Abstract
Background
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) on the surface of Streptococcus dysgalactiae, coded with gapC, is a glycolytic enzyme that was reported to be a moonlighting protein and virulence factor.
Objective
This study assessed GAPDH as a potential immunization candidate protein to prevent streptococcus infections.
Methods
Mice were vaccinated subcutaneously with recombinant GAPDH and challenged with S. dysgalactiae in vivo. They were then evaluated using histological methods. rGAPDH of mouse bone marrow-derived dendritic cells (BMDCs) was evaluated using immunoblotting, reverse transcription quantitative polymerase chain reaction, and enzyme-linked immunosorbent assay methods.
Results
Vaccination with rGAPDH improved the survival rates and decreased the bacterial burdens in the mammary glands compared to the control group. The mechanism by which rGAPDH vaccination protects against S. dysgalactiae was investigated. In vitro experiments showed that rGAPDH boosted the generation of interleukin-10 and tumor necrosis factor-α. Treatment of BMDCs with TAK-242, a toll-like receptor 4 inhibitor, or C29, a toll-like receptor 2 inhibitor, reduced cytokines substantially, suggesting that rGAPDH may be a potential ligand for both TLR2 and TLR4. Subsequent investigations showed that rGAPDH may activate the phosphorylation of MAPKs and nuclear factor-κB.
Conclusions
GAPDH is a promising immunization candidate protein for targeting virulence and enhancing immune-mediated protection. Further investigations are warranted to understand the mechanisms underlying the activation of BMDCs by rGAPDH in a TLR2- and TLR4-dependent manner and the regulation of inflammatory cytokines contributing to mastitis pathogenesis.
Keywords: Streptococcus dysgalactiae, GAPDH, BMDCs, IL-10, intramammary infection
INTRODUCTION
Streptococcus dysgalactiae is a common environmental pathogen causing intramammary infections in dairy bovines [1] that lead to financial losses in the dairy industry [2]. The bacteria can survive within the mammary gland for extended periods [3]. Vaccines and medications have been developed to prevent and cure invasive mastitis [4]. S. dysgalactiae produces surface proteins that initiate an immune response in the host. These proteins have been evaluated as vaccine candidates with limited protection against infection [1,2,3].
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is an enzyme found in all major bacterial groups. The enzyme is involved in energy production and metabolism and is considered a housekeeping molecule. GAPDH is also a “moonlighting protein” because of its ability to catalyze the transformation of glyceraldehyde 3-phosphate and its involvement in adhesion, invasion, immunomodulation, and immunological evasion [5,6]. Nevertheless, these multiple functions cannot be attributed to gene fusion, splice variants, or distinct protein domains [7]. GAPDH is also functionally conserved in mammals, with homologs identified in mice and rabbits that share up to approximately 50% amino acid identity with S. agalactiae [6]. The S. dysgalactiae GAPDH protein can provide cross-protection for S. agalactiae and S. uberis [3]. GAPDH also plays a significant role in the pathogenesis of various bacteria and acts as an immunomodulatory antigen associated with virulence, eliciting innate and adaptive immune responses [8]. The GAPDH activity can stimulate the proliferation and differentiation of T cells and B cells, increase the expression of interleukin (IL)-10 and CD69 cytokines, and play an important role in the survival of bacteria [9]. GAPDH binds to host actin and myosin, particularly myofibers, facilitating the adherence of S. pyogenes to damaged or injured tissue and enhancing colonization and invasiveness. This is particularly relevant in cases where the judgment of basic myofibers is compromised within the tissue [10]. From another perspective, probiotic GAPDH also adheres to the host intestinal mucosa by binding to fibronectin or plasminogen. Some studies have suggested that probiotic-secreted GAPDH is an immunomodulator, and probiotics can interact with the host plasminogen through GAPDH to induce immunoregulatory effects [11,12]. For example, GAPDH exerts a beneficial effect on the adhesion of Lactobacillus reuteri to intestinal cells [11]. Subsequent studies into the underlying mechanism showed that GAPDH from Lactobacillus gasseri induces a phenotypic shift in macrophages from M2 to M1, induces PPARγ activation, and inhibits Th2 activation. This results in the attenuation of airway inflammation in a murine model [12].
S. agalactiae GAPDH enhanced spleen mononuclear cell proliferation in vitro, resulting in B-cell differentiation into immunoglobulin (Ig)-secreting cells, and boosted IL-10 generation in mice, allowing for easier colonization [6]. S. suis GAPDH has heightened immunogenicity in swine. A trivalent protein against S. suis SS2, SS7, and SS9 conferred immune protection in zebrafish and mice against SS2 infections and offered adequate security against SS2 strain diseases in piglets [13,14]. GAPDH or S. agalactiae culture supernatant induces apoptosis in mouse bone marrow-derived macrophages [15]. In H9C2 cells with cardiac injury, the knockdown GAPDH decreases intracellular ROS and promotes autophagy, reducing apoptosis and necrosis [16]. Streptococcosis is an area of concern in flounder aquaculture. Subunit vaccines containing B-cell epitope peptides from S. iniae virulence factors could curb infections. GAPDH-based peptide vaccines potentially elicit high-titer antibodies and protective immunity in flounder fish against S. iniae [17]. A multi-epitope vaccine composed of epitopes from S. dysgalactiae GAPDH induced stronger cellular and humoral immune responses and offered better protection against S. dysgalactiae infection [18].
GAPDH induces maturation of bone marrow-derived dendritic cells (BMDCs) by TLR2 and TLR4, activating MAPKs, nuclear factor (NF)-κB, and PI3K-Akt, and regulating microRNAs expression [19]. GAPDH linked to S. pneumoniae DNA was released by autolysis and produced considerably greater IL-6 and tumor necrosis factor (TNF) levels in peritoneal macrophages and THP-1-derived macrophage-like cells by TLR4, possibly causing host tissue damage and influencing the development of pneumococcal diseases [20]. GAPDH controls TNF receptor-associated factor 2 (TRAF2) polyubiquitination through various interactions. The NleB-interacting protein alters GAPDH by acting as a translocated O-GlcNAc transferase [21].
