Abstract
Background
Compelling evidence from preclinical and clinical studies supports the therapeutic role of cannabidiol (CBD) in several medical disorders. We reviewed the scientific evidence on CBD-related toxicity and adverse events (AEs) in 2019, at the beginning of the spike in clinical studies involving CBD. However, CBD safety remained uncertain.
Objective
With the benefit of hindsight, we aimed to provide an update on CBD-related toxicity and AEs in humans.
Methods
A systematic literature search was conducted following PRISMA guidelines. PubMed, Cochrane, and Embase were accessed in October 2022 to identify clinical studies mentioning CBD-related toxicity/AEs from February 2019 to September 2022. Study design, population characteristics, CBD doses, treatment duration, co-medications, and AEs were compiled.
Results
A total of 51 reports were included. Most studies investigated CBD efficacy and safety in neurological conditions, such as treatment-resistant epilepsies, although a growing number of studies are focusing on specific psychopathological conditions, such as substance use disorders, chronic psychosis, and anxiety. Most studies report mild or moderate severity of AEs. The most common AEs are diarrhea, somnolence, sedation, and upper respiratory disturbances. Few serious AEs have been reported, especially when CBD is co-administered with other classes of drugs, such as clobazam and valproate.
Conclusion
Clinical data suggest that CBD is well tolerated and associated with few serious AEs at therapeutic doses both in children and adults. However, interactions with other medications should be monitored carefully. Additional data are needed to investigate CBD's long-term efficacy and safety, and CBD use in medical conditions other than epilepsy syndromes.
Keywords: Cannabidiol, clinical study, toxicity, adverse event, psychosis, anxiety, treatment-resistant epilepsy
1. INTRODUCTION
Cannabidiol (CBD) is a prevalent bioactive compound in the Cannabis sativa plant, second to Δ9-tetrahydrocannabinol (THC) but without eliciting psychoactive effects [1-4]. CBD's precise molecular mechanism is still not fully elucidated. Compared to THC, CBD shows a very low affinity for endogenous cannabinoid receptors CB1 and CB2 [5] and actually seems to antagonize the CB1 receptor by allosteri CBD is the first cannabis-based medicine approved by both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as an add-on treatment for two rare and severe forms of childhood epilepsies: Lennox-Gastaut Syndrome (LGS) and Dravet Syndrome (DS) [13]. Recent clinical trials have also investigated CBD as a potential treatment for diabetes [14], fatty liver disease [15], insomnia [16, 17], psychosis [18, 19], autism [20, 21], chronic pain [22], cancer [23], and neurodegenerative disorders, such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) [24-26]. CBD is also available in over-the-counter preparations that have not been evaluated in clinical trials [27].
Considering the recent approval by the FDA and EMA for childhood epilepsy syndrome and the wide range of pharmacological effects with a broad spectrum of potential clinical use, CBD safety needs to be investigated. In 2019, we reviewed the scientific evidence on CBD-related toxicity and adverse events (AEs) [28]. However, most clinical studies involving CBD were published in recent years, providing essential data on CBD safety at specific controlled doses in humans. The aim of the present review was to provide an update on CBD toxicity and AEs, specifically focusing on clinical trials.
2. MATERIALS AND METHODS
A systematic literature search was conducted according to the PRISMA guidelines [29]. Report identification, screening, and assessment for eligibility and inclusion were conducted by two authors independently after agreeing on the search term list. The results were cross-checked after each step of the investigation.
2.1. Report Identification
PubMed (US National Library of Medicine), Cochrane, and Embase bibliographic databases were accessed in October 2022 to identify scientific reports of CBD-related toxicity and AEs. Other potential reports were searched from international agencies or institutional websites including ClinicalTrials.gov and US FDA. The search terms were: “cannabidiol”, “CBD”, or “Epidiolex”; in combination with “adverse effects”, “side effects”, “adverse reactions”, “adverse events”, “toxicology”, “toxicity”, or “safety”. Further manuscripts were retrieved through the reference lists of selected articles. Duplicates were excluded based on the articles’ DOI using Microsoft Excel (v. 2211); the results were manually checked before removal. Only the studies published after February 2019 were included to avoid redundancy with our previously published article [28].
2.2. Report Screening and Assessment for Eligibility
From the list of identified reports, clinical studies with a title and abstract mentioning CBD use were eligible for full-text reading. If a title/abstract was insufficient to understand whether the corresponding report should be included, the report was eligible for full-text reading. Clinical studies on CBD with THC co-administration only and case reports were excluded, as observed adverse events and toxicity can hardly be linked to CBD use. Literature reviews, commentaries, letters to the editor, viewpoints, or erratum; purely analytical (method development and chemical analysis), in vitro or preclinical studies; and reports that were not written in English, Italian, or French were excluded.
2.3. Report Inclusion
The full text of manuscripts reporting CBD toxicity or AEs in clinical studies was included. Clinical studies on CBD with THC co-administration; case reports; literature reviews, commentaries, letters to the editor, viewpoints, or erratum; purely analytical, in vitro, or preclinical studies; and reports that were not written in English, Italian, or French were excluded, if not excluded during the report identification and screening steps.
Authorship, date of publication, study title, study design, population characteristics, CBD doses, treatment duration, co-medications, and AEs were compiled.
The studies were compared based on their location and date of occurrence to avoid duplicates. If it arose, data were merged into a single entry in the database. The authors’ affiliations, funding sources, and disclosures were verified for each report to avoid potential bias linked to conflicts of interest.
3. RESULTS
The results of the systematic review are presented in a flow diagram of the literature search (Fig. 1). The initial search strategy yielded a total of 1053 articles that were screened by title and abstract for eligibility. One hundred duplicates were removed. Of the 953 remaining records, 707 were removed as they included preclinical studies, inappropriate study design/population, and THC co-administration. A total of 246 studies were considered for full-text review. Forty-nine reports referring to clinical trials in a recruiting, not yet recruiting, or completed status with no results posted, and 29 abstracts were excluded because of missing results or not enough data on population characteristics and CBD toxicity. Likewise, six proof-of-concept papers and five case reports were excluded as they did not meet the inclusion criteria. Finally, a total of 51 reports met the inclusion criteria of this systematic review (Table 1). Study design, population characteristics, CBD doses, treatment duration, co-medications, and AEs are depicted in Table 1. The database compiling all the retrieved data is available on demand.
Fig. (1).
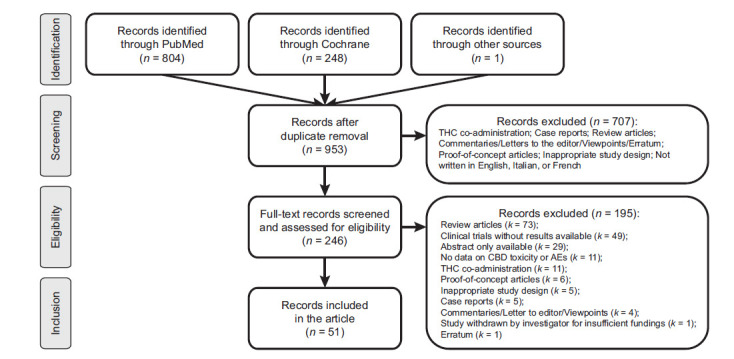
Flow diagram of the literature search.
Table 1.
