Abstract
Objective:
To describe the pattern of recurrence, treatments received, as well the oncological outcomes, of pancreatic neuroendocrine tumors (PanNETs) following curative surgery.
Background:
PanNETs recur in 10–15% of cases following surgery. Information on the natural history and management of recurring disease is lacking.
Methods:
Patients with PanNET that underwent curative surgery at 4 institutions between 2000–19 were identified. Patients with poorly-differentiated tumors, unknown tumor grade and differentiation, hereditary syndromes, unknown margin or R2 status, metastatic, and those that had neoadjuvant treatment or perioperative mortality were excluded. Clinical variables were assessed including: first site of recurrence, treatment received, and survival outcomes.
Results:
1402 patients were included: 957 (74%) had G1, 322 (25%) had G2, and 13 (1%) had G3 tumors. Median follow-up was 4.8 (IQR:2–8.2) years. Cumulative incidence of recurrence at 5 years was 13% (95%CI:11%-15.2%) for distant disease, 1.4% (95%CI:0.8%-2.3%) for locoregional recurrence, and 0.8% (95%CI: 0.4%-1.5%) for abdominal nodal recurrence. Patients who recurred had 2.89 increased risk of death (95%CI:2–4.1) as compared to patients who did not recur. Therapy post-recurrence included: somatostatin analogues in 111 (61.0%), targeted-therapies in 48 (26.4%), liver-directed therapies in 61 (33.5%), PRRT in 30 (16.5%) and surgery in 46 (25.3%) patients. Multiple treatments were used in 103 (57%) cases. After the first recurrence, 5-year overall survival was 74.6% (95%CI: 67.4–82.5).
Conclusion:
Recurrence following surgery is infrequent but reduces survival. Most recurrences are distant and managed with multiple therapies. Prospective studies are needed to establish strategies for surveillance and the sequence of treatment to control the disease and prolong survival.
Mini-abstract
Pancreatic neuroendocrine tumors recur in 10–15% of cases following surgery. Information on the natural history of recurring disease is lacking. We described the rate, timing, and patterns of recurrence after surgery providing a full picture of the sequence of treatments received by patients developing recurrence and their oncological outcomes.
Introduction
Pancreatic neuroendocrine tumors (PanNETs) are rare neoplasms, with heterogeneous biology and clinical behaviors. Surgery is curative in the majority of patients, but in 10–15% of cases, recurrent disease develops, with variable patterns and oncological outcomes [1,2]. Clinical and biological predictors of recurrence have been extensively studied and a nomogram to accurately predict recurrence following surgery is now available [1]. However, information on the natural history of recurrent disease is lacking, and management of these patients is still not well-defined. Treatment of recurrent PanNETs depends on the tumor grade, burden of disease, and performance status; it includes surgery, locoregional treatments, such as liver embolization and systemic therapies such as somatostatin analogs (SSAs), peptide receptor radionuclide therapy (PRRT), and targeted therapies (Everolimus, Sunitinib), as well as cytotoxic chemotherapy [3].
Previous studies have tried to elucidate both the pattern and the management of recurrence, but pieces of evidence are limited by the small number of cases per institution [4–6]. Few studies on population registries have been conducted, however, those tend to overestimate the recurrence rate and tumor aggressiveness, as they do not differentiate between well- and poorly- differentiated neuroendocrine carcinomas (PanNEC) [7,8]. WHO recommends that PanNEC have distinct genetic alterations and biology and therefore are now managed differently from well-differentiated PanNET. [3,7]
In this study, we describe the rate, timing, and patterns of recurrence in patients with well-differentiated PanNET who underwent surgery with curative intent in four high-volume North- American pancreatic centers. Also, we aim to provide a full picture of sequence of treatments received by patients developing PanNETs recurrence and their oncological outcomes.
Materials and Methods
Patients and Data Collection
Patients with pancreatic neuroendocrine neoplasms who underwent surgery between 2000 and 2019 from 4 high volume centers which make up the Pancreatic Neuroendocrine Disease Alliance (PANDA): Johns Hopkins, Massachusetts General Hospital, University of Pittsburgh, and Memorial Sloan Kettering were identified. Inclusion criteria were resected patients who underwent curative surgery, age ≥18 and histopathologically confirmed diagnosis of pancreatic neuroendocrine neoplasm. Patients with PanNEC, both unknown tumor grade and differentiation status, hereditary syndromes, unknown margin or R2 status following surgery, metastatic at diagnosis, and those that received neoadjuvant treatment or had perioperative mortality were excluded. Medical records were retrospectively reviewed for patient demographics and clinical characteristics, pathology, post-surgical oncological treatments, and outcomes. Tumors were graded according to the WHO classification system and staged according to the AJCC 8th staging system specific for well-differentiated neoplasms [7,9]. Pattern of recurrence was defined according to the first site of recurrence and classified into (1) “local recurrence” for those patients presenting with isolated recurrence localized to the pancreatic remnant or pancreatic surgical bed, (2) “abdominal lymph node recurrence” when a patient presented with intrabdominal nodal disease only, and (3) “distant recurrence” when a patient had distant metastases, regardless the concomitant presence of pancreatic or abdominal nodal disease. This study was approved by a waiver authorization from all centers institutional review boards.