The contribution of BMDCs to TLR2 and TLR4 signaling for the TNF-α and IL-10 levels in reaction to S. dysgalactiae rGAPDH activation has not been reported. To the best of the authors’ knowledge, only one study examined how GAPDH affects IL-10, a fundamental anti-inflammatory cytokine that negatively regulates immune responses to microbial antigens in BMDCs [22]. The present study focused on the protective effect of rGAPDH in a mouse model and the underlying mechanisms of activation and modulation of innate immunity.
MATERIALS AND METHODS
Bacterial strain
E. coli DH5α (Invitrogen, USA) served as plasmid cloning. E. coli Rosetta (DE3) (Novagen) was used for recombinant protein production. HLJ2019 was obtained from a clinical mastitis test in Heilongjiang Province, China. Sequence analysis revealed HLJ2019 to have 98.5% to 100.0% nucleotide identity to S. dysgalactiae strain ATCC 43078 (accession number: NR_115147.1) registered in the NCBI database.
Statement of ethics
Mice were housed in germ-free isolators and provided food and water ad libitum in a controlled environment with a 12 h light-dark cycle. The study involving animals was conducted in strict accordance with the recommendations of the Ethical Committee for Animal Experiments at Northeast Agricultural University, Harbin, China (NEAUEC20230319). All animal experiments were designed to minimize suffering.
Preparation of recombinant protein GAPDH
Recombinant GAPDH was produced using the HLJ2019 strain. The full-length gene was obtained by PCR amplification with primers 5-CCCCATGGTAGTTAAAGTTGG-3 and 5-CCCCTCGAGTTATTTAGCGATTTTTGCAAA-3. The PCR products were digested with NcoI and XhoI, and cloned into the pET32a vector (Novagen) with the same enzymes. The plasmid pET32a-GAPDH with an N-terminal His6 tag was expressed in E. coli Rosetta (DE3). The rGAPDH protein was expressed with isopropyl-d-thiogalactoside after induction and purified using a nickel nitrilotriacetic acid column (GE Healthcare) according to the manufacturer’s instructions. LPS was eliminated from rGAPDH using an EtEraser SE Kit (Xiamen Bioendo Technology) according to the manufacturer’s instructions. rGAPDH was purified by SDS-PAGE and western blot with anti-His monoclonal antibody. The aliquots were stored at −80°C.
Generation of murine BMDCs
The BMDCs were separated from the femurs and tibias of wild-type (WT) C57BL/6 mice (Yisi Laboratory Animal Technology, China). These bones were soaked in a dish with 70% alcohol for one minute and washed with RPMI 1640 (Gibco, Thermo Fisher Scientific) twice. The tissue was suspended and passed through a nylon mesh to remove the tissue pieces. The cells were lysed using a red blood cell lysing buffer and resuspended with RPMI 1640 containing 10% FBS, 100 U/mL penicillin/streptomycin, 20 ng/mL GM-CSF (Novoprotein, China), and 10 ng/mL IL-4 (Novus, Bio-Techne). Fresh RPMI 1640 media was added on days three and five of culture. The nonadherent and adherent cells were harvested for the following experiments on day seven.
Estimations of the cytokine concentrations and counteracting agent titers using enzyme-linked immunosorbent assay (ELISA)
The concentrations of IL-10 and TNF-α in culture supernatants were measured using an ELISA kit from Novus Biologicals according to the manufacturer’s instructions. ELISA also detected the anti-rGAPDH-specific antibody titers in the serum. The BMDCs were pretreated with TLR inhibitors (TLR2 inhibitor C29 and TLR4 inhibitor TAK-242) and signaling pathway inhibitors (p38 MAPK inhibitor SB203580, IκBα phosphorylation inhibitor BAY11-7082, and BMS-345541) for 2 h. The appropriate amounts of rGAPDH, LPS (TLR4 agonist), or PGN (TLR2 agonist) were then added for 24 h with inhibitors added every 8 h during the incubation period. The above inhibitors were purchased from MedChemExpress (MCE, USA).
RNA extraction and reverse transcription polymerase chain reaction
The total RNA was extracted from the BMDCs using TRIzol reagent (Invitrogen) according to the manufacturer’s protocol. cDNA was produced using random primers on an API7500 real-time PCR machine with SYBR Green. BGI (China) provided the primers; Table 1 lists the reported sequences.
Table 1. Primers used in this study.
| Primer | Sequence (5′-3′) | Amplicon size (bp) |
|---|---|---|
| TNF-α | F-GTCGTAGCAAACCACCAAGT | 149 |
| R-TTGAAGAGAACCTGGGAGTAGA | ||
| IL-10 | F-ATACTGCTAACCGACTCCTTAAT | 140 |
| R-TCAAATGCTCCTTGATTTCTGG | ||
| β-actin | F-AACTCCATCATGAAGTGTGA | 248 |
| R-ACTCCTGCTTGCTGATCCAC |
TNF, tumor necrosis factor; IL, interleukin.
Cytotoxicity analyses
BMDCs were treated with 10 or 50 μg/mL rGAPDH or 1 μg LPS for 24 h and stained with propidium iodide and FITC-Annexin V (eBioscience, USA). Flow cytometry and immunofluorescence microscopy were used to examine the BMDC death.
Western blot analyses
The BMDCs were plated on 12-well plates and treated with 50 μg/mL of rGAPDH for the indicated times (0, 10, 20, 30, 40, and 60 min). The harvested cells were lysed in a lysis buffer (RIPA containing PMSF and phosphatase inhibitor mixed with 5 × SDS loading buffer for 20 min on ice. The cell lysates at the same concentrations were separated by 12% SDS-PAGE and transferred to a PVDF membrane. After being blocked with TBST containing 5% BSA for 2 h, the membranes were incubated with primary antibodies (anti-mouse p38 MAPK and phosphor-p38MAPK [Cell Signaling Technology, USA], anti-mouse NF-κB p65 and phosphor-NF-κB p65, and β-actin antibody [Abmart, China]) overnight at 4°C. The membranes were then incubated with HRP-conjugated secondary antibody (ZSGB-bio, China) for 1 h. The ECL western blot system was used to detect the target bands.