Cannabidiol adverse events in clinical studies.
| Study Design | Population | CBD Dose; Administration Route | Time | Concomitant Medication | Reported AEs | Authors |
|---|---|---|---|---|---|---|
| Pharmacokinetics and Drug-Drug Interaction | ||||||
| RCT, crossover, placebo-controlled trial | Age ≥ 18 (mean 26 [8]); 14 healthy subjects randomized to take at each visit a different CBD formulation: (725 Water soluble. 088 Not water soluble. 126 Water soluble. Contains gum Arabic and maltodextrin 213 water-soluble. Contains gum Arabic and sorbitol 625 Not water soluble. Pure CBD as a crystalline powder (>99% purity) | 30 mg; oral | Single dose | Not reported | No AEs related to liver or kidney dysfunction | Abbotts et al., 2022 [70] |
| Open-label, crossover trial | Age 18-60; 30 healthy subjects: high-fat/calorie meal (n = 15), a low-fat/calorie meal (n = 14), whole milk (n = 15), or alcohol (n = 14), relative to the fasted state (n = 29). | 750 mg; oral | Single dose; days follow-up |
None | AEs mild/moderate in 27/30 (90%); moderate AEs after administration with alcohol (n = 2 (13%)): headache (n = 13 (43%)), somnolence and diarrhea (n = 5 (17%)); headache more frequent when taking CBD with alcohol (n = 8 (53%)) versus other groups (7%-21%); dizziness more frequent when taking CBD with alcohol (n = 3 (20%)) | Crockett et al., 2020 [71] |
| Open-label, four-way crossover trial (Single dose study) RCT, double-blind, placebo-controlled trial (Multiple-dose study) |
Single-dose study: Age 18-50 (mean 33 [32-49]); 12 healthy subjects randomized to receive a sequence of four different CBD single doses as a sublingual wafer (25 or 50 mg CBD), oil solution (50 mg CBD), or nabiximols oromucosal spray (20 mg CBD, 21.6 mg THC) Multiple-dose study: Age 18-50 (mean 30 [27-34]); 6 healthy subjects randomized in ratio 4:2 to receive CBD or placebo. |
Single-dose study: 25 or 50 mg CBD; sublingual 50 mg in oil; oral 20 mg CBD/21.6 mg THC in liquid spray; oromucosal Multiple-dose study: 50 mg CBD twice daily; sublingual4 |
Single-dose study: 1 day Multiple-dose study: 5 days |
Not reported | AEs mild/moderate Single-dose study: somnolence (k = 5 in n = 4 (17%)), sedation (k = 2 in n = 2 (8%)), altered mood (k = 2 in n = 2 (8%)) in sublingual and oral CBD groups Multiple-dose study: 1 participant withdrew from treatment due to altered mood but remained in the study |
Hosseini et al., 2021 [72] |
| Open-label, fixed-single sequence trial | Age 18-55 (mean range from 26.2 to 35.1); 78 healthy subjects: 12 clobazam + CBD; 12 CBD + clobazam; 12 stiripentol + CBD; 12 CBD + stiripentol; 12 valproate + CBD; 14 CBD + valproate | 750 mg twice daily; oral | 3-25 days | Clobazam, stiripentol, valproate | AEs mild/moderate: 7/77 withdrawal: 1 withdrew consent to participate; 6 (7.8%) discontinuations due to AEs. Most common AE was rashes (5 of 6) in subjects taking clobazam concomitantly with steady-state cannabidiol (2 subjects) or valproate concomitantly with steady-state cannabidiol (3 subjects) | Morrison et al., 2019 [77] |
| RCT, double-blind, placebo-controlled trial | Age 18-48; 24 healthy subjects assuming CBD after a high-fat meal: Cohort 1 (5mg/kg CBD), n = 6 CBD or n = 2 placebo; cohort 2 (10mg/kg CBD), n = 6 CBD or n = 2 placebo (cohort 2); cohort 3 (20mg/kg CBD), n = 6 CBD or n = 2 placebo (cohort 2) |
5, 10, 20 mg/kg/day; oral | Single dose, 15 days follow-up | None | AEs mild/moderate in 42% (10/24). Most common: headache (17%) (CBD group 3/18 versus placebo group 1/6) and diarrhea (8%) (CBD group 3/18 versus placebo group 0/6) | Perkins et al., 2020 [73] |
| RCT, double-blind, placebo-controlled trial | Age 18-45 (mean 25.3 [6.7]). 30 healthy subjects were randomized to receive 750 mg CDB twice daily (b.i.d.) for 4 weeks (Part 1) followed by 2 weeks of 750 mg b.i.d. CBD (Part 2, Arm1) or matched placebo (Part 2, Arm2) to assess the occurrence of withdrawal symptoms induced by abrupt cessation of CBD after prolonged administration | 750 mg twice daily; oral | 4-6 weeks | Not reported | Part 1 (CBD only): AEs in 97% (29/30); most common AEs diarrhea (63%), headache (50%), abdominal pain (47%), nausea (43%), and fatigue (33%); 4 (13%) severe AEs, and 6 (20%) moderate AEs. Discontinuation in 30% (9/39) in Part 1 because of AEs due to skin rash (7 (23.3%)), liver disorder (2 (6.7%)), and eosinophilia (1 (3.3%)). Part 2, Arm 1 (CBD): AEs in 67% (6/9) Part 2, Arm 2 (placebo) AEs in 75% (9/12); Increased incidence of headaches in Arm2 (7 (58%) in Arm2 vs. 2 (22%) in Arm 1). All AEs mild in intensity were comparable between Arm 1 and Arm 2. Most common AEs in Arm 1: diarrhea (4 (44%)), nausea, and headache (2 ((22%)); in Arm2, headache (7 (58%)) | Taylor et al., 2020 [74] |
| Open-label, fixed single-sequence trial | Age 18-60 (mean 32.6 (12.9)); 16 healthy subjects | 250 mg/day-750 mg twice daily; oral | 28 days | Caffeine | AEs moderate/mild occurred in 87.5%. (14/16). Most common AEs: is diarrhea. Elevated liver transaminase levels occurred in one subject. Six subjects (37.5%) were discontinued from the trial drug because of AEs: 3 increased ALT and (AST) ; 1 increased ALT and AST and eosinophilia; 1 increased ALT, abdominal discomfort, vomiting, and increased percentage of eosinophils; and 1 increased ALT and AST, nausea, and syncope | Thai et al., 2021 [75] |
| Open-label, fixed single-sequence trial | Age 18-60 (mean 29 [range from 23.5 to 37.8]); 16 healthy subjects | 1500 mg/day; oral | 27 days | Acetaminophen, oral contraceptive | AEs mild/moderate in 88% (14/16). Most common diarrhea in eight (50%) participants and abdominal discomfort in five (31%) participants. Peak serum alanine aminotransferase (ALT) values greater than the upper limit of normal (ULN) in 44% (7/16); for five (31%) participants, the value exceeded 5 × ULN |
Watkins et al., 2021 [76] |
| Open-label trial | Age 18-75 (mean range from 52.7 to 57.5); 30 subjects: 1 of 4 subject groups (mild [n = 8], moderate [n = 8], or severe [n = 6] hepatic impairment or normal hepatic function [n = 8]) |
200 mg; oral | 3 days | Most common: beta-blockers agents, diuretics, drugs for acid-related disorders | AEs moderate/mild. In the mild hepatic impairment group AEs occurred in 12.5% (1/8): 1 diarrhea, dizziness; in the moderate hepatic impairment group AEs occurred in 12.5% (1/8): 1 diarrhea, 1 low platelet count; no AEs in the severe hepatic impairment group. There was no increase in AE frequency or severity with increasing degrees of hepatic impairment | Taylor et al., 2019 [78] |
| An open-label, parallel-group trial | Age 18-75 (mean from 58.8 to 64.6 years); 32 subjects with mild [n = 8], moderate [n = 8], or severe [n = 8] renal impairment or normal renal function [n = 8] | 200 mg; oral | Single dose, 15 days follow-up | Beta-Blockers, xanthine oxidase inhibitors, calcium channel blockers, thyroid hormones, diuretics | All AEs mild. Only five mild AEs were reported by two subjects 1 in the mild renal impairment group and 1 in the normal renal function group. There was no increase in AE frequency or severity with increasing degree of renal impairment | Tayo et al., 2020 [79] |