Statistical Analysis
Disease and treatment characteristics were summarized using median and interquartile range (IQR) for continuous variables, and frequency and percentages for categorical variables. Fisher exact test and Wilcoxon rank-sum test for categorical and continuous variables, respectively, were used to comparing subgroups. Recurrence-free survival (RFS) was calculated from the date of surgery until the date of the first recurrence and overall survival (OS) was calculated from the date of surgery until the date of last contact or death. OS after recurrence was computed from the date of the first recurrence until the date of the last contact or death among the subset of patients who experienced a recurrence during study. Time to event outcomes were estimated using Kaplan-Meier methods and compared between subgroups using the log-rank test. To examine the impact of recurrence on OS on all patients post-surgery, recurrence was treated as time-dependent covariate in a univariate Cox proportional hazards model. A Cox proportional hazards model was also used to study the association between possible risk factors and RFS, as well as OS following the first recurrence. A multivariable Cox proportional hazards model was built for patients with recurrence following the identification of risk factors with a significant univariate association with OS. Cumulative incidences of patterns of first recurrence site were estimated using competing risks method and Fine and Gray regression model was used to examine factors associated with first site of distant recurrence Patients who died without a recurrence were censored at the date of death. All analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria). P-values were 2-sided and were considered statistically significant if less than 0.05.
Results
Study Population and oncological outcomes after surgery
Of the 1,402 patients included in the study, 723 (52%) were women and median age at surgery was 59 years (IQR: 50,68). Overall, 957 (74%) patients had a G1 neoplasm, 322 (25%) a G2, and 13 (1%) a G3; grading was unknown for 110 patients. Median Ki-67 value was 2.0% (IQR: 1.0, 4.0), median tumor diameter was 2.2 cm (IQR: 1.5–3.5), and 171 (12%) neoplasms were functional. Vascular and perineural invasion was present in 373 (28%) and 320 (24%) neoplasms, respectively. Metastatic nodal disease was detected in 291 (21%) patients. Margins were negative (R0) in 1,260 (90%) cases (Supplemental Digital Content 1).
The median follow-up time for alive patients was 4.8 years (IQR: 2.0, 8.2). The overall survival at 3, 5, and 10 years was 97.4% (95% CI: 96.5, 98.3), 93.6% (95% CI: 92.1, 95.2), and 81.3% (95% CI: 78.1, 84.7), respectively. At 3 years 89.9% (95% CI: 88.2, 91.7) of patients were recurrence-free whereas RFS was 84.8% (95% CI: 82.5%, 87%) at 5 years, and 77.4% (95% CI: 74.1, 80.8) at 10 years (Supplemental Digital Content 2).
Clinical characteristics of recurring patients
At the last follow up, 193 patients experienced a recurrence. The median age at the time of recurrence was 59 (IQR 52, 68) years and the median size of the primary resected tumor was 3.85 (IQR 2.50, 6.20) cm. Seventy-seven (42%) recurring patients had a G1, 97 (53%) a G2, and 9 (4.9%) a G3 PanNET; tumor grade was unknown for 10 of the patients with recurrence. The number of patients who had vascular and perineural invasion at the time of surgery was 124 (68%) and 91 (49%), whereas a metastatic nodal disease was found in 102 (53%) cases. Most patients recurred despite an R0 resection (n= 157, 81%) (Supplemental Digital Content 3).
Pattern of Recurrence
During study follow-up, we observed 193 recurrences: 161 relapsed at a distant site, 19 experienced a local recurrence, and 13 recurred in the abdominal lymph nodes. In detail, the liver was involved in 154 (80%) cases, and in 115/154 cases it was the only site of first recurrence. Other sites of recurrence were lymph nodes that was observed in 39 (20%) patients, pancreas remnant in 35 (18%), bone in 8 (4%), peritoneum in 4 (2.1%) and lungs in 3 (1.5%). The cumulative incidence of distant disease increased over time, measuring 8.4% (95% CI: 6.8, 10.1) at 3 years, 13% (95% CI: 11.0, 15.2) at 5 years, and 18.3% (95% CI: 15.5, 21.3) at 10 years, with a trend similar to the liver-only first recurrence (Supplemental Digital Content 4). The cumulative incidences at 3, 5 and 10 years were 1% (95% CI: 0.5, 1.7), 1.4% (95% CI: 0.8, 2.3), and 2.4% (95% CI: 1.4, 2.8) for local recurrence, and 0.7% (95% CI: 0.3, 1.3), 0.8% (95% CI: 0.4, 1.5), and 1.8% (95% CI: 0.8, 3.5) for abdominal recurrence, respectively (Figure 1). Clinical and pathologic characteristics of patients according to the pattern of recurrence are shown in (Supplemental Digital Content 3).
