colorectal carcinomas and demonstrates that, besides morphology, high expression of INSM1 distinguishes true colorectal MANEC/NEC from conventional CRC with diffuse expression of synaptophysin (>30% positive tumor cells) [10]. MANEC/NEC usually showed high or at least moderate expression of INSM1 (91%, 9% with low INSM1 expression), while almost 90% of conventional CRC with diffuse synaptophysin expression were either INSM1 negative or INSM1 low. These general observations from our main cohort were then validated in two independent collectives of conventional CRC and MANEC/NEC.
In line with our previous observations for synaptophysin [7], the survival of conventional CRC patients was not influenced by the expression of INSM1, regardless of its intensity, while patients suffering from true MANEC/NEC showed a stage independent, highly decreased survival on all comparisons. These findings strengthen the assumption that the expression of any neuroendocrine marker in colorectal carcinomas has to be correlated with the underlying morphology and is not automatically equal to a NEC. Interestingly, a recent study from Fassan et al [29] revealed synaptophysin‐expressing conventional CRC to be a prognostic subgroup in BRAF‐mutated CRC, indicating that the molecular context may also be of importance when the clinical relevance of endocrine markers in conventional CRC is considered.
The spectrum of neuroendocrine differentiation in colorectal and extra‐colorectal carcinomas with non‐neuroendocrine morphology varies from scattered cells to neoplasms with diffuse expression of (traditional) neuroendocrine markers, especially synaptophysin. Although most previous studies including our own [7, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40] considered this phenomenon to be of minor prognostic relevance, these cases frequently cause diagnostic and clinical confusion over whether to classify those tumors as MANECs or NECs and to treat them accordingly [1, 2, 41]. Although the WHO understandably requires NEC morphology for the diagnosis of MANEC or NEC, some conventional CRCs with diffuse expression of synaptophysin that exceeds the 30% threshold may be misinterpreted as MANEC or NEC [42, 43]; this should be avoided at all costs, because of the vast prognostic differences and the diverging treatment modalities between conventional CRC and NECs. The data from our study may be helpful in diagnostically challenging cases. For example, in carcinomas that are morphologically ambiguous regarding possible neuroendocrine differentiation (e.g. medullary CRC, poorly differentiated adenocarcinoma NOS) and show weak but diffuse immunoreactivity for synaptophysin, negative staining for INSM1 can increase confidence for the diagnosis of a conventional CRC. Conversely, in carcinomas that are morphologically consistent with a poorly differentiated NEC, which is the most important requirement for the diagnosis, but only show weak or focal expression of traditional neuroendocrine markers, high expression of INSM1 can greatly increase the certainty of the diagnosis of a true MANEC/NEC. The results of our study are concordant with data on gynecological carcinomas by Zou et al [44], who also found no or only weak INSM1 expression in conventional ovarian or uterine carcinomas (e.g. high‐grade serous carcinoma or poorly differentiated endometrioid carcinoma) with diffuse expression of neuroendocrine markers, but non‐neuroendocrine morphology. In addition to INSM1, our study also evaluated the expression of chromogranin A immunoreactivity in conventional CRCs and MANECs/NECs. High or moderate chromogranin A expression was quite specific for MANEC/NEC, but not very sensitive, as the majority of MANEC/NEC showed weak expression of chromogranin A or were entirely negative.
Our study has some limitations. First, it is of retrospective nature. However, to minimize this disadvantage, we performed our analyses on a very large cohort of more than 1,054 colorectal carcinomas including 21 MANECs/NECs, which is close to the incidence of these tumors described in the literature [3, 6], and added two substantial validation cohorts comprising conventional CRC as well as MANEC/NEC. Another shortcoming of our work is that our analyses were performed on TMAs because of the large size of our cohort. However, comparing our TMA data with full block slides revealed an almost perfect concordance, thus supporting the robustness of our approach.
In conclusion, our study shows that high INSM1 expression in conventional colorectal carcinoma has no prognostic significance, but is helpful for distinguishing colorectal MANEC/NEC from conventional colorectal carcinoma with diffuse synaptophysin expression. Furthermore, our data suggest that INSM1 has a higher specificity for colorectal MANEC/NEC than synaptophysin and a higher sensitivity than chromogranin A, further strengthening the role of INSM1 as a complementary marker for the diagnosis of poorly differentiated neuroendocrine neoplasms. Further studies are needed to investigate the molecular basis of diffuse synaptophysin expression in conventional colorectal carcinomas and to clarify whether this phenomenon is due to the recognition of synaptophysin‐like proteins or whether it is another, more benign form of neuroendocrine differentiation that is not associated with a worse clinical outcome.