| Neurology | ||||||
| RCT, double-blind, placebo-controlled trial | Age 16-55 (mean 29.5, range from 17.4 to 54.5); 35 subjects with epilepsy already receiving a stable dose of stiripentol or valproate randomized in a 4:1 ratio to receive concomitant CBD or placebo. Stiripentol group: 14 patients (2 placebo; 12 CBD); Valproic acid group: 20 patients (4 placebo; 16 CBD; one withdrawal before treatment). |
20 mg/kg/d; oral | 10-days, followed by a 14-day maintenance period | Stiripentol, valproic acid, lacosamide, clobazam, ethosuximide, lamotrigine, topiramate, levetiracetam, rufinamide, zonisamide, clonazepam, lorazepam, oxcarbazepine, caramazepine |
Stiripentol group: AEs mild in 8/12 (67%) all in CBD patients: diarrhea and fatigue, severe rash leading to withdrawal (n = 1 (8%)), increased transaminase (n = 2 (17%)) Valproic acid group: AEs mild/moderate in 14/16 (88%) in the CBD group and 1/4 (25%) in the placebo group: diarrhea, 1 increased transaminase level |
Ben-Menachem et al., 2020 [50] |
| Cohort study | Age 2-16 (mean 10.5); 50 subjects with drug-resistant EE | 2-5 mg/kg/day; oral | 1 year | Not reported | AEs mild/moderate: drowsiness (k = 16 (32%)), decreased appetite (k = 15 (30%)), diarrhea (k = 14 (28%)), irritability or behavior disturbances (k = 12 (26%)), weight loss (k = 10 (20%)), vomiting (k = 7 (14%)), mood changes (k = 3 (6%)), insomnia (k = 5 (10%)), blurred vision (k = 1 (2.6%)), dry mouth (k = 1 (2.6%)), fever (k = 1 (2.6)) | Caraballo et al., 2020 [39] |
| RCT, double-blind, placebo-controlled trial | Age ≥ 18 (mean 57); 36 subjects with Parkinson's disease and comorbid REM behavior disorder (RBD): 20 CBD group, 16 placebo group | 75-300 mg daily in a fixed progressive dose scheme: 75 mg, 1st week; 150 mg, 2nd week; 300 mg, 3rd week until the 12th week of treatment; oral | 12 weeks | Antidepressants, clonazepam, melatonin | No differences between CBD and placebo groups: epigastric pain (n = 1/20 (5%)), headache (n = 1/20 (5%)), drowsiness (n = 1/20 (5%)), sadness (n = 2/20 (10%)), dizziness (n = 1/20 (5%)) in CBD group |
de Almeida et al., 2021 [24] |
| OLE trial GWPCARE5 (NCT02224573) | Age 2-55 (mean 9.8 [4.4]); 264 subjects with DS | 2.5-30 mg/kg/day, mean modal dose 21 mg/kg/day; oral | 274 days | Clobazam, valproic acid, stiripentol, levetiracetam, topiramate | AEs mild/moderate in 246/264 (93%) and serious in 77/264 (29%): diarrhea (n = 91 (34%)), decreased appetite (n = 67 (25.4%)), somnolence (n = 65 (25%)), nasopharyngitis (n = 41 (16%)), vomiting (n = 37 (14%)), upper respiratory tract infection (n = 36 (14%)), fatigue (n = 27 (10%)), status epilepticus (n = 29 (11%)), convulsion (n = 40 (15%)), pyrexia (n = 72 (27%)), pneumonia (n = 7 (3%)), aspartate aminotransferase increased (n = 5 (2%)), dehydration (n = 4 (2%)), influenza (n = 4 (2%)), generalized tonic-clonic seizure (n = 4 (2%)) | Devinsky et al., 2019 [47] |
| Retrospective chart review | Age 3-45 (mean 17); 25 subjects with TSC | 5-20 mg/kg/day; oral | Not reported | Sirolimus, everolimus, bupropione, felbamate, lamotrigine, quetiapine, trazodone, lysine, taurine, topiramate, zonisamide, clobazam, rufinamide, bivaracetam, risperidone, lacosamide, vigabatrin, clonazepam | AEs in 10/25 (40%): diarrhea (n = 3/25 (12%)); drowsiness, increased and severe mouth sores, increased acne, ankle swelling, sinusitis, abdominal pain, mild elevation of transaminases and increased phenytoin level | Ebrahimi-Fakhari et al., 2020 [44] |
| Open-label trial | Mean age 20.8 (15) years (10 [5] years for children and 33 [14] years for adults); 169 subjects with TRE: 89 children, 80 adults | 5-50 mg/kg/day; oral | 2 years | Clobazam, valproate | AEs mild/moderate: 3042 AEs (severe in 44/3042), of which 1998 (971 children, 1027 adults) CBD-related: diarrhea (k = 737/3042), sedation (k = 391/3042), decreased appetite (k = 107/3042), increased liver function tests (k = 21/3042 in n = 11 (6.5%), of which n = 5 (45%) were also taking valproate), death (n = 1 (1%), not CBD-related); 109 serious AEs (48 children, 61 adults), of which 27 CBD-related: hospital admissions, skin rashes | Gaston et al., 2021 [32] |
| Open-label trial | Age 2-16 (mean 8); 9 subjects with ES | 25, 45, 50 mg/kg/day; oral | 12 months | Low glycemic treatment, valproate, vigabatrin |
AEs mild in 8/9 (89%): drowsiness (n = 7 (78%)), diarrhea (n = 2 (22%)), ataxia (n = 2 (22%)), appetite loss (n = 2 (22%)), elevated liver enzymes (n = 1 (11%)), agitation (n = 2 (22%)), twitchiness (n = 1 (11%)), irritability (n = 1 (11%)) | Herlopian et al., 2020 [34] |
| Retrospective chart review | Age 2-18 (mean 5.1, range from 1.2 to 15.8); 34 subjects with LGS and 10 subjects with DS | 10-20 mg/kg/day; oral | 6 months | Antiepileptics: most frequent valproate, levetiracetam | Gastrointestinal problems (mild liver enzyme elevation, vomiting, and diarrhea; n = 7 (16%)), behavioral changes (irritability, hyperactivity, excessive alertness, sleep disturbances; n = 5 (11.4%)), increased seizure frequency (n = 2 (5%)) in LGS and DS groups; AEs leading to discontinuation: drowsiness (n = 1 (2%)), gastrointestinal problems with acute pancreatitis (n = 1 (2%)), and behavioral changes (n = 1 (2%)) | Koo et al., Oct 2020 [45] |
| Open-label, multicenter trial | Age (mean 12.8 [1.7-51]); 152 subjects: 94 with LGS, 54 with DS; Age (mean 13.3[0.4-62.1]); 455 subjects with other TRE | 2-50 mg/kg/day; oral | 144 weeks | Clobazam, felbamate, lamotrigine, levetiracetam, rufinamide, stiripentol, topiramate, valproic acid | AEs mild/moderate. AEs in 91% LGS/DS group, of whom 41% reported serious AEs. Most common: somnolence (30%), convulsion (24%), and diarrhea (24%). Most common all-cause serious AEs: convulsion (14%), status epilepticus (9%), pneumonia (5%), and pyrexia (4%). Increased liver transaminase in 15% (22/152), of these 82% (18/22) reported concomitant valproate. Somnolence in 38% (38/101) subjects taking concomitant clobazam, and in 18% (9/51) subjects not taking clobazam. 12 deaths reported were deemed not-treatment-related by the investigator | Laux et al., 2019 [31] |
| Open-label trial | Age ≥ 19 (mean 34 [14]); 27 subjects with TRE |
Mean modal dose of 36.5 mg/kg/day; oral | 1 year | Not reported | Not reported | Martin et al., 2019 [33] |
| Open-label trial | Age 0-59 (mean 16.7 [13.4]); 48 subjects with TRE | 4-25 mg/kg/day; oral | 3 months | Antiepileptics | No significant AEs related to add-on CBD treatment | Metternich et al., 2021 [30] |