Figure 1.
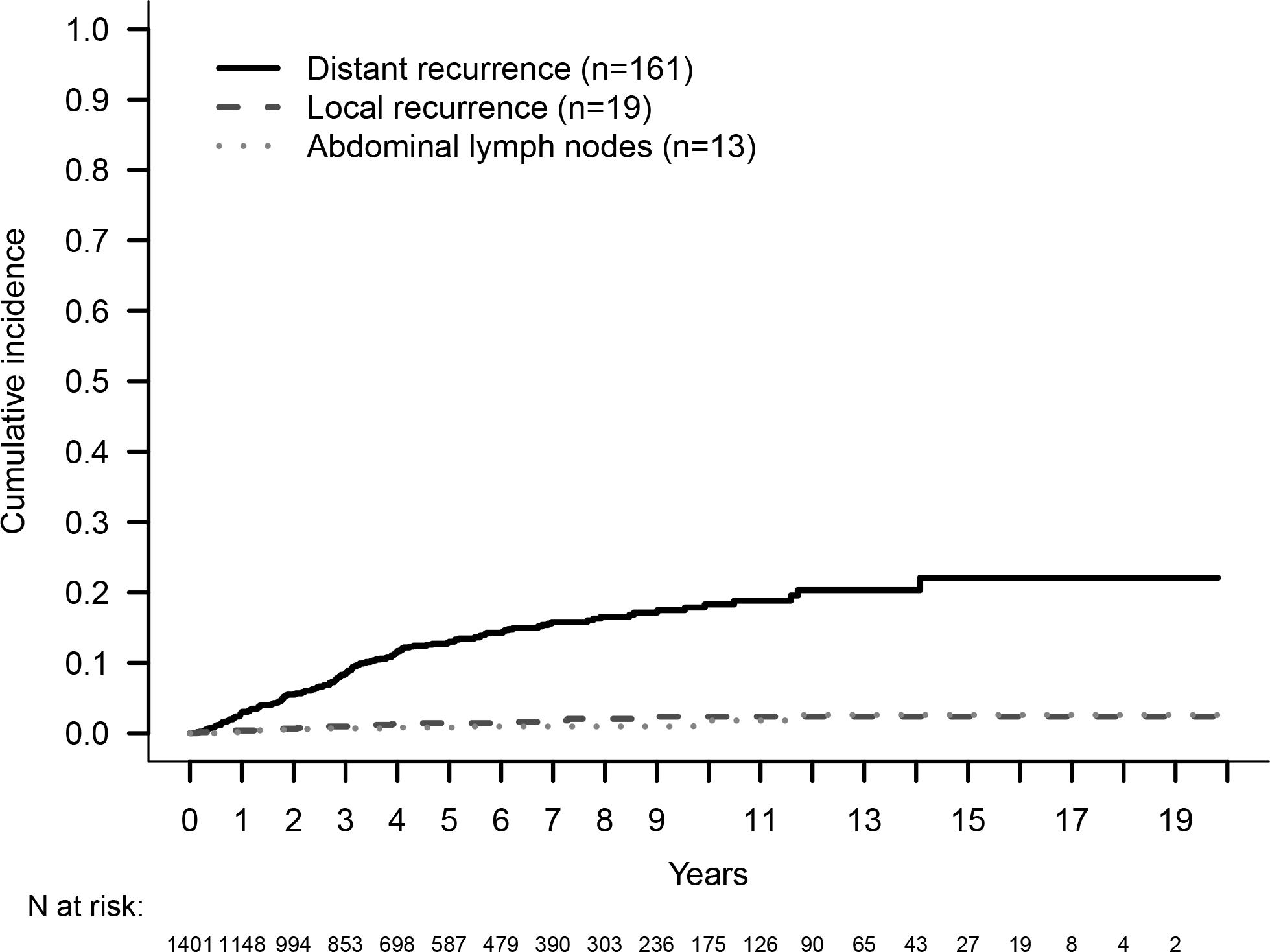
Cumulative Incidence of Recurrence by Site of First Recurrence.