Author contributions statement
MJ and BK designed this study. MJ and ASL wrote the manuscript with assistance from GK, AK, CD and WW. SF, MS, FK, TG, BL, DKB, AR and PJ collected clinical or pathological data. ASL, MJ, BK, AK and BK performed histopathological or immunohistochemical analyses.
Supporting information
Table S1. Correlation between INSM1 expression and expression of chromogranin A in conventional adenocarcinomas
Table S2. Correlation between INSM1 expression and expression of chromogranin A in MANEC/NEC
Table S3. Survival associations of INSM1 expression groups among conventional adenocarcinomas
Table S4. Survival associations of INSM1 expression groups among conventional adenocarcinomas compared with colorectal MANEC/NEC
Table S5. Multivariate analysis for OS including INSM1 expression groups in conventional adenocarcinomas compared with MANECs
Table S6. Multivariate analysis for DFS including INSM1 expression groups in conventional adenocarcinomas compared with MANECs
Table S7. Cohort characteristics and expression of synaptophysin, chromogranin A and INSM1 in the conventional CRC validation cohort
Table S8. Cohort characteristics and expression of synaptophysin, chromogranin A and INSM1 in the MANEC/NEC validation cohort
Acknowledgements
We would like to thank Prof. Dr. Martin Anlauf and Dr. Rebekka Eschmann from the Institute of Pathology ÜGP MVZ Mittelhessen for kindly providing the MANEC/NEC validation cohort. We are grateful to Marion Kalden, Manuela Gerber, and Viktoria Wischmann for excellent technical assistance. Open Access funding enabled and organized by Projekt DEAL.
No conflicts of interest were declared.
Data availability statement
Data from this study are available from the corresponding author upon reasonable request.
References
- 1. Yamaguchi T, Machida N, Morizane C, et al. Multicenter retrospective analysis of systemic chemotherapy for advanced neuroendocrine carcinoma of the digestive system. Cancer Sci 2014; 105: 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tanaka T, Kaneko M, Nozawa H, et al. Diagnosis, assessment, and therapeutic strategy for colorectal mixed adenoneuroendocrine carcinoma. Neuroendocrinology 2017; 105: 426–434. [DOI] [PubMed] [Google Scholar]
- 3. WHO Classification of Tumours Editorial Board. Digestive system tumours. In WHO Classification of Tumours Series, Volume 1 (5th edn). International Agency for Research on Cancer: Lyon, France, 2019; 157–162. [Accessed 10 August 2023]. Available from: http://publications.iarc.fr/579 [Google Scholar]
- 4. Jesinghaus M, Konukiewitz B, Keller G, et al. Colorectal mixed adenoneuroendocrine carcinomas and neuroendocrine carcinomas are genetically closely related to colorectal adenocarcinomas. Mod Pathol 2017; 30: 610–619. [DOI] [PubMed] [Google Scholar]
- 5. Jesinghaus M, Schmitt M, Lang C, et al. Morphology matters: a critical reappraisal of the clinical relevance of morphologic criteria from the 2019 WHO classification in a large colorectal cancer cohort comprising 1004 cases. Am J Surg Pathol 2021; 45: 969–978. [DOI] [PubMed] [Google Scholar]
- 6. Ilett EE, Langer SW, Olsen IH, et al. Neuroendocrine carcinomas of the gastroenteropancreatic system: a comprehensive review. Diagnostics (Basel) 2015; 5: 119–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Konukiewitz B, Kasajima A, Schmitt M, et al. Neuroendocrine differentiation in conventional colorectal adenocarcinomas: incidental finding or prognostic biomarker? Cancers (Basel) 2021; 13: 5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagtegaal ID, Odze RD, Klimstra D, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76: 182–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mellitzer G, Bonné S, Luco RF, et al. IA1 is NGN3‐dependent and essential for differentiation of the endocrine pancreas. EMBO J 2006; 25: 1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bellizzi AM. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol 2020; 96: 8–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuji S, Watanabe R, Sato Y, et al. A new marker, insulinoma‐associated protein 1 (INSM1), for high‐grade neuroendocrine carcinoma of the uterine cervix: analysis of 37 cases. Gynecol Oncol 2017; 144: 384–390. [DOI] [PubMed] [Google Scholar]