| RCT, double-blind, placebo-controlled trial (GWPCARE2) | Age 2-18; 199 patients with DS: n = 67 10mg/kg/d CBD10 group; n = 67 20 mg/kg/d CBD20 group; n=65 placebo |
10-20 mg/kg/day; oral | 14 weeks | Antiepileptics: most common valproate, clobazam | AEs moderate/mild occurred in 176 of 198 patients (88.9%): 58 of 65 (89.2%) in the placebo group, 56 of 64 (87.5%) in the CBD10 group, and 62 of 69 (89.9%) in the CBD20 group. Common AEs decreased appetite, diarrhea, somnolence, pyrexia, and fatigue. Elevated liver transaminase levels occurred more frequently in the CBD20 (n = 13) than the CBD10 (n = 3) group, with all affected patients given concomitant valproate sodium |
Miller et al., 2020 [51] |
| RCT, double-blind, placebo-controlled, multicenter trial (STAR 1), with OLE (STAR 2) | Age 18-70 (mean 39.2 [12.78]); 188 subjects with drug-resistant focal epilepsy receiving a stable regimen of up to 3 antiseizure medications randomized in a ration 1:1:1 (195mg CBD, 63 participants; 390mg CBD, 62 participants; placebo, 63 participants) | 195, 390 mg; transdermal | 12 weeks, followed by an open-label extension study for up to 2 years | Three concomitant antiepileptics | AEs in 50.4% (63/125) CBD group, and in 41.3% (26/63) in the placebo group. Similar rates in the CBD groups. Discontinuation in 7% (14/188) due to Aes |
O'Brien et al., 2022 [48] |
| Open-label, multicenter trial | Age 1-18 (mean 10.4); 50 subjects with TRE | 25-50 mg/kg/day, mean modal dose of 35 mg/kg/day; oral | 36 months | Clobazam, levetiracetam, lamotrigine, topiramate zonisamide, rufinamide oxcarbazepine, phenytoin, lacosamide phenobarbital, vigabatrin, ethosuximide, perampanel, valproic acid, prorenata (as needed) |
All AEs mild/moderate; Common AEs: upper respiratory infection, gastrointestinal disorders, pyrexia, somnolence | Park et al., 2020 [42] |
| OLE trial of the RCT, placebo-controlledtrial GWPCARE5 (NCT02224573) | Age 1-65 years (mean 15.9 [9.5]); 366 subjects with LGS | 5-30 mg/kg/day; mean modal dose of 24 mg/kg/day; oral | Mean of 826 days (range, 3-1421) | Clobazam, valproate, lamotrigine, levetiracetam, rufinamide | AEs in 96% of patients, serious AEs in 42%, and AE-related discontinuations in 12%. Common AEs were convulsion (39%), diarrhea (38%), pyrexia (34%), and somnolence (29%). 55 (15%) patients experienced liver transaminase elevations; 40 (73%) were taking concomitant valproate | Patel et al., Jun 2021 [40] |
| Open-label trial | Age 1-18 (mean range 1.9-16.3 [4.7]); 29 subjects with DEE | 2-25 mg/kg/day; oral | Minimum 6 months | Clobazam | All AEs mild/moderate; Common AEs: somnolence, decreased appetite and diarrhea | Pietrafusa et al., 2019 [38] |
| Open-label trial | Age 3-18 (mean 10.5 [3.8]); 48 subjects, Age: 3-18 years with DEE | 125-500 mg; transdermal | 6.5 months | Valproate, clobazam, levetiracetam, lamotrigine, topiramate | All AEs mild/moderate in 48 of 48 subjects; Common AEs: upper respiratory tract infection (n = 42 (42%)), nasopharyngitis (n = 21 (10%)), somnolence (n = 13 (6%)), vomiting (n = 10 (5%)), application site dryness (n = 8 (4%)), application site pain (n = 8 (4%)), decreased appetite (n = 8 (4%)), diarrhea (n = 8 (4%)), gastroenteritis (n = 8 (4%)), pyrexia (n = 8 (4%)), viral infection (n = 8 (4%)), viral upper respiratory tract infection (n = 8 (4%)), contusion (n = 6 (3%)), cough (n = 6 (3%)), ear infection (n = 6 (3%)), fall (n = 6 (3%)), fatigue (n = 6 (3%)), pneumonia (n = 6 (3%)), scratch (n = 6 (3%)), seizure (n = 6 (3%)) |
Scheffer et al., 2021 [37] |
| OLE trial GWPCARE5 (NCT02224573) | Age 2-18 (mean 9.7 [4.4]); 315 subjects with DS | 2.5-30 mg/kg/day; mean modal dose of 22 mg/kg/day; oral | 444 days (range = 18-1535) | Three concomitant antiepileptics | AEs occurred in 97% (306/315) of patients (mild, 23%; moderate, 50%; severe, 25%). The most common AEs: diarrhea (43%), pyrexia (39%), decreased appetite (31%), and somnolence (28%). 28 (9%) patients discontinued due to AEs. 69 (22%) liver transaminase elevations, 84% were on concomitant valproic acid |
Scheffer et al., 2021 [52] |
| OLE trial GWPCARE5 (NCT02224573) | Age 2-55 (mean 15.9 [9.5]); 66 subjects with LGS | 2.5-30 mg/kg/day; mean modal dose of 23 mg/kg/day; oral | 38 weeks | Clobazam, valproic acid | AEs (92.1%): mild/moderate; Common AEs: Diarrhea (26.8%), somnolence (23.5%), convulsion (21.3%), Liver transaminase elevations (10.1%) |
Thiele et al., 2019 [41] |
| OLE trial of the RCT, placebo-controlled trial GWPCARE6 (NCT02544763) | Age 1-65 (mean age 13.5 [10.5]); 199 TSC associated seizures subjects: 75 placebo; 64 CBD 25mg/kg/d; 60 CBD 50mg/kg/d | 5-50 mg/kg/d; mean modal dose of 27 mg/kg/day; oral | Mean of 267 days (range, 18-910) | Valproate, vigabatrin, clobazam, levetiracetam, lamotrigine, lacosamide, oxcarbazepine | AEs in 92% of patients (184/199): most AEs were mild or moderate severity. Most common AEs were diarrhea (42%), seizure (22%), and decreased appetite (20%). AEs led to permanent discontinuation in 6% of patients. One death was deemed not-treatment-related by the investigator. Elevated liver transaminases occurred in 17 patients (9%) patients; 12 were taking valproate |
Thiele et al., 2021 [46] |
| RCT, double-blind, parallel-controlled, multicenter trial (GWPCARE6) (NCT02544763) | Age 1-65 (mean 11.4 [1.1-56.8]); 224 subjects with TSC and TRE randomized in 76 placebo; 75 CBD 25mg/kg/d; 73 CBD 50mg/kg/d | 25 or 50 mg; oral | 16 weeks: 4 weeks titration phase, 12 weeks maintenance phase, up to 10 days taper phase |
Valproate, vigabatrin, clobazam, levetiracetam, lamotrigine, everolimus |
AEs were reported by 70 patients (93%) in the CBD25 group, 73 patients (100%) in the CBD50 group, and 72 patients (95%)in the placebo group; 88% of AEs were mild or moderate. Most common AEs: diarrhea (placebo group, 19 (25%); CBD25 group, 23 (31%); CBD50 group, 41 (56%)), somnolence (placebo group, 7 (9%); CBD25 group, 10 (13%); CBD50 group, 19 (26%)), occurred more frequently with CBD than placebo. Discontinuation because of AEs 8 in the CBD25 group, 10 in the CBD50 group, and 2 in the placebo group. Elevated liver transaminase levels in 28 subjects (18.9%) taking CBD vs. none taking placebo |
Thiele et al., 2021 [43] |
| Open-label trial | Age 3-19 (mean 14); 38 subjects with TRE | 5-50 mg/kg/day; oral | 1 year | Not reported | No significant AEs related to add-on CBD treatment | Thompson et al., 2020 [36] |
| RCT, double-blind, placebo-controlled trial | Age 18-65 (mean 36.6 CBD group; 37.6 placebo); 20 patients with uncontrolled epilepsy on stable doses of CLB randomize in a ratio 4:1 to receive CBD or placebo | 20 mg/kg/day; oral | 32 days: 1-10 day titration phase, 11-32 maintenance phase | Clobazam | CBD group: most AEs were mild (5 (31.3%)) or moderate (7 (43.8%)), and 1 (6.3%) was severe (seizure cluster leading to withdrawal); 2 (12.5%) increased transaminase level with concomitant valproate; 3 (18.9%) reduction of CBD dose due to occurrence of rash, diarrhea, and multiple events of sedation, slurred speech, and word-finding difficulties. Placebo group: all AEs were mild. Most common AEs overall: diarrhea, nausea, vomiting, sedation, somnolence |