Univariate analyses suggested that tumor size [HR 1.15 (95% CI: 1.1,1.2), p<0.001], tumor grade [Ref: G1, G2: HR 4.79 (95% CI: 3.45, 6.65), G3: HR 50.78 (95% CI: 24.15–106.76), p<0.001], Ki-67 [HR 1.16 (95% CI: 1.13, 1.18), p<0.001], presence of perineural [HR 3.79 (95% CI: 2.77, 5.18), p<0.001] and vascular invasion [HR 6.37 (95% CI: 4.54, 8.94), p<0.001], as well as the presence of metastatic nodal disease at surgery [HR 5.34 (95% CI: 3.92, 7.28), p<0.001] were associated with an increased risk to develop distant recurrence; age at surgery [HR 0.99 (95% CI: 0.98,1), p=0.04] was also univariately associated with the risk of developing distant recurrence. The same risk factors were found to be predictive for liver-only recurrence (Supplemental Digital Content 5). Given the small numbers, an analysis to examine predictors of local and abdominal nodal recurrence could not be performed.
Oncological outcomes of recurring patients
Post-surgery, the risk of dying for patients who experienced recurrence was almost 3-fold (HR: 2.98 [95%CI 2.0,4.1) as compared to patients who did not develop a recurrence during follow up. Among 193 patients who recurred, OS from the first recurrence was 86.2% (95% CI: 80.9, 91.8) at 3 years, 74.6% (95% CI: 67.4, 82.5) at 5 years, and 58.9% (95% CI: 49.1, 70.6) at 10 years (Figure 2A). OS from first recurrence was not significantly different when compared by site of recurrence (p=0.91, Figure 2B). Age at recurrence, tumor grade, R status, and tumor site were univariately associated with OS after recurrence. When evaluating these four predictors in a multivariable model, age at recurrence [HR 1.03 (95% CI: 1.01, 1.06), p=0.017) and tumor grade [Ref: G1, G2: HR 0.76 (95% CI: 0.38, 1.49), G3: HR 4.85 (95% CI: 1.50, 15.7), p=0.018] continued to be statistically significant.
Figure 2.
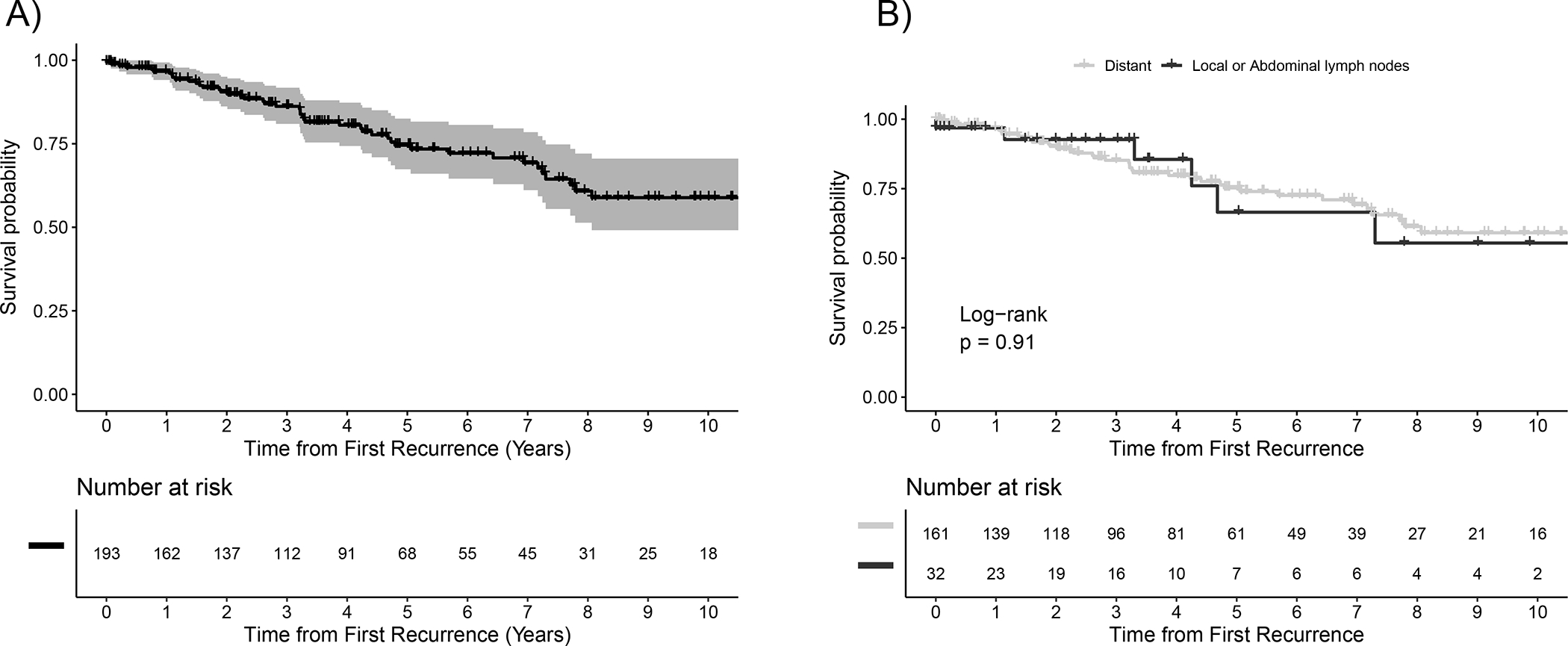
Survival Following First Recurrence (A) in the Whole Population (B) According to the Pattern of Recurrence.