- 12. Rooper LM, Sharma R, Li QK, et al. INSM1 demonstrates superior performance to the individual and combined use of synaptophysin, chromogranin and CD56 for diagnosing neuroendocrine tumors of the thoracic cavity. Am J Surg Pathol 2017; 41: 1561–1569. [DOI] [PubMed] [Google Scholar]
- 13. Rosenbaum JN, Guo Z, Baus RM, et al. INSM1: a novel immunohistochemical and molecular marker for neuroendocrine and neuroepithelial neoplasms. Am J Clin Pathol 2015; 144: 579–591. [DOI] [PubMed] [Google Scholar]
- 14. González I, Lu HC, Sninsky J, et al. Insulinoma‐associated protein 1 expression in primary and metastatic neuroendocrine neoplasms of the gastrointestinal and pancreaticobiliary tracts. Histopathology 2019; 75: 568–577. [DOI] [PubMed] [Google Scholar]
- 15. Tanigawa M, Nakayama M, Taira T, et al. Insulinoma‐associated protein 1 (INSM1) is a useful marker for pancreatic neuroendocrine tumor. Med Mol Morphol 2018; 51: 32–40. [DOI] [PubMed] [Google Scholar]
- 16. Mukhopadhyay S, Dermawan JK, Lanigan CP, et al. Insulinoma‐associated protein 1 (INSM1) is a sensitive and highly specific marker of neuroendocrine differentiation in primary lung neoplasms: an immunohistochemical study of 345 cases, including 292 whole‐tissue sections. Mod Pathol 2019; 32: 100–109. [DOI] [PubMed] [Google Scholar]
- 17. McHugh KE, Mukhopadhyay S, Doxtader EE, et al. INSM1 is a highly specific marker of neuroendocrine differentiation in primary neoplasms of the gastrointestinal tract, appendix, and pancreas. Am J Clin Pathol 2020; 153: 811–820. [DOI] [PubMed] [Google Scholar]
- 18. Couvelard A, Cros J. An update on the development of concepts, diagnostic criteria, and challenging issues for neuroendocrine neoplasms across different digestive organs. Virchows Arch 2022; 480: 1129–1148. [DOI] [PubMed] [Google Scholar]
- 19. Zhang Q, Huang J, He Y, et al. Insulinoma‐associated protein 1 (INSM1) is a superior marker for the diagnosis of gastroenteropancreatic neuroendoerine neoplasms: a meta‐analysis. Endocrine 2021; 74: 61–71. [DOI] [PubMed] [Google Scholar]
- 20. Remmele W, Stegner HE. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER‐ICA) in breast cancer tissue. Pathologe 1987; 8: 138–140. [PubMed] [Google Scholar]
- 21. Konukiewitz B, Schmitt M, Silva M, et al. Loss of CDX2 in colorectal cancer is associated with histopathologic subtypes and microsatellite instability but is prognostically inferior to hematoxylin–eosin‐based morphologic parameters from the WHO classification. Br J Cancer 2021; 125: 1632–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitt M, Silva M, Konukiewitz B, et al. Loss of SATB2 occurs more frequently than CDX2 loss in colorectal carcinoma and identifies particularly aggressive cancers in high‐risk subgroups. Cancers (Basel) 2021; 13: 6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kaemmerer D, Peter L, Lupp A, et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol 2012; 5: 187–194. [PMC free article] [PubMed] [Google Scholar]
- 24. Regierer AC, Wolters R, Kurzeder C, et al. High estrogen receptor expression in early breast cancer: chemotherapy needed to improve RFS? Breast Cancer Res Treat 2011; 128: 273–281. [DOI] [PubMed] [Google Scholar]
- 25. Wang HW, Muguira M, Liu WD, et al. Identification of an INSM1‐binding site in the insulin promoter: negative regulation of the insulin gene transcription. J Endocrinol 2008; 198: 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang T, Chen C, Breslin MB, et al. Extra‐nuclear activity of INSM1 transcription factor enhances insulin receptor signaling pathway and Nkx6.1 expression through RACK1 interaction. Cell Signal 2014; 26: 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang T, Wang H, Saunee NA, et al. Insulinoma‐associated antigen‐1 zinc‐finger transcription factor promotes pancreatic duct cell trans‐differentiation. Endocrinology 2010; 151: 2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gierl MS, Karoulias N, Wende H, et al. The zinc‐finger factor Insm1 (IA‐1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev 2006; 20: 2465–2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fassan M, Milione M, Maddalena G, et al. Synaptophysin expression in (V600EBRAF‐)mutated advanced colorectal cancers identifies a new subgroup of tumours with worse prognosis. Eur J Cancer 2021; 146: 145–154. [DOI] [PubMed] [Google Scholar]