VanLan-dingham et al., 2020 [49] |
| Open-label trial | Age 1-17 (mean 7.6); 61 subjects with TRE | 5-40 mg/kg; oral | 2 months | Clobazam | Most AEs mild/moderate; Common AEs: somnolence (21.3%), anemia (18.0%), diarrhea (16.4%), Less common SAEs: thrombophlebitis, apnea, and skin rash |
Wheless et al., 2019 [56] |
| Open-label trial | Age 12-25; 31 subjects with treatment-resistant anxiety disorders | 800 mg; oral | 12 weeks | SSRI, SNRI, tricyclic antidepressants, tetracyclic antidepressants, fluoxetine, citalopram, sertraline, mirtazapine, venlafaxine, desvenlafaxine, dosulepin | AEs mild/moderate (80.6%): fatigue, low mood, hot flushes, cold chills | Berger et al., 2022 [63] |
| Open-label trial | Age mean 33.6; 120 frontline healthcare workers with COVID-19 to reduce burnout and exhaustion symptoms | 300 mg; oral | 28 days | Not reported | Most AEs mild/moderate (>10%): somnolence (n = 34/120 (29%)), fatigue (n = 27/120 (23%)), increased appetite (n = 19/120 (16%)), diarrhea (n = 13/120 (11%)), weight gain (n = 12/120 (10%)), lethargy (n = 12/120 (10%)); less common AEs elevated liver enzymes (critical n = 1/120 (1%), mild n = 3/120 (2%)), pharmacodermia (n = 1 (1%)) | Crippa et al., 2021 [66] |
| RCT, double-blind, placebo-controlled trial | Age mean 32.9 (6.8); 31 subjects with cocaine use disorder: 14 CBD group; 17 placebo group | 300mg; oral | 10 days | Not specified | AEs mild/moderate. Sleepiness and increased sleep duration in 5/14 of CBD group and 3/17 of control group; nausea in 2/14 CBD group and 1/17 of control group; headache in 2/14 CBD group and 1/17 of the control group. No serious AEs. | de Meneses-Gaya et al., 2021 [61] |
| RCT, double-blind, placebo-controlled trial | Age 8-16 (range from 11 to 16.9); 8 children with intellectual disability and severe behavioral problems randomized 1:1 in CBD (4) or placebo (4) group | 20 mg/kg/day, maximum dose 500 mg, twice daily; oral | 8 weeks with 9 days titration period | Not reported | AEs mild/moderate (all n = 1 (25%) if not specified otherwise): eyes rolled up, tics/grimace, ear ringing, drooling/pooling, abdominal pain, decreased appetite, increased appetite, constipation, decreased weight, increased weight), restlessness/pacing/cannot sit still, jitter/jumpiness/nervousness, acne, urination incontinence/nocturnal enuresis, crying/feelings of sadness, drowsiness/lethargy/sedation, excessive sleep, insomnia in CBD group; headache, nose congestion/running nose, increased appetite, crying/feelings of sadness, insomnia, increased weight (n = 3 (75%)) in the placebo group | Efron et al., 2021 [59] |
| Retrospective, open-label case series | Age (mean 39.1 [17.39]); 11 subjects with PTSD | 22-28 mg in capsules or 425-575 mg in liquid spray; oral | 8 weeks | Antiepileptics, antidepressants, antipsychotics, anxiolytic/sedatives, beta-blockers | AEs in 5/21 (24%): gastrointestinal bloating or pain (n = 2 (10%)), fatigue (n = 2 (10%)), daytime fogginess (n = 1 (5%)), impaired concentration (n = 1 (5%)), worsening reflux (n = 1 (5%)) | Elms et al., 2019 [65] |
| RCT, double-blind, placebo-controlled trial | Age 16-60 (range from 18.35 to 43.35); 48 subjects with Cannabis Use Disorder (CUD) were randomized into 12 placebo groups, 12 CBD 200mg group, 12 CBD 400 mg group, and 12 CBD 800mg group | 200, 400, 800 mg; oral | 4 weeks | Not reported | AEs mild/moderate; no differences between placebo versus CBD 200, 400, 800 mg CBD: 65 mild and 9 moderate AEs in the placebo group; 42 mild and 4 moderate AEs in CBD 200 mg group, 96 mild and 8 moderate AEs in CBD 400 mg group, 78 mild and 8 moderate AEs in the CBD 800 mg group | Freeman et al., 2020 [57] |
| RCT, double-blind, placebo-controlled trial |
Age 21-65 (mean 49.8 [9.2]); 50 drug-abstinent subjects with heroin use disorder was assessed to test acute (1 hour, 2 hours, and 24 hours), short-term (3 consecutive days), and protracted (7 days after the last of three consecutive daily administrations) effects of CBD administration (400 or 800 mg, once daily for 3 consecutive days) on drug cue-induced craving and anxiety | 400, 800 mg; oral | Acute (1, 2, and 24 h), short-term (3 consecutive days), and protracted (7 days after the last of 3 consecutive daily administrations) | Not reported | No difference between CBD and placebo groups: diarrhea (n = 1 (4%)), headache (n = 1 (4%)), tiredness or fatigue (n = 1 (4%)) in the CBD group; diarrhea (n = 2 (13%)) headache (n = 2 (13%)), tiredness or fatigue (n = 1 (7%)) in the placebo group | Hurd et al., 2019 [60] |
| Open-label trial | Age 18-65 (mean 35.1 [10.58]); 31 acutely psychotic subjects with comorbid tobacco use disorder (16 CBD-group; 15 placebo) |
20 mg/cigarette; smoking | 0-28 days | Antipsychotics | Death (n = 1 (6%)), not CBD-related), headache (n = 1 (6%)) in the CBD group | Kock et al., Nov 2021 [19] |
| RCT, double-blind, placebo-controlled, multicenter trial | Age 18-65 (mean of 36.7 [10.5]); 80 subjects with a treatment-refractory social anxiety disorder or panic disorder with agoraphobia (39 CBD group; 41 placebo) | 300 mg; oral | 8 weeks, once a week, 2 h before therapist-assisted outpatient sessions | Not reported | CBD group 4/39: 1 isolated dizziness; 1 isolated drowsiness; 1 recurrent tiredness; 1 recurrent feeling of a strong blood flow placebo group 6/41: 1 isolated sweating, hot flushes, nausea, blurred vision, and a bad taste in the mouth; 1 isolated flu and gout attack; 1 isolated suicidal thoughts; 1 recurrent tiredness; 1 recurrent drowsiness; 1 recurrent headache | Kwee et al., 2022 [58] |
| Open-label trial | Age (mean 37.8 [7.8]); 5 subjects, who were taking buprenorphine for an average of 37.8 months and had psychiatric comorbidities |
600 mg; oral | 3 days | Buprenorphine | No significant AEs related to add-on CBD treatment apart from moderate sedation (n = 1) | Suzuki et al., 2020 [64] |
| Open-label, multi-site trial | Age 6-17; 20 subjects with X-fragile Syndrome |
50-250 mg/day; transdermal | Twice daily, 12 weeks | Valproate, lamotrigine | AEs mild/moderate in 17/20 (85%); 6/20 (30%) with at least 1 AE possibly/probably CBD-related, including 2 patients with application site disorders (mild dryness, moderate rash): gastroenteritis (n = 5 (25%)), vomiting (n = 2 (10%)), upper respiratory tract infection (n = 2 (10%)) |
Heussler et al., 2019 [54] |
| Open-label trial | Age 2-18; 3 subjects with SYNGAP1 mutation. |
10-23 mg/kg/day as add-on therapy; oral | 11-14 months | Valproate, lamotrigine, clobazam, zonisamide | Not reported | Kuchenbuch et al., 2020 [55] |
| Pain Management and Inflammatory Response | ||||||