Mice subcutaneous immunization
The protective effects of rGAPDH were examined by immunizing groups of 4–6 week-old female C57BL/6 mice (n = 6 per group) subcutaneously with 50 μg rGAPDH emulsified with an equal volume of complete Freund’s adjuvant (Sigma–Aldrich and Merck KGaA, Germany). The mice were given PBS and pET32a emulsified in the same adjuvant as a mock and negative control, respectively. The mice in all groups were immunized four times at two-week intervals via a subcutaneous injection. Booster injections of the same antigen emulsified with incomplete Freund’s adjuvant were administered after the primary immunization. The samples were harvested from the tail vein of each animal one week after the last vaccine, and mammary gland samples were collected 10 days after a challenge with HLJ2019.
Mammary gland challenge
A mouse model of intramammary challenge with bovine mastitis pathogens has been used to measure bacterial infection and tissue damage [23]. In the focal infection model, pups were removed 2 h before intramammary inoculation, and six mice from each group were anesthetized three days after parturition. The mammary gland ducts of the fourth pair of mammary glands were exposed by cutting the teat tip, and the sample was injected slowly intraductally at a volume of 50 μL with a 33 gauge blunted needle. The mice were challenged with HLJ2019 (1 × 108 CFU/mouse/gland) in the intramammary region after subcutaneous immunization. The mammary glands were collected 10 days after the challenge, serially diluted in sterile PBS, plated on THA (Hopebio, China), and incubated overnight at 37°C. The bacterial burdens were counted after 24 h. In the lethal-infection models, six mice from each group were challenged with HLJ2019 (1 × 1012 CFU/mouse) intraperitoneally after immunization. The survival rates and body weights were tracked for 21 days. All surviving mice were sacrificed at the end of the monitoring period.
Histopathologic examination of the mammary gland
Anesthetized C57BL/6 mice were used, and the mammary tissues from the separate groups were extracted and preserved in 4% PFA for 24 h. Paraffin-embedded slices were examined by optical microscopy after hematoxyling and eosin staining.
Statistical analyses
The data are reported as means ± SD and analyzed using GraphPad Prism8 (GraphPad Software, USA). One-way or two-way ANOVA was used to compare the cytokine levels, mRNA relative expression, bacterial burdens, and antibody titers. A Log-rank test was used to analyze the survival rates. P values < 0.05 or < 0.01 were considered significant.
RESULTS
Purification and identification of recombinant GAPDH
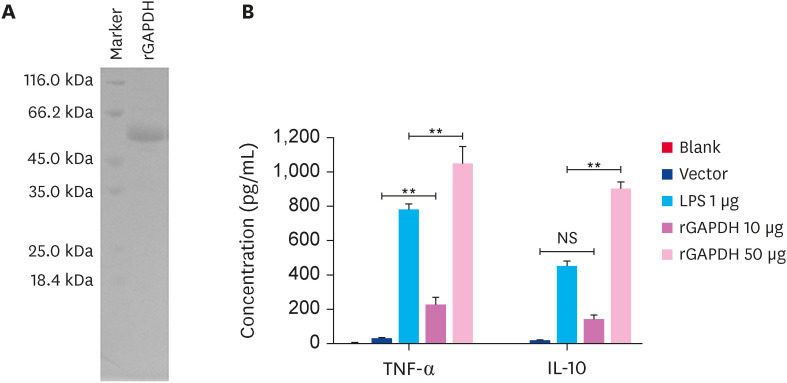
rGAPDH was expressed in E. coli, purified by Ni2+-charged column chromatography, and analyzed by 12% SDS-PAGE with an estimated molecular weight of approximately 55 kDa (Fig. 1A). LPS contamination was prevented using an EtEraser SE Endotoxin Removal Kit. rGAPDH had a comparable effect on TNF-α and IL-10 induction as LPS (Fig. 1B). Exposure to 10 μg/mL of rGAPDH for 24 h caused an increase in the TNF-α levels, while there was no significant difference in the IL-10 levels. On the other hand, the biological activity of the cytokines was reflected when the BMDCs were stimulated with 50 μg of rGAPDH. These findings suggest that rGAPDH was well prepared for further experiments.
Fig. 1. Purification of recombinant GAPDH and induction of TNF-α and IL-10 expression. (A) SDS-PAGE and stained assays were used to detect the purification of rGAPDH from pET32a. (B). Approximately 3 × 105 cells of BMDCs were exposed to 10 or 50 μg rGAPDH, 50 μg pET32a, and 1 μg LPS for 24 h. The levels of TNF-α and IL-10 in supernatants were detected by enzyme-linked immunosorbent assay.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TNF, tumor necrosis factor; IL, interleukin.
**p < 0.01 compared with the blank group, NS, not significant differences.
Effects of rGAPDH on cytotoxicity of BMDCs
BMDCs were tested for rGAPDH-induced cytotoxicity. After 24 h of stimulation with 10 μg or 50 μg/mL rGAPDH, the cells were stained with PI and Annexin V and analyzed by flow cytometry and immunofluorescence (Fig. 2A and B). rGAPDH was not hazardous to BMDCs at concentrations less than 50 μg/mL.
Fig. 2. Cytotoxicity was determined by Annexin V-FITC/PI staining. The BMDCs were activated with 10 or 50 μg/mL rGAPDH and 1 μg/mL LPS. The cells were collected after 24 h and then stained with PI and Annexin V for analysis of flow cytometry (A). 10 or 50 μg/mL rGAPDH and 1 μg/mL LPS induce the cell apoptosis and necrosis of immature BMDCs by Annexin V-FITC/PI staining (B).
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; BMDC, bone marrow-derived dendritic cell.
rGAPDH induces the generation of TNF-α and IL-10 via TLR2 and TLR4
TLR2 and TLR4 play important roles in bacterial inflammatory infections. This study examined whether TLR2 and TLR4 were involved in producing TNF-α and IL-10 by rGAPDH. The BMDCs were pretreated with C29 or/and TAK-242 and stimulated with rGAPDH. As shown in Fig. 3A, D, G protein expression was reduced markedly after the dose. rGAPDH, an extracellular secretory protein of S. dysgalactiae, is possibly one of the pathogen-associated molecular patterns recognized by TLR2 and TLR4. rGAPDH might upregulate cytokines in a TLR4- and TLR2-dependent manner. An inhibitor-mediated knockdown experiment evaluated the function of TLR2 and TLR4 in cytokine production triggered by rGAPDH. BMDCs were treated with various doses of C29 or TAK-242 to suppress TLR2 or TLR4 expression and stimulated with rGAPDH, LPS, or PGN. This process partly abrogated the rGAPDH-induced TNF-α and IL-10 production at the mRNA levels (Fig. 3B, E, H). Similarly, TNF-α and IL-10 were substantially higher in the WT than in those pretreated with inhibitors (Fig. 3C, F, I). Hence, rGAPDH-induced cytokine production may be partially dependent on TLR2 and TLR4. In addition, rGAPDH stimulates the activation and maturation of BMDCs in vitro.