- 30. Chavez‐Blanco A, Taja‐Chayeb L, Cetina L, et al. Neuroendocrine marker expression in cervical carcinomas of non‐small cell type. Int J Gynecol Pathol 2002; 21: 368–374. [DOI] [PubMed] [Google Scholar]
- 31. Chen Y, Liu F, Meng Q, et al. Is neuroendocrine differentiation a prognostic factor in poorly differentiated colorectal cancer? World J Surg Oncol 2017; 15: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shinji S, Naito Z, Ishiwata T, et al. Neuroendocrine cell differentiation of poorly differentiated colorectal adenocarcinoma correlates with liver metastasis. Int J Oncol 2006; 29: 357–364. [PubMed] [Google Scholar]
- 33. Lloyd RV, Schroeder G, Bauman MD, et al. Prevalence and prognostic significance of neuroendocrine differentiation in colorectal carcinomas. Endocr Pathol 1998; 9: 35–42. [DOI] [PubMed] [Google Scholar]
- 34. Foley EF, Gaffey MJ, Frierson HF Jr. The frequency and clinical significance of neuroendocrine cells within stage III adenocarcinomas of the colon. Arch Pathol Lab Med 1998; 122: 912–914. [PubMed] [Google Scholar]
- 35. Yao GY, Zhou JL, Lai MD, et al. Neuroendocrine markers in adenocarcinomas: an investigation of 356 cases. World J Gastroenterol 2003; 9: 858–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suresh PK, Sahu KK, Pai RR, et al. The prognostic significance of neuroendocrine differentiation in colorectal carcinomas: our experience. J Clin Diagn Res 2015; 9: EC01–EC04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ogimi T, Sadahiro S, Kamei Y, et al. Distribution of neuroendocrine marker‐positive cells in colorectal cancer tissue and normal mucosal tissue: consideration of histogenesis of neuroendocrine cancer. Oncology 2019; 97: 294–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sterlacci W, Fiegl M, Hilbe W, et al. Clinical relevance of neuroendocrine differentiation in non‐small cell lung cancer assessed by immunohistochemistry: a retrospective study on 405 surgically resected cases. Virchows Arch 2009; 455: 125–132. [DOI] [PubMed] [Google Scholar]
- 39. Howe MC, Chapman A, Kerr K, et al. Neuroendocrine differentiation in non‐small cell lung cancer and its relation to prognosis and therapy. Histopathology 2005; 46: 195–201. [DOI] [PubMed] [Google Scholar]
- 40. Ionescu DN, Treaba D, Gilks CB, et al. Nonsmall cell lung carcinoma with neuroendocrine differentiation – an entity of no clinical or prognostic significance. Am J Surg Pathol 2007; 31: 26–32. [DOI] [PubMed] [Google Scholar]
- 41. Mitry E, Baudin E, Ducreux M, et al. Treatment of poorly differentiated neuroendocrine tumours with etoposide and cisplatin. Br J Cancer 1999; 81: 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith JD, Reidy DL, Goodman KA, et al. A retrospective review of 126 high‐grade neuroendocrine carcinomas of the colon and rectum. Ann Surg Oncol 2014; 21: 2956–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ramage JK, De Herder WW, Delle Fave G, et al. ENETS consensus guidelines update for colorectal neuroendocrine neoplasms. Neuroendocrinology 2016; 103: 139–143. [DOI] [PubMed] [Google Scholar]
- 44. Zou Q, Zhang L, Cheng Z, et al. INSM1 is less sensitive but more specific than synaptophysin in gynecologic high‐grade neuroendocrine carcinomas: an immunohistochemical study of 75 cases with specificity test and literature review. Am J Surg Pathol 2021; 45: 147–159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlation between INSM1 expression and expression of chromogranin A in conventional adenocarcinomas
Table S2. Correlation between INSM1 expression and expression of chromogranin A in MANEC/NEC
Table S3. Survival associations of INSM1 expression groups among conventional adenocarcinomas
Table S4. Survival associations of INSM1 expression groups among conventional adenocarcinomas compared with colorectal MANEC/NEC
Table S5. Multivariate analysis for OS including INSM1 expression groups in conventional adenocarcinomas compared with MANECs
Table S6. Multivariate analysis for DFS including INSM1 expression groups in conventional adenocarcinomas compared with MANECs
Table S7. Cohort characteristics and expression of synaptophysin, chromogranin A and INSM1 in the conventional CRC validation cohort
Table S8. Cohort characteristics and expression of synaptophysin, chromogranin A and INSM1 in the MANEC/NEC validation cohort
Data Availability Statement
Data from this study are available from the corresponding author upon reasonable request.