Treatment of Recurrent Disease
At the last follow-up, 182 patients had at least one known treatment post recurrence: 103 (56.6%) had undergone multimodal therapies, 62 (34.1%) received one treatment, 17 (9.3%) patients with recurrence were managed by radiology surveillance only. Overall, SSAs were used to treat the recurrence in 111 (61.0%) of patients, liver-directed therapies in 61 (33.5%); chemotherapy was administered in 56 (30.8%) cases, targeted therapies in 48 (26.4%), PRRT in 30 (16.5%), radiotherapy (RT) in 19 (10.4%) and 46 (25.3%) patients had resection for curative attempt or debulking. Of the 46 patients who had surgery 4 (9 %) had a local recurrence, 5 (11%) abdominal nodal disease and 37 (80%) distant metastases. Surgery was performed with curative intent in 35 (76%) cases.
The sequence of the first three treatments for recurrence is summarized in Figure 3. Radiological surveillance with an expectant management was used as first approach in 25 (13.7%) patients. SSAs were the most frequent first line of treatment, being employed in 78 (42.9%) of patients. Surgery was used in 33 (18.1%) cases, and liver-directed therapies, in 18 (9.9%) cases. Patients receiving SSAs tended to stay on this treatment, with only a minority of patients switching treatment (mostly towards targeted therapy, liver-directed therapy, or chemotherapy). Patients treated by surgery either didn’t get other therapies or switched to SSAs or targeted therapy; those who initially underwent liver-directed therapy mostly received it also as second and third line, whereas only a minority switched to SSAs as second line, and chemotherapy or PRRT as third line. Targeted therapy and chemotherapy were used as the first line in 7 (3.8%) and 14 (7.7%) patients but their use progressively increased as second and third lines of treatment. The sequence of treatments for patients with distant recurrence is summarized in Supplemental Digital Content 6.
Figure 3.
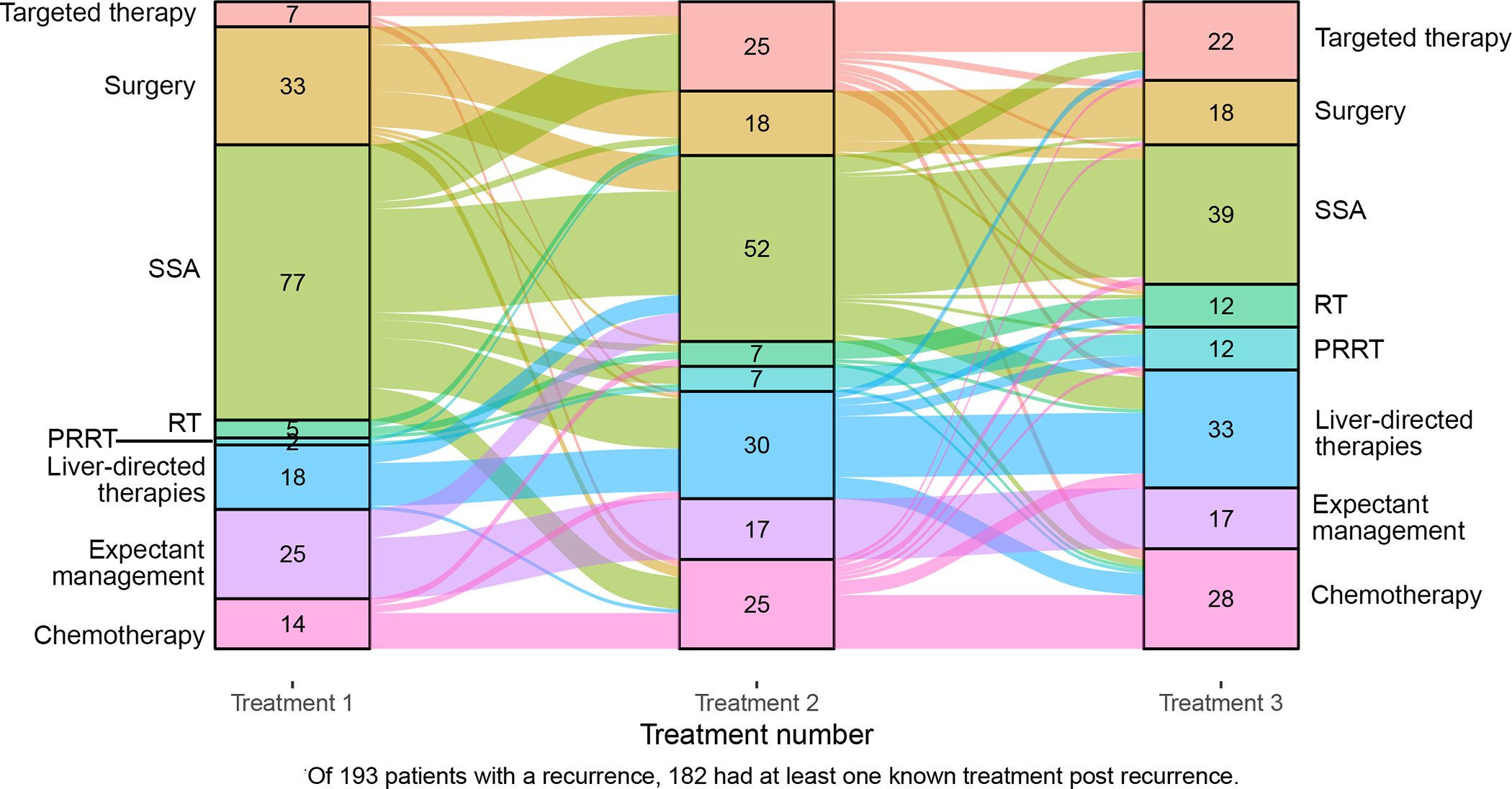
Sequence of first three treatments following first recurrence represented by Sankey diagram. The columns display the number of patients who underwent different treatments at different time-points. The arcs linking the columns represent the patients shifting from one treatment to another. The width of each arc is proportional to the number of patients.
Discussion
PanNETs include a group of neoplasms with mixed behavior that, when localized to the pancreas, can be curable by surgery alone, however recurrence can still occur. In the present study, we evaluated a large multicenter cohort of 1,402 patients with PanNET that had undergone curative pancreatic resection.
We report that the cumulative incidence of recurrence at 5 years was 15.3% (95% CI: 13.1, 17.6), with most of the cases recurring within the third year after surgery. We delineated three different types of recurrence based on the site of the first relapse: distant recurrence, local recurrence, and intra-abdominal nodal recurrence.