Fig. 3. rGAPDH induces the generation of cytokines via TLR2 and TLR4. Bone marrow-derived dendritic cells were pretreated with three different C29 and TAK-242 doses for 2 h. The efficiency of the inhibitor was determined using western blotting and grayscale analysis (A, D, G). BMDCs were pretreated with C29 (TLR2 inhibitor) and TAK-242 (TLR4 inhibitor) for 2 h and stimulated with 50 μg/mL rGAPDH. TNF-α and IL-10 mRNA expression was analyzed by reverse transcription polymerase chain reaction (B, E, H), and the levels of cytokines in the supernatant were detected by enzyme-slinked immunosorbent assay (C, F, I).
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TNF, tumor necrosis factor; IL, interleukin.
*p < 0.05, **p < 0.01 compared with blank group, NS, not significant differences.
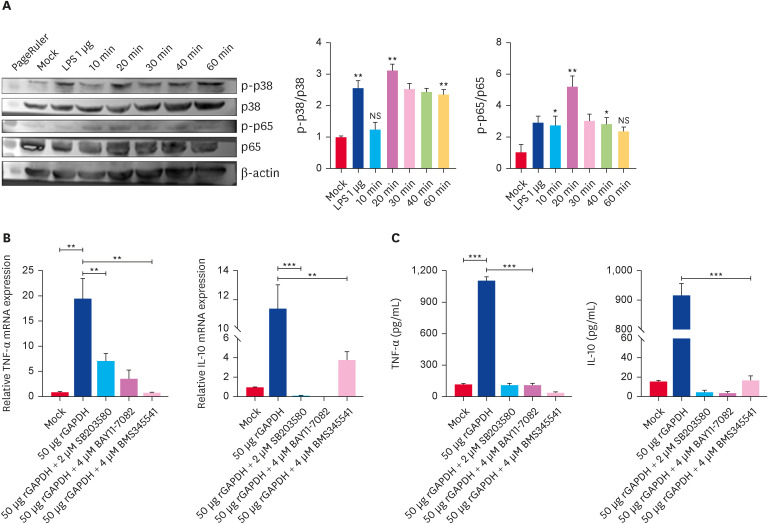
rGAPDH activates the MAPKs and NF-κB pathways in BMDCs
MAPKs and NF-κB pathways are required for BMDC cytokines generation. Western blot was used to assess their phosphorylation after stimulating BMDCs with rGAPDH at indicated times. The cells were treated with rGAPDH within 60 min to determine the synthesis of pathway-related protein and demonstrate the functional activities of these kinases in the rGAPDH-induced BMDCs pathways. rGAPDH triggered the rapid phosphorylation of p65 from 10 min, peaking at 20 min before decreasing at 30 min (Fig. 4A). The level of p38 phosphorylation was higher after 20 min, followed by a significant increase from 30 to 60 min (Fig. 4A). Significant increases in the levels of p38 and p65 phosphorylation were observed after a 60 min treatment of BMDCs with LPS. The above involvement of kinases in rGAPDH-induced BMDC activation was confirmed by pretreating the cells with suitable doses of pharmacological inhibitors before the rGAPDH treatment and measuring the TNF-α and IL-10 mRNA and cytokine production. The mRNA levels were consistent with the level of cytokines. TNF-α was partially blocked with p38 and NF-κB inhibitors (Fig. 4B and C). Unlike the former, a treatment with p38 and NF-κB inhibitors quenched IL-10 production (Fig. 4B and C). The MAPKs and NF-κB pathways are implicated in the rGAPDH-induced inflammatory signaling pathways from BMDCs.
Fig. 4. rGAPDH activates MAPKs and NF-κB pathways. Bone marrow-derived dendritic cells were incubated with 50 μg/mL rGAPDH for the indicated times. Western blot analysis was applied to detect the phosphorylation of MAPKs and NF-κB (A). The relative band intensities are presented in the histograms. TNF-α and IL-10 mRNA expression was analyzed by reverse transcription polymerase chain reaction (B), and enzyme-slinked immunosorbent assay (C) detected the cytokine levels in the supernatant.
TNF, tumor necrosis factor; IL, interleukin; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; NF, nuclear factor.
*p < 0.05, **p < 0.01, ***p < 0.001 compared with Mock, NS, not significant differences.
rGAPDH vaccination protects mice from lethal S. dysgalactiae infection
The mice were given rGAPDH, pET32a, or PBS subcutaneously on days 0, 14, 28, and 42 to test the protective effectiveness of GAPDH. The serum samples were taken one week after the last vaccination (Fig. 5A). The mice immunized with rGAPDH exhibited higher levels of polyclonal antibodies than those in the negative groups (Fig. 5B, C, and D). The mice were challenged intraperitoneally with HLJ2019 after their last immunization to test the protective impact of rGAPDH against a fatal infection. The survival rate of the rGAPDH-immunized mice reached 83%. In contrast, all the mice in the control groups died in four and eight days, respectively (Fig. 5E). Furthermore, the mice in the PBS and the Vector groups lost substantial weight and fur ruffling (Fig. 5F). These findings suggest that immunization with rGAPDH protects mice from S. dysgalactiae infections.
Fig. 5. rGAPDH vaccination protects mice from a streptococcus infection. Pregnant C57BL/6 mice (six mice per group) were subcutaneously immunized according to the immunization protocol (A). Antibodies to rGAPDH were assessed in serum one week after the second and third immunization using goat anti-mouse immunoglobulin G (B) and the OD450 value determination of polyclonal antibody to rGAPDH (C), and the P/N ratio of rGAPDH (D). After immunization, mice were challenged intraperitoneally with HLJ2019 (1 × 1012 CFU/mouse) two weeks after the last immunization. The survival rate (E) and body weight (F) were monitored for 21 consecutive days after the challenge.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
*p < 0.05 compared with the blank group, NS, not significant differences.