| RCT, double-blind, placebo-controlled trial | Age 18-75; 99 opioid-naive subjects undergoing Arthroscopic Rotator Cuff Repair to assess CBD analgesic effect: 47 placebo group, 54 CBD group | 25 or 50 mg (three times a day); oral | 14 days | Opioid | AEs in 4/54 in the CBD group and 4/47 in the placebo group (increased transaminase level); no differences between CBD and placebo groups |
Alaia et al., 2022 [80] |
| RCT, double-blind, placebo-controlled trial | Age 21-50 (mean 32 [8]); 17 healthy non-cannabis-using subjects receiving 0-200-400-800 mg CBD in a randomized order | 200, 400, or 800 mg single dose; oral | Single dose | Not reported | AEs mild/moderate; no differences between placebo and CBD groups: Lethargy (n = 5 (29%)), upset stomach including gas and cramps (n = 1 (6%)), subtle mood change (n = 1 (6%)) in the placebo group; lethargy (n = 5 (29%)), upset stomach including gas and cramps (n = 1 (6%)) in the CBD 200 mg group; lethargy (n = 5 (29%)), upset stomach including gas and cramps (n = 4 (24%)), frequent urination (n = 1 (6%)), wooziness (n = 1 (6%)) in the CBD 400 mg group; lethargy (n = 5 (29%)), upset stomach including gas and cramps (n = 4 (24%)), wooziness (n = 1 (6%)) in the CBD 800 mg group |
Arout et al., 2021 [22] |
| Open-label, single-center trial | Age mean 64.2 (11.0); 18 subjects with symptomatic thumb basal joint arthritis | 2 mL; transdermal | 2 weeks | Not reported | No significant AEs related to CBD | Heinemann et al., 2022 [82] |
| RCT, double-blinded, placebo-controlled trial | Age (mean 62.0 [56.25-68] CBD group; mean 61.5 [53-70.75] placebo group); 136 subjects with hand osteoarthritis or psoriatic arthritis: 70 CBD group; 66 placebo group. | 20, 30 mg; oral | 12 weeks | Paracetamol, NSAIDs, antiepileptics, codeine, tramadol, opioids |
4 SAEs reported; Placebo Group SAEs: acute shoulder fracture, malignant hypertension; CBD Group: ductal carcinoma, lipothymia | Vela et al., 2022 [81] |
| RCT, double-blind, placebo-controlled trial | Age (mean 37.8 [11]); 42 subjects with COVID-19 on a placebo Age (mean 40.9 [10.9]); 49 subjects with COVID-19 on CBD |
300 mg; oral | 2 weeks | Paracetamol, acetaminophen, dipyrone | AEs mild/moderate: somnolence (n = 38 (78%)), fatigue (n = 38 (78%)), decreased appetite (n = 38 (78%)), lethargy (n = 25 (51%)), weight loss (n = 24 (49%)), nausea (n = 23 (47%)), diarrhea (n = 21 (43%)), increased appetite (n = 17 (35%)), fever (n = 11 (22%)), weight gain (n = 10 (20%)), vomiting (n = 6 (12%)), headache (n = 4 (8%)), abdominal pain (n = 4 (8%)), rash (n = 3 (6%)), bitter mouth (n = 3 (6%)) in 49/49 in CBD group; somnolence (n = 33 (79%)), fatigue (n = 33 (79%)), decreased appetite (n = 32 (76%)), lethargy (n = 15 (36%)), weight loss (n = 22 (52%)), nausea (n = 16 (38%)), diarrhea (n = 20 (48%)), increased appetite (n = 10 (24%)), fever (n = 15 (46%)), weight gain (n = 8 (19%)), vomiting (n = 4 (10%)), headache (n = 3 (7%)), abdominal pain (n = 2 (5%)), bitter mouth (n = 4 (10%)) in 42/42 in placebo group |
Crippa et al., 2021 [62] |
Abbreviations: AD, Alzheimer’s disease; AEs, adverse events; AEDs, antiepileptic drugs; CBD, cannabidiol; CYP, cytochrome P450; DEE, Developmental and Epileptic Encephalopathy; DDI, drug-drug interaction; DS, Dravet syndrome; ES, epileptic spasm; LGS, Lennox-Gastaut syndrome; PD, Parkinson’s disease; OLE, open-label extension; RBD, REM behavior disorder; RCT, randomized controlled trial; THC, Δ9-tetrahydrocannabinol ; TRE, treatment-resistant epilepsy; TSC, Tuberous Sclerosis Complex.
3.1. Neurological Studies
Since the FDA and EMA approval of CBD for childhood epileptic syndrome, there has been an increase in clinical studies focusing on the long-term efficacy and safety of CBD, and its use in several forms of treatment-resistant epilepsies (TREs). Of 51 included reports, 24 studies evaluated CBD use as an add-on treatment for severe TREs, including LGS, DS, Tuberous Sclerosis complex (TSC), and other resistant epileptic syndromes. The study designs included: open-label trials [30-39], observational cohort studies “open-label extension” (OLE) [40-43], retrospective chart reviews [44, 45], and randomized controlled trials (RCTs) with placebo group comparison [46-51]. Most studies aimed to assess the efficacy and safety of CBD treatment, and study endpoints included seizure frequency reduction, seizure response rate, seizure freedom, change in seizure severity, treatment discontinuation, and occurrence of AEs.
In the OLE studies, LGS, DS, and TSC patients who completed the RCTs were enrolled in a long-term open-label extension trial for each pivotal study allowing the evaluation of treatment-emergent AEs related to CBD administration. Across the trials, a pharmaceutical formulation of highly purified CBD (Epidiolex; 100 mg/mL) in oral solution was administered as an add-on treatment to the pre-existing antiepileptic regimen at up to 50 mg/kg/day with a follow-up time of 48 weeks. Most patients reported at least one AE, whose severity was mostly mild or moderate. The most commonly reported AEs in patients with LGS, DS, and TSC were pyrexia and gastroenteric tract disorders, including diarrhea, vomiting, and decreased appetite [40, 41, 43, 47, 52]. Somnolence, sedation, drowsiness, and convulsion were the most frequent neurological AEs. Overall, the reported AEs, frequencies, and severity, are comparable with previous observations in the RCTs [46, 51]. Treatment discontinuation was associated with increased liver transaminase and seizures. Elevation of liver transaminase was more frequent in patients taking concomitant valproate and higher dosages of CBD.
An open-label, multicenter, expanded access trial reported similar findings in children with TRE, ineligible to be included in RCTs that only included patients with LGS or DS [42]. All 47 enrolled patients experienced at least one AE during the 36-month treatment. Most AEs were mild or moderate in severity. Serious AEs were reported by twelve children; none of them were related to CBD according to the authors. The most common AEs were upper respiratory infections, gastrointestinal disorders (diarrhea, nausea, vomiting), pyrexia, and somnolence [42]. A well-tolerated safety profile of oral CBD and a good response rate for controlling seizures was confirmed by an expanded access program trial involving a large cohort of 607 patients (58 with DS, 94 with LGS, and 455 with other TREs) over a 96-week treatment [31]. Patients received oral CBD titrated until tolerability to a maximum dose of 25-50 mg/kg/day and were taking a median of three concomitant antiepileptic drugs (AEDs) (range 0-10). After 144 weeks, the safety analysis revealed that 91% of patients reported at least one AE. Most AEs were mild or moderate in severity; diarrhea and somnolence were the most common (30% and 24%, respectively).