The most common recurrence pattern was distant, occurring with cumulative incidence of 13% [95%CI: 11%,15.2%] at 5 years, and having in the majority of cases the liver has the first organ involved. Cumulative incidence of distant recurrence continued to increase during follow-up, with values increasing so far as 10 years after initial surgery. This has an impact on the duration of the surveillance, which could not be discontinued because of the persistent risk of developing metastasis. However, given the increasing incidence of PanNETs and the relatively small risk of recurrence, a lifelong surveillance approach presents unclear benefit to patient survival and could lead to an increase in health care burden and costs, including consequences on patients’ mental health.[10,11] Additionally, our data suggest that early frequent scans in the first 1–2 years may also not be helpful. Therefore, an optimal approach on surveillance should be tailored to patients’ individual risk to recur. Our group has previously developed and validated a nomogram that can accurately predict a patient’s risk of recurrence at 5 years and, more importantly, identify those with minimal risk to recur.[1] All variables included in the nomogram (presence of metastatic lymph nodes, Ki-67 value, tumor size and presence of vascular and perineural invasion), are confirmed to be predictors for recurrence also by the present study. Such tools can be used in the clinical practice to develop a personalized surveillance protocol with a longer follow-up only in selected high-risk patients. Also, further steps towards more precise management of those patients are expected to come from new molecular tissue and blood-based biomarkers such as the ALT detection on tissue or the NETest, a multi-analyte transcript-based biomarker measured on blood samples.[2,12–14]
Local recurrence and intrabdominal nodal recurrence shared a low incidence, estimated at 1.4% (95% CI: 0.8, 2.3) and 0.8% (95% CI: 0.4, 1.5) at 5 years, were observed only in G1/G2 neoplasms, and had the tendency to occur earlier in follow up post-surgery; cumulative incidence reached a plateau for both sites as time from surgery increased. These findings suggest that these types of recurrence may represent residual disease, the result of non-radical surgery, rather than relapse of disease. Regardless, local and intra-abdominal nodal recurrences remain clinically relevant as they carry the same prognosis of patients with distant sites of recurrence. Given the small frequency of these two patterns of recurrence, a specific sub-analysis to identify risk factors for these recurrences was not performed. However, we observed that only 11% of patients with local recurrence had positive resection margins, whereas 85% of patients with abdominal nodal recurrence were staged as N1 at the time of surgery. However, while a radical pancreatic resection with R0 margins is advocated for PanNETs, it is unclear whether an extended lymphadenectomy would prevent the development of a distant recurrence, and perhaps improve overall survival. To date no randomized controlled trial has been conducted to investigate this topic, but retrospective data suggests that extended lymphadenectomy does not offer any advantages in terms of staging and treatment as nodal metastases outside the stadard lymphadenectomy area are rare (1%).[15] The North American Neuroendocrine Tumor Society guidelines recommend harvesting 11–15 lymph nodes during formal resections and performing nodal sampling during parenchyma sparing procedure, for staging purposes.[16] Indeed, it should be noted that given the current lack of indications for adjuvant treatments, the retrieval of metastatic lymph nodes will likely only help define the risk of recurrence without any possibility of intervention to reduce such risk. More insight on the possible role of adjuvant treatment, also in patients with metastatic nodes, is expected in the next years from the ongoing SWOG S2104 trial, which is investigating the impact of adjuvant capecitabine and temozolomide on recurrence-free survival.[17]