Immunization with rGAPDH can minimize mammary colonization
Following vaccination in the focal mastitis model, the mice were challenged with HLJ2019 in the intramammary region. The bacterial burdens in mammary glands were evaluated 10 days post-challenge, as shown in Fig. 6A. Immunization with rGAPDH reduced the mammary gland colonization levels. The mammary glands in the three groups were collected after the intramammary challenge with HLJ2019 for a histological evaluation. The PBS and Vector immunized group had congested inflammatory cells. In contrast, the rGAPDH group displayed rare pathological alterations, less neutrophil and monocyte infiltration, and less mammary necrotic debris (Fig. 6B). These findings show that rGAPDH immunization provides protection.
Fig. 6. Immunization with rGAPDH shows lower colonization in the mammary gland. Pregnant C57BL/6 mice (six mice per group) were vaccinated according to Fig. 6A, and subsequently challenged with HLJ2019 (1 × 108 CFU/gland). On day 10 after the challenge, mammary gland homogenates (A) were collected to detect the bacterial burdens. Pathological analyses of gland tissues were conducted through hematoxyling and eosin staining at a magnification of 100 (B). Unchallenged control; PBS group; Vector group; rGAPDH group). Inflammatory cell infiltration is indicated by red arrows. The data are shown as the mean ± SD.
GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
**p < 0.01 when compared with the PBS group, NS, not significant difference.
DISCUSSION
GAPDH is a protein found in all types of cells. The protein helps infections evade the host immune system by attaching to the host proteins. GAPDH was discovered as a novel pathogenic factor and potential vaccine target antigen. S. dysgalactiae requires the highly conserved GAPDH for development and energy generation. As a recombinant subunit vaccine, GAPDH lacks a signal peptide derived from surface immunogenic proteins that regulate the host immune responses and induce higher lysozyme activity and antibody titers. GAPDH is a promising vaccine candidate for preventing severe infections caused by S. dysgalactiae in cobia [24]. The recombinant GapC protein of Staphylococcus aureus has high GAPDH activity and immunogenicity. It offers protection against an S. aureus challenge and could serve as a promising target for further genetic engineering vaccines [25]. GAPDH, when used as a vaccine against S. uberis mastitis, elicited specific IgG antibodies but had little effect on the mortality in vaccinated mice [26]. Freund’s adjuvant, which is considered the gold standard for animal immunization to develop high antibody titers to proteins in mice, was used in this investigation [27]. Subcutaneous immunization with Streptococcus equi GAPDH-L/S adjuvanted with Freund’s resulted in greater survival rates and significantly decreased bacterial burdens in the lung, liver, kidney, and spleen after a challenge with S. equi [5]. In contrast, a controversial study reported that GAPDH1-150 aa, located in S. dysgalactiae, is the dominant fragment of the GAPDH protein. Furthermore, an epitope vaccine was constructed using one T cell and two B cell epitopes of S. dysgalactiae GAPDH. The T cell epitope was located strategically at the N-terminus, while the B cell epitopes were positioned at the C-terminus. Mouse immunization studies showed that the epitope-based vaccine elicited a robust immune response. Incorporating a T cell epitope into an epitope-based vaccine enhanced the cellular and humoral immune responses, expediting bacterial clearance [18]. Hence, the differences in its immunomodulatory activity may be related to its glycolytic activity, involving different structural domains of the protein, including the NAD-binding domain at the N-terminal and the catalytic active domain at the C-terminal. Tilapia immunized with rGAPDH showed a significantly higher survival rate and superior antibody production than fish inoculated with WC+ISA [28]. Mice inoculated subcutaneously with rGAPDH had a higher survival against S. pneumococci and reduced colonization in the nasopharynx and lung tissue [19].
Previous studies reported that mice immunized intraperitoneally with rGAPDH and Freund’s adjuvant were protected against Streptococcus zooepidemicus [29]. In the present study, HLJ2019 was used to confirm the protective effect and demonstrate the positive benefits of rGAPDH in the lethal infection model. Previous research from the authors’ laboratory showed that HLJ2019 produces chronic infection symptoms in the mammary gland of the mice [30]. Recombinant GAPDH adjuvanted with Freund's adjuvant was synthesized and injected subcutaneously into mice. A subcutaneous injection of rGAPDH offered excellent protection because the vaccinated mice survived longer and had smaller bacterial burdens in the mammary glands after being challenged with a fatal dosage of HLJ2019 than the control group. TNF-α, IL-6, and IL-1β, as conventional proinflammatory cytokines, are involved in streptococcus host defense. TNF-α, the first cytokine to emerge in the circulation, promotes S. pneumoniae proliferation and migration, followed by IL-1β [19]. The excessive production of proinflammatory cytokines, however, results in a powerful inflammatory response that is harmful to the host. In this study, reduced inflammatory cytokine production was observed in the rGAPDH group compared to the other groups, and pathological analysis of the mammary tissues suggested that rGAPDH can alleviate the HLJ2019 mammary challenge because there was less neutrophil and monocyte infiltration in the mammary tissues.
Interestingly, the immunosuppressive cytokine IL-10 concentration increased considerably in mammary tissues and BMDCs. In line with previous studies, exogenous GAPDH is anti-inflammatory in LPS-stimulated macrophages. This is achieved by suppressing TNF-α production and elevating the IL-10 levels, dependent primarily on its dehydrogenase activity [31]. These results showed that the extracellular GAPDH of S. agalactiae induces significant IL-10 production early in neonatal infection [6]. This suggests that early IL-10 synthesis, rather than being a physiological response to increased levels of pro-inflammatory cytokines, could be a risk factor for illness [6]. Subcutaneous vaccination with rGAPDH stimulates the mouse immunological responses and offers protection against S. dysgalactiae, including better survival rates and lower mammary inflammation.