A good antiseizure response to CBD, defined as ≥ 50% reduction in seizure frequency, and an acceptable safety profile have been demonstrated by studies evaluating both children and adults with other forms of TREs [32, 45], such as developmental and epileptic encephalopathy [37, 38, 53], refractory childhood-onset epileptic spasm [34], X-fragile syndrome [54], and SYNGAP1 developmental and epileptic encephalopathy [55]. Most patients were taking a highly purified oral CBD formulation; in only one study, a transdermal CBD gel at a dosage range of 150-500 mg/day was administered [37]. Most AEs were mild or moderate. The most common AEs were related to gastrointestinal (diarrhea, nausea, decreased appetite), and respiratory or neurological disturbances (somnolence and sedation) [37]. Overall, a few serious AEs, including increased transaminase level, sedation, and convulsion, were associated with the discontinuation of treatment.
Considering the increasing interest in CBD therapy for epilepsies, concerns regarding potential cognitive effects have been raised by treating physicians. Three open-label trials assessed whether CBD has long-term cognitive effects in both children and adults with TRE [30, 33, 36]. 27 adults and 38 subjects between the age of 3 and 19 years with TRE enrolled in open-label CBD studies were evaluated prior to initiating CBD treatment and after one year. There were no statistically significant changes in cognitive functions, as measured by the National Institutes of Health Toolbox Cognition Battery, or functional adaptive skills, as measured by the Adaptive Behavior Assessment System - Second edition after a one-year trial with CBD [33, 36]. Similarly, no significant CBD-related behavioral effects were found in 39 patients with TRE, including both children and adults, evaluated at baseline and after three months of CBD treatment [30].
Finally, four studies were primarily aimed at describing pharmacokinetic analyses or DDI between CBD and antiseizure or non-antiseizure medications [44, 49, 50, 56]. An open-label, multiple-ascending-dose, phase I/II trial characterized the pharmacokinetic parameters and short-term tolerability of a pharmaceutical-grade synthetic CBD oral solution in 61 pediatric patients (aged 1 to ≤ 17 years) with TRE as an add-on to their AED regimen [56]. Patients received a single dose (5, 10, or 20 mg/kg) on day 1 and twice-daily dosing on days 4 through 10 (10-mg/kg (cohort 1), 20-mg/kg (cohort 2), or 40-mg/kg (cohort 3) total daily dose). A steady-state concentration of CBD was reached at 2-6 days of twice-daily dosing. Systemic CBD exposure generally increased linearly with increases in dose. A bidirectional DDI occurred with CBD and clobazam. Concomitant administration of clobazam with oral CBD at 40 mg/kg/day resulted in a 2.5-fold increase in mean CBD exposure. All doses were generally well tolerated, and common AEs in over 10% of patients were somnolence (21.3%), anemia (18.0%), and diarrhea (16.4%) [56].
A retrospective chart review study investigated a DDI between CBD and mTOR inhibitors, such as everolimus and sirolimus, in a cohort of 25 patients with TSC (18 with everolimus, 7 with sirolimus) [44]. The median age at treatment initiation was 17 years (range 3-45 years; 13 children, 12 adults). CBD was orally administered, and follow-up mTOR inhibitor levels were drawn after a therapeutic dose of 5-20 mg/kg/day was achieved. Both everolimus and sirolimus plasma levels were significantly higher in 76% of patients after CBD treatment. AEs occurred in 10 of 25 patients (40%) with diarrhea being the most frequent AEs in three patients (12%). Other AEs included drowsiness, increased and severe mouth sores, increased acne, ankle swelling, sinusitis, abdominal pain, mild elevation of transaminases, and increased phenytoin level [44].
A phase II, two-arm, parallel-group, double-blind, randomized, placebo-controlled trial was conducted to evaluate pharmacokinetic DDI between CBD and stiripentol or valproate in 35 patients with epilepsy aged 16-55 [50]. Patients receiving a stable dose of stiripentol or valproate were randomized 4:1 to receive concomitant double-blind CBD or placebo. Oral CBD was administered at a dose of 20 mg/kg/day from day 12 to 26, following a 10-day dose escalation. The combination of CBD and stiripentol led to a small increase in exposure to stiripentol, while the combination with valproate did not result in significant changes in the pharmacokinetics of valproate or its metabolite, 2-propyl-4-pentenoic acid (4-ene-VPA). The most common AE was diarrhea; most AEs were mild. Two patients discontinued CBD because of serious AEs (rash (n = 1) in the stiripentol arm; hypertransaminasemia (n = 1) in the valproate arm) [50].
A similar trial investigated potential DDIs between CBD and clobazam in 20 patients with refractory epilepsy aged 18-65 on stable clobazam doses [49]. No statistically significant pharmacokinetic changes were found in patients taking concomitant CBD and clobazam for 32 days. A well-tolerated safety profile for CBD was reported, with all AEs being mild (31.3%) or moderate (43.8%) in severity. Most common AEs were mild (5 (31.3%)) or moderate (7 (43.8%)). The most common AEs were diarrhea, nausea, vomiting, sedation, and somnolence. One severe AE (6.3%) occurred as a seizure cluster leading to withdrawal. There were two cases (12.5%) of increased transaminase level with concomitant valproate, and three cases (18.9%) required a reduction of CBD dose due to the occurrence of rash, diarrhea, and multiple events of sedation, slurred speech, and word-finding difficulties [49].
CBD is a modulator of the endogenous cannabinoid system, involved in the homeostatic regulation of many physiological processes, such as mood, anxiety, sleep, and neuroprotection, which seem to be involved in mechanisms underlying the pathophysiology of PD. A phase II/III, double-blind, placebo-controlled clinical trial was conducted in 33 PD patients with rapid eye movement behavior disorder. Patients were randomized 1:1 to CBD in doses of 75 to 300 mg or matched capsules placebo and were followed up for 14 weeks [24]. CBD showed no reduction of rapid eye movement behavior disorder manifestation in PD patients; AEs were similarly reported in both groups.
A transdermal CBD gel was also evaluated for efficacy and safety profile in a RCTs, double-blind, placebo-controlled, multicenter trial [48]. A total of 188 patients (mean age 39.2 (12.78)) with focal resistant epilepsy were randomized (1:1:1) to 195-mg or 390-mg transdermal CBD or placebo daily for 12 weeks, after which they could enroll in an OLE study for up to 2 years. AEs occurred in 50.4% of the CDB group vs. 41.3% of the placebo group at a similar rate [48].
3.2. Psychiatric Studies
In psychiatry, CBD has been suggested to have antipsychotic, antidepressant, anxiolytic, anti-craving, and precognitive effects [1-3]. Our systematic search has led to the inclusion of twelve clinical trials, of which five are RCTs, double-blind, placebo-controlled studies [57-61], six open-label trials [19, 62-64], and one retrospective case series [65].