We also evaluated the oncological outcomes of patients experiencing disease recurrence. We found that patients who developed a recurrence during follow-up had a ~3-fold risk of dying compared to patients who did not have a recurrence, yet OS at 5 years after the first recurrence was 75% (95% CI: 67.4, 82.5). This OS is still remarkable considering that this population accounted for 80% of cases of disease metastatic to the liver, and a G2/G3 tumor in 58% of cases. Factors associated with OS from time of recurrence were increased tumor grade, confirming tumor biology as the main driver of prognosis in this population, and older age at recurrence, suggesting a different impact of age according to the life expectancy. Recurrent PanNET represent slow but progressive disease. Like patients with initial metastatic disease, these patients should be approached by considering the PanNET proliferative activity, tumor bulk, and the presence of hormonal syndrome. To date, randomized trials comparing head-to-head treatments for metastatic PanNET are lacking, yet trials are ongoing. [18,19] In our real-world study, recurrence of PanNET was managed with sequential use of different types of treatment in ~60% of cases, and in 1/3 of patients’ systemic chemotherapy was used; it should be noted that, compared to patients with metastatic disease at diagnosis, recurring patients are usually diagnosed during radiological surveillance and might present with lower volume of disease. Whether earlier intervention leads to better outcomes is unknown and side effects of therapy should always be weighed with biology of the disease when deciding on timing of treatment. [20]
This study presents several limitations. Because of the long study period, more treatments became available over the time given robust development of systemic therapies for this disease in recent years, and the sequence of treatments we reported might not represent the current standard of care. Also, it is difficult to provide recommendations on the ideal sequence of treatments based on this study. Finally, despite the large cohort and the multi-institutional nature of the study, data on recurrence are still relatively small to delineate definitive conclusions.
In conclusion, recurrence following curative surgery is uncommon, but is associated with decreased overall survival. Most recurrences present with distant disease involving liver and are managed with sequential different treatments. Isolated local and abdominal nodal recurrences have a low cumulative incidence and develop early but still negatively affect prognosis.
Prospective multicentric studies, as well as randomized trials, are needed to establish the role of adjuvant therapy, the optimal strategy for surveillance and the sequence of treatment, to further control the disease and prolong survival.