Nothing is known regarding the role of rGAPDH in the innate immune responses, and some studies suggest that the regulatory role of bacterial GAPDH protein in host adaptive immunity is minimal [12]. DCs perform antigen recognition, antigen capture, inflammatory cytokine release, chemotactic migration, and T-cell priming and regulation. Despite the greater knowledge of the immunological roles and molecular mechanisms of DCs migration during inflammation and immunity, overlap between the migratory processes of DCs and other aspects of DCs function is required to guarantee the most efficient and beneficial immunological outcome. On the other hand, the underlying mechanisms remain unknown [32]. DCs are immune system sentinels that recognize invading pathogens and tissue damage through pattern recognition receptors (PRRs) and have a traditional dendritic architecture and robust expression of MHC II molecules [32]. Fewer studies have addressed the biology of GAPDH in the host cellular responses. Hence, measurements of cell death would be of relevance given the previously observed cytotoxic effects [33]. Moreover, rGAPDH could induce IL-10 and TNF-α, suggesting that DCs are functionally maturing. IL-10 production can stimulate CD4+ T cells differentiating down the Th1 pathways through STAT4 and ERK-dependent. Moreover, Notch signaling requires STAT4 [34]. rGAPDH may stimulate developed DCs and excite naive T cells to Th1-type CD4+ T cells induced IFN-γ. Overall, rGAPDH can trigger DCs to mature in both phenotype and function.
TLRs detect invading pathogens and initiate the host innate immunity [35]. TLR2 and TLR4 have been implicated in a streptococcus infection, detecting peptidoglycan and lipoteichoic acid, and streptolysin, respectively. rGAPDH may affect the host immunity via TLR2 and TLR4. This study showed that BMDCs stimulated with rGAPDH and inhibited for TLR2 and TLR4 exhibited a significant decrease in TNF-α and IL-10 production compared to rGAPDH-stimulated WT BMDCs. In particular, BMDCs inhibited for TLR4 showed a marked decrease in TNF-α and IL-10 production. Further research will be needed to determine if rGAPDH is a TLR2 and TLR4 ligand. TLR stimulation attracts particular adaptors, initiating the downstream signaling cascades and generating inflammatory cytokines and chemokines [36]. Phosphorylation of the MAPK and NF-κB pathways was confirmed using western blot, suggesting their involvement in DC maturation following rGAPDH.
Bacterial transformation, cellular signaling, and activation are required for bacteria to initiate and maintain a chronic infection in the presence of antimicrobial immunity. Inducing IL-10 production is one technique for immune evasion, infection, and illness [37]. IL-10 is an anti-inflammatory cytokine that minimizes the inflammatory response and avoids host damage [34]. TLR2 agonists induce IL-10 production by antigen-presenting cells, such as macrophages and myeloid dendritic cells, with macrophages producing the most IL-10 [38]. TLR2/MyD88 signaling and the PI3K pathway are required for the bacterial activation of IL-10-producing B cells to regulate T-cell-mediated colitis [39]. In addition to the MAPKs pathway and NF-κB pathway, the PI3K/AKT pathway also serves IL-10 expression in myeloid cells, and mTOR may influence IL-10 synthesis via STAT3 activation [22]. Interestingly, STAT1 or STAT3 recruitment to the Il10 promoter is required for type I IFN to enhance the IL-10 function. Type I IFN also activates the transcription factor interferon regulatory factor (IRF) 1, which increases IL-10 synthesis. In contrast, IRF5 inhibits IL-10 production [40]. The pleiotropism, complexity, and paradoxical anti-inflammatory effects of recombinant IL-10 have been studied in human clinical trials, but the safety–efficacy results were unsatisfactory. The ability to carry out infectious tolerogenic programs via the IL-10/IL-10R axis is essential for maintaining immunological tolerance–homeostasis and restoring the effector–regulatory balance during the immune responses, which is a basic immune function that avoids immunopathology. Many innate and adaptive immune cells can express IL-10 and IL-10R, establishing a network through which the tolerogenic signals are conveyed, maintained, and reinforced via autocrine and paracrine feedback loops [41]. Another intriguing theory that merits further study is that the GAPDH-targeted cells are intermediates that promote IL-10 synthesis rather than the primary source of IL-10.
S. dysgalactiae may instigate a potentially lethal resistance against the host cells because of the high levels of pro-inflammatory cytokines like TNF-α. On the other hand, a cytokine storm is prevented by strong IL-10 release, which is essential for the chronic inflammation phase. This experiment had some limitations. GAPDH-induced innate immunity was evaluated in mice and BMDCs, not living cows. Further studies will be needed to investigate the in vivo effects. In addition, various PRRs can mediate streptococcus virulence proteins [42]. This study could not exclude other TLRs in GAPDH-induced cytokine production. Future studies will assess relevant PRRs recognizing GAPDH in macrophages. S. agalactiae GAPDH negligibly affects bacterial persistence in the female reproductive tract of mice [33]. GAPDH in S. dysgalactiae contributes to bacterial colonization and adhesion. Further investigation is needed to determine the mechanisms of GAPDH regulation following the gapgh gene deletion and its release as a non-signal peptide protein. Neither rGAPDH nor its derivatives are used to prevent bovine mastitis. Further studies, including in vivo studies, should improve the understanding of the GAPDH potential in preventing bovine mastitis.
In conclusion, purified GAPDH from S. dysgalactiae induces a strong host defense response in vivo and in vitro. Subcutaneous inoculation with rGAPDH prolonged survival against HLJ2019 in mice and decreased streptococcus colonization in mammary gland tissue. This study investigated how rGAPDH elicited immune response and protection against S. dysgalactiae in mice. rGAPDH stimulates BMDCs through TLR2 and TLR4, partially via the MAPK and NF-κB signaling pathways. Targeting GAPDH would be a suitable vaccine candidate and provide insights into S. dysgalactiae pathogenesis and therapeutic strategies to combat invasive mastitis disease.
ACKNOWLEDGEMENTS
The authors would like to thank YueYang Yu for helping to isolate Streptococcus dysgalactiae.
Footnotes
Funding: Earmarked Fund for China Agriculture Research System (CARS36).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Gao M, Wang J.
- Data curation: An R.
- Formal analysis: An R.
- Funding acquisition: Gao M and Wang J.
- Investigation: An R.