Supplementary Material
Pancreatic Neuroendocrine Disease Alliance (PANDA)
Aatur D Singhi, Alessandra Pulvirenti, Alessandro Paniccia, Alice Wei, Amer H. Zureikat, Ammar A Javed, Caitlin A McIntyre, Carlos Fernandez del Castillo, Christopher L Wolfgang, Cristina R Ferrone, David S Klimstra, Diane L Reidy-Lagunes, Elizabeth Thompson, Elliot K Fishman, Hannah L Kalvin, Jian Zheng, Jin He, Joanne F Chou, John L Cameron, Keith D Lillemoe, Kenneth K Lee, Kevin C Soares, Laura H Tang, Martina Nebbia, Marty A Makary, Matthew J Weiss, Michael I D’Angelica, Mithat Gonen, Motaz Qadan, Nitya Raj, Ralph H Hruban, Richard A Bukhart, Samrah Razi, T Peter Kingham, Theodoros Michelakos, Vikram Deshpande, Vinod P Balachandran, William R Burn III, William R Jarnagin, Yurie Sekigami
References
- 1.Pulvirenti A, Javed AA, Landoni L, et al. Multi-institutional Development and External Validation of a Nomogram to Predict Recurrence After Curative Resection of Pancreatic Neuroendocrine Tumors. Ann Surg. 2021;274(6):1051–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pulvirenti A, Pea A, Chang DK, et al. Clinical and Molecular Risk Factors for Recurrence Following Radical Surgery of Well-Differentiated Pancreatic Neuroendocrine Tumors. Front Med. 2020;7(August):6–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulvirenti A, Raj N, Cingarlini S, et al. Platinum-Based Treatment for Well- and Poorly Differentiated Pancreatic Neuroendocrine Neoplasms. Pancreas. 2021;50(2):138–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marchegiani G, Landoni L, Andrianello S, et al. Patterns of recurrence after resection for pancreatic neuroendocrine tumors: who, when, and where? Neuroendocrinology. 2018; [DOI] [PubMed] [Google Scholar]
- 5.Dong D-H, Zhang X-F, Lopez-Aguiar AG, et al. Resection of pancreatic neuroendocrine tumors: defining patterns and time course of recurrence. HPB. 2020;22(2):215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partelli S, Landoni L, Andreasi V, et al. Pattern of disease recurrence and treatment after surgery for nonfunctioning well-differentiated pancreatic neuroendocrine tumors. Surg (United States). 2020;168(5):816–24. [DOI] [PubMed] [Google Scholar]
- 7.Klimstra DS, Kloppell G, La Rosa S RG. Classification of neuroendocrine neoplasms of the digestive system. In: Board WC of TE, editor. WHO Classification of Tumours: Digestive System Tumours. 5th ed. Lyon: International Agency for Research on Cancer; 2019. p. 16. [Google Scholar]
- 8.Singh S, Moody L, Chan DL, et al. Follow-up Recommendations for Completely Resected Gastroenteropancreatic Neuroendocrine Tumors. JAMA Oncol. 2018;4(11):1597. [DOI] [PubMed] [Google Scholar]
- 9.Edge SB, American Joint Committee on Cancer. AJCC Cancer Staging Manual. AJCC Cancer Staging Manual. Springer; 2017. 648 p. [Google Scholar]
- 10.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;26(13):2124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Wang L, Dai S, et al. Epidemiologic Trends of and Factors Associated with Overall Survival for Patients with Gastroenteropancreatic Neuroendocrine Tumors in the United States. JAMA Netw Open. 2021;4(9):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pea A, Yu J, Marchionni L, et al. Genetic Analysis of Small Well-differentiated Pancreatic Neuroendocrine Tumors Identifies Subgroups With Differing Risks of Liver Metastases. Ann Surg. 2020;271(3):566–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marinoni I, Kurrer AS, Vassella E, et al. Loss of DAXX and ATRX Are Associated With Chromosome. Gastroenterology. 2014;146(2):453–460.e5. [DOI] [PubMed] [Google Scholar]
- 14.Modlin IM, Kidd M, Frilling A, et al. Molecular Genomic Assessment Using a Blood-based mRNA Signature (NETest) is Cost-effective and Predicts Neuroendocrine Tumor Recurrence With 94% Accuracy. Ann Surg. 2021;274(3):481–90. [DOI] [PubMed] [Google Scholar]
- 15.Partelli S, Muffatti F, Andreasi V, et al. A Single-center Prospective Observational Study Investigating the Accuracy of Preoperative Diagnostic Procedures in the Assessment of Lymph Node Metastases in Nonfunctioning Pancreatic Neuroendocrine Tumors. Ann Surg. 2022;276(5):921–8. [DOI] [PubMed] [Google Scholar]
- 16.Howe JR, Merchant NB, Conrad C, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Vol. 49, Pancreas. 2020. 1–33 p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testing the Use of Chemotherapy After Surgery for High-Risk Pancreatic Neuroendocrine Tumors - NCT05040360, ClinicalTrials.gov [Internet]. [cited 2022 Jun 6]. Available from: https://clinicaltrials.gov/ct2/show/NCT05040360 [DOI] [PubMed]
- 18.Halfdanarson TR, Reidy DL, Vijayvergia N, et al. Lutetium 177Lu-Edotreotide Versus Best Standard of Care in Well-differentiated Aggressive Grade-2 and Grade-3 GastroEnteroPancreatic NeuroEndocrine Tumors (GEP-NETs) - COMPOSE (COMPOSE). NCT04919226, ClinicalTrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT04919226
- 19.Study to Evaluate the Efficacy and Safety of Lutathera in Patients With Grade 2 and Grade 3 Advanced GEP-NET (NETTER-2). NCT03972488, ClinicalTrials.gov [Internet]. Available from: https://clinicaltrials.gov/ct2/show/NCT03972488
- 20.Bertani E, Fazio N, Radice D, et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1–G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann Surg Oncol. 2016;23(September):981–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