- Methodology: An R.
- Project administration: Gao M.
- Resources: Guo Y.
- Supervision: Gao M.
- Validation: Gao M.
- Visualization: An R.
- Writing - original draft: An R.
- Writing - review & editing: Gao M.
References
- 1.Zhang L, Zhang H, Fan Z, Zhou X, Yu L, Sun H, et al. Production of mouse monoclonal antibody against Streptococcus dysgalactiae GapC protein and mapping its conserved B-cell epitope. Res Vet Sci. 2015;98:39–41. doi: 10.1016/j.rvsc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Yao D, Zhang H, Wang X, Yu S, Wei Y, Liu W, et al. Identification and characterization of CD4+ T-cell epitopes on GapC protein of Streptococcus dysgalactiae . Microb Pathog. 2016;91:46–53. doi: 10.1016/j.micpath.2015.11.025. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Zhang H, Fan Z, Zhou X, Yu L, Sun H, et al. Identification of a conserved linear B-cell epitope of Streptococcus dysgalactiae GapC protein by screening phage-displayed random peptide library. PLoS One. 2015;10(6):e0131221. doi: 10.1371/journal.pone.0131221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kabelitz T, Aubry E, van Vorst K, Amon T, Fulde M. The role of Streptococcus spp. in bovine mastitis. Microorganisms. 2021;9(7):1497. doi: 10.3390/microorganisms9071497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao YN, Wang H, Su LL, Wang HQ, Zhang BJ, Su Y. Optimized GAPDH-truncated immunogen of Streptococcus equi elicits an enhanced immune response and provides effective protection in a mouse model. Vet Microbiol. 2021;254:108953. doi: 10.1016/j.vetmic.2020.108953. [DOI] [PubMed] [Google Scholar]
- 6.Madureira P, Andrade EB, Gama B, Oliveira L, Moreira S, Ribeiro A, et al. Inhibition of IL-10 production by maternal antibodies against Group B Streptococcus GAPDH confers immunity to offspring by favoring neutrophil recruitment. PLoS Pathog. 2011;7(11):e1002363. doi: 10.1371/journal.ppat.1002363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang G, Xia Y, Cui J, Gu Z, Song Y, Chen YQ, et al. The roles of moonlighting proteins in bacteria. Curr Issues Mol Biol. 2014;16:15–22. [PubMed] [Google Scholar]
- 8.Kopeckova M, Pavkova I, Stulik J. Diverse localization and protein binding abilities of glyceraldehyde-3-phosphate dehydrogenase in pathogenic bacteria: the key to its multifunctionality? Front Cell Infect Microbiol. 2020;10:89. doi: 10.3389/fcimb.2020.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Zhang W, Wang C, Wang H. Mechanisms of glyceraldehyde 3-phosphosphate dehydrogenaseis in bacteria adhesion - a review. Wei Sheng Wu Hsueh Pao. 2016;56(9):1398–1405. [PubMed] [Google Scholar]
- 10.Seidler KA, Seidler NW. Role of extracellular GAPDH in Streptococcus pyogenes virulence. Mo Med. 2013;110(3):236–240. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang WM, Wang HF, Gao K, Wang C, Liu L, Liu JX. Lactobacillus reuteri glyceraldehyde-3-phosphate dehydrogenase functions in adhesion to intestinal epithelial cells. Can J Microbiol. 2015;61(5):373–380. doi: 10.1139/cjm-2014-0734. [DOI] [PubMed] [Google Scholar]
- 12.Chen PC, Hsieh MH, Kuo WS, Wu LS, Kao HF, Liu LF, et al. Moonlighting glyceraldehyde-3-phosphate dehydrogenase (GAPDH) protein of Lactobacillus gasseri attenuates allergic asthma via immunometabolic change in macrophages. J Biomed Sci. 2022;29(1):75. doi: 10.1186/s12929-022-00861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YB, Wang S, Wang C, Huang QY, Bai JW, Chen JQ, et al. Emodin affects biofilm formation and expression of virulence factors in Streptococcus suis ATCC700794. Arch Microbiol. 2015;197(10):1173–1180. doi: 10.1007/s00203-015-1158-4. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Guo M, Kong L, Gao Y, Ma J, Cheng Y, et al. TLR4 agonist combined with trivalent protein JointS of Streptococcus suis provides immunological protection in animals. Vaccines (Basel) 2021;9(2):184. doi: 10.3390/vaccines9020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira L, Madureira P, Andrade EB, Bouaboud A, Morello E, Ferreira P, et al. Group B streptococcus GAPDH is released upon cell lysis, associates with bacterial surface, and induces apoptosis in murine macrophages. PLoS One. 2012;7(1):e29963. doi: 10.1371/journal.pone.0029963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang S, Figtree G, Aiqun M, Ping Z. GAPDH-knockdown reduce rotenone-induced H9C2 cells death via autophagy and anti-oxidative stress pathway. Toxicol Lett. 2015;234(3):162–171. doi: 10.1016/j.toxlet.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 17.Sheng X, Zhang H, Liu M, Tang X, Xing J, Chi H, et al. Development and evaluation of recombinant B-cell multi-epitopes of PDHA1 and GAPDH as subunit vaccines against Streptococcus iniae infection in flounder (Paralichthys olivaceus) Vaccines (Basel) 2023;11(3):624. doi: 10.3390/vaccines11030624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma J, Wang L, Fan Z, Liu S, Wang X, Wang R, et al. Immunogenicity of multi-epitope vaccines composed of epitopes from Streptococcus dysgalactiae GapC. Res Vet Sci. 2021;136:422–429. doi: 10.1016/j.rvsc.2020.12.019. [DOI] [PubMed] [Google Scholar]
- 19.Sun X, Wang J, Zhou J, Wang H, Wang X, Wu J, et al. Subcutaneous immunization with Streptococcus pneumoniae GAPDH confers effective protection in mice via TLR2 and TLR4. Mol Immunol. 2017;83:1–12. doi: 10.1016/j.molimm.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Nagai K, Domon H, Maekawa T, Oda M, Hiyoshi T, Tamura H, et al. Pneumococcal DNA-binding proteins released through autolysis induce the production of proinflammatory cytokines via toll-like receptor 4. Cell Immunol. 2018;325:14–22. doi: 10.1016/j.cellimm.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Gao X, Wang X, Pham TH, Feuerbacher LA, Lubos ML, Huang M, et al. NleB, a bacterial effector with glycosyltransferase activity, targets GAPDH function to inhibit NF-κB activation. Cell Host Microbe. 2013;13(1):87–99. doi: 10.1016/j.chom.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rutz S, Ouyang W. Regulation of interleukin-10 expression. Adv Exp Med Biol. 2016;941:89–116. doi: 10.1007/978-94-024-0921-5_5. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Yin J, Han B, Barkema HW, Shahid M, De Buck J, et al. Adherent/invasive capacities of bovine-associated Aerococcus viridans contribute to pathogenesis of acute mastitis in a murine model. Vet Microbiol. 2019;230:202–211. doi: 10.1016/j.vetmic.2019.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Thu Nguyen TT, Nguyen HT, Vu-Khac H, Wang PC, Chen SC. Identification of protective protein antigens for vaccination against Streptococcus dysgalactiae in cobia (Rachycentron canadum) Fish Shellfish Immunol. 2018;80:88–96. doi: 10.1016/j.fsi.2018.05.052. [DOI] [PubMed] [Google Scholar]
- 25.Kerro-Dego O, Prysliak T, Perez-Casal J, Potter AA. Role of GapC in the pathogenesis of Staphylococcus aureus . Vet Microbiol. 2012;156(3-4):443–447. doi: 10.1016/j.vetmic.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Collado R, Prenafeta A, González-González L, Pérez-Pons JA, Sitjà M. Probing vaccine antigens against bovine mastitis caused by Streptococcus uberis . Vaccine. 2016;34(33):3848–3854. doi: 10.1016/j.vaccine.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 27.Hawksworth D. Advancing Freund’s and AddaVax adjuvant regimens using CpG oligodeoxynucleotides. Monoclon Antib Immunodiagn Immunother. 2018;37(5):195–199. doi: 10.1089/mab.2018.0022. [DOI] [PubMed] [Google Scholar]
- 28.Trung Cao T, Tsai MA, Yang CD, Wang PC, Kuo TY, Gabriel Chen HC, et al. Vaccine efficacy of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Edwardsiella ictaluri against E. tarda in tilapia. J Gen Appl Microbiol. 2014;60(6):241–250. doi: 10.2323/jgam.60.241. [DOI] [PubMed] [Google Scholar]
- 29.Fu Q, Wei Z, Liu X, Xiao P, Lu Z, Chen Y. Glyceraldehyde-3-phosphate dehydrogenase, an immunogenic Streptococcus equi ssp. zooepidemicus adhesion protein and protective antigen. J Microbiol Biotechnol. 2013;23(4):579–585. doi: 10.4014/jmb.1209.09037. [DOI] [PubMed] [Google Scholar]
- 30.An R, Gao M, Meng Y, Tong X, Chen J, Wang J. Infective mastitis due to bovine-associated Streptococcus dysgalactiae contributes to clinical persistent presentation in a murine mastitis model. Vet Med Sci. 2021;7(5):1600–1610. doi: 10.1002/vms3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakano T, Goto S, Takaoka Y, Tseng HP, Fujimura T, Kawamoto S, et al. A novel moonlight function of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) for immunomodulation. Biofactors. 2018;44(6):597–608. doi: 10.1002/biof.1379. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Zhang X, Cheng Y, Cao X. Dendritic cell migration in inflammation and immunity. Cell Mol Immunol. 2021;18(11):2461–2471. doi: 10.1038/s41423-021-00726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan MJ, Goh KG, Thapa R, Chattopadhyay D, Ipe DS, Duell BL, et al. Streptococcus agalactiae glyceraldehyde-3-phosphate dehydrogenase (GAPDH) elicits multiple cytokines from human cells and has a minor effect on bacterial persistence in the murine female reproductive tract. Virulence. 2021;12(1):3015–3027. doi: 10.1080/21505594.2021.1989252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10(3):170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 35.Nilsen NJ, Vladimer GI, Stenvik J, Orning MP, Zeid-Kilani MV, Bugge M, et al. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J Biol Chem. 2015;290(6):3209–3222. doi: 10.1074/jbc.M114.593426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: a comprehensive review. Clin Rev Allergy Immunol. 2014;47(2):136–147. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 37.Quan H, Kim J, Na YR, Kim JH, Kim BJ, Kim BJ, et al. Human cytomegalovirus-induced interleukin-10 production promotes the proliferation of Mycobacterium massiliense in macrophages. Front Immunol. 2020;11:518605. doi: 10.3389/fimmu.2020.518605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ricci-Azevedo R, Mendonça-Natividade FC, Santana AC, Alcoforado Diniz J, Roque-Barreira MC. Microneme proteins 1 and 4 from Toxoplasma gondii induce IL-10 production by macrophages through TLR4 endocytosis. Front Immunol. 2021;12:655371. doi: 10.3389/fimmu.2021.655371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mishima Y, Oka A, Liu B, Herzog JW, Eun CS, Fan TJ, et al. Microbiota maintain colonic homeostasis by activating TLR2/MyD88/PI3K signaling in IL-10-producing regulatory B cells. J Clin Invest. 2019;129(9):3702–3716. doi: 10.1172/JCI93820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, et al. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12(3):231–238. doi: 10.1038/ni.1990. [DOI] [PubMed] [Google Scholar]
- 41.Tabares-Guevara JH, Jaramillo JC, Ospina-Quintero L, Piedrahíta-Ochoa CA, García-Valencia N, Bautista-Erazo DE, et al. IL-10-dependent amelioration of chronic inflammatory disease by microdose subcutaneous delivery of a prototypic immunoregulatory small molecule. Front Immunol. 2021;12:708955. doi: 10.3389/fimmu.2021.708955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bhattarai D, Worku T, Dad R, Rehman ZU, Gong X, Zhang S. Mechanism of pattern recognition receptors (PRRs) and host pathogen interplay in bovine mastitis. Microb Pathog. 2018;120:64–70. doi: 10.1016/j.micpath.2018.04.010. [DOI] [PubMed] [Google Scholar]