CBD has been evaluated for its anxiolytic properties in an RCT, double-blind placebo-controlled, in which 80 patients (aged 18-65 years) with a treatment-refractory social anxiety disorder or panic disorder with agoraphobia were enrolled. Patients underwent eight therapist-assisted exposure in vivo sessions (weekly, outpatient). Prior to each session, patients were administered 300 mg oral CBD (n = 39) or placebo (n = 41). Although no differences were found in treatment outcome over time between CBD and placebo as measured by the Fear Questionnaire assessed at baseline, mid-, and post-treatment, and at 3- and 6-month follow-up, only a few AEs were reported, evenly distributed between the CBD (n = 4) and the placebo groups (n = 6). Most reported AEs were dizziness, drowsiness, recurrent tiredness, the recurrent feeling of a strong blood flow, and recurrent headache [58]. In an open-label study enrolling 31 young subjects aged 12-25 years with treatment-refractory anxiety disorder, anxiety severity scores significantly decreased after receiving CBD for 12 weeks at 400-800 mg. CBD demonstrated an acceptable safety profile with no serious AEs reported. Most patients (80.6%) experienced mild or moderate AEs. The most common AEs were fatigue, low mood, hot flashes, and cold chills [63]. A more recent open-label trial used CBD for the reduction of emotional an effective agent for the reduction of emotional exhaustion and burnout symptoms in frontline healthcare professionals working with patients with COVID-19. A total of 120 participants were randomized to receive either CBD, 300 mg, plus standard care (treatment arm; n = 61) or standard care alone (control arm; n = 59) for 28 days. Five participants who received CBD plus standard care experienced serious AEs, including increased liver transaminase levels and skin rash, with full recovery after discontinuation [66]. Most AEs were mild or moderate in severity (> 10%). Somnolence (n = 34 (28.8%)), fatigue (n = 27 (22.9%)), increased appetite (n = 19 (16.1%)), diarrhea (n = 13 (11.0%)), weight gain (n = 12 (10.2%)), lethargy (n = 12 (10.2%)) were frequently reported.
In a retrospective case series, data from 11 patients diagnosed with post-traumatic stress disorder were evaluated to investigate CBD efficacy and safety profile. CBD was given in a flexible dosing regimen in two different formulations, capsular and liquid spray (n = 4 only capsular; n = 1 only liquid spray; n = 6 both capsular and liquid spray). The mean total starting dose of CBD (liquid or capsular or both) was 33.18 mg (standard deviation = 23.34). The mean total dose of CBD at the conclusion of the study period was 48.64 mg (range 2-100). Patients were taking concomitant medications of different classes; the most relevant were AEDs, antidepressants, antipsychotics, anxiolytic/sedatives, and beta-blockers. CBD was well tolerated with no serious AEs reported and few mild/moderate AEs [65].
Likewise, CBD showed an acceptable safety profile in an infantile population with severe behavioral problems and intellectual disabilities in an RCT, double-blind, placebo-controlled trial conducted by Efron et al. [59]. Eight children aged 8-16 years receiving oral CBD at a maximum dose of 500 mg/day reported only a few mild/moderate AEs. No reduction adjustments due to AEs were necessary, and no withdrawals or serious AEs were recorded [59]. No significant serious AEs were also reported by studies evaluating oral CBD at a range dose of 200-800 mg for a duration of 3 to 28 days in a cohort of subjects with different types of addiction, such as cannabis use disorder [57], opioid use disorder [60, 64], cocaine use disorder [61], and tobacco use disorder comorbid to psychotic syndromes [19]. Across the studies, most AEs reported were mild or moderate in severity. Nausea, diarrhea, fatigue, tiredness, and headache were commonly reported.
3.3. Drug-Drug Interaction and Pharmacokinetic Studies
CBD is metabolized in the liver and the intestine by cytochrome P450 (CYP) CYP2C19 and CYP3A4, and 5'-diphosphoglucuronosyltransferase (UGT) UGT1A7, UGT1A9, and UGT2B7 isoforms, mainly producing hydroxylated and carboxylated metabolites. CBD inhibits barbiturate metabolism, increasing barbiturate-induced sleep duration in mice, and phenazone hepatic metabolism [67] due to the inhibition of CYP3A and CYP2C microsomal enzymes [68]. It has been suggested that CBD also induces hepatic CYP3A, CYP2B, and CYP2C [69]. Considering the complexity of CBD pharmacology, available clinical trials address the occurrence of AEs and DDIs in a cohort of healthy subjects [70-77]. Liver safety has been investigated in an open-label DDI trial enrolling 16 healthy subjects aged 18-60 years [76]. Therapeutic oral CBD at a dose of 1500 mg was administered daily for about 3.5 weeks, and the effects on CYP1A2 activity were monitored. Of 16, seven subjects (44%) experienced peak serum alanine aminotransferase values greater than the upper limit of normal. Five subjects (31%) reached the international consensus criteria for drug-induced liver injury. There was no correlation between transaminase elevations and baseline characteristics, CYP2C19 genotype, or CBD plasma concentrations. All alanine aminotransferase elevations above the upper limit of normal began within 2-4 weeks of initial exposure to CBD. DDIs and liver impairment may occur mostly when CBD is co-administered with AEDs, such as clobazam, valproate, and stiripentol, as they share similar metabolic pathways. An open-label DDI study investigated the reciprocal impact on the steady-state pharmacokinetics of CBD, clobazam (and N-desmethylclobazam), stiripentol, and valproate; and CBD safety and tolerability when co-administered with each AED. Concomitant cannabidiol had little effect on clobazam exposure, N-desmethyl clobazam exposure increased, and stiripentol exposure increased slightly, while no clinically relevant effect on valproate exposure was observed. Concomitant clobazam with CBD increased 7-OH-CBD exposure, without notable 7-COOH-CBD or CBD increases. Stiripentol decreased 7-OH-CBD exposure by 29% and 7-COOH-CBD exposure by 13%. There was no effect of valproate on CBD or its metabolites. CBD was moderately well tolerated, with similar incidences of AEs with concomitant clobazam, stiripentol, or valproate. There were no deaths, serious AEs, pregnancies, or other clinically significant safety findings [77].
A pharmacokinetic DDI trial investigated the effects of repeated dosing of oral CBD on caffeine clearance via modulation of CYP1A2 activity in healthy adults. Sixteen healthy subjects received a single 200 mg caffeine dose and placebo on day 1. CBD was then titrated to reach the dosage of 750 mg twice daily through 28 days of treatment. CBD affected the exposure of caffeine, a CYP1A2 substrate, leading to an elevation of 15% for Cmax, 88% for AUC0-t, and 95% for AUC0-∞, and showed a good safety profile with no unexpected AEs. Diarrhea was the most common, and six subjects discontinued because of elevated liver transaminases [75].
Two studies with a crossover or RCT double-blind design focused on the pharmacokinetic effects of different oral [70] or sublingual formulations of CBD [72]. Fourteen healthy subjects, aged ≥ 18 years (mean 26 [8]), were randomized to take each visit (5 in total) a different CBD formulation: (725 Water soluble. Contains sorbitol; 088 Not water soluble. Contains medium chain triglyceride coconut oil; 126 Water soluble. Contains gum Arabic and maltodextrin; 213 water-soluble. Contains gum Arabic and sorbitol; 625 Not water soluble. Pure CBD as a crystalline powder (>99% purity)) [70]. Comparing the pharmacokinetics of (CBD) and CBD metabolites (6-OH-CBD, 7-OH-CBD, and CBD-COOH) following ingestion of five different CBD formulations, considerable variability was found among the different formulations. When delivered in an aqueous beverage, water-soluble CBD formulations typically evoked the greatest exposure. CBD was not influencing the thermic effect of food; however, it did lower insulin and triglyceride concentrations during the first 30-min following food ingestion. Moreover, a single 30 mg dose of CBD did not influence most of the circulating markers of liver and kidney function. Consistently, no serious AEs were reported [70]. The pharmacokinetic properties and safety profile of a novel CBD wafer formulation compared with an oil formulation were evaluated by Hosseini et al. [72]. First, they conducted an open-label, single-dose, crossover study in which 12 healthy volunteers were randomized to receive a sequence of four different single doses of CBD as a sublingual wafer (25 or 50 mg CBD), oil solution (50 mg CBD), or nabiximols oromucosal spray (20 mg CBD, 21.6 mg THC). Then, they carried out a double-blind study where a sublingual wafer (50 mg CBD) was administered twice a day for five days. The extract was generally well tolerated by participants when administered in either wafer or oil form, with some adverse events, including mild or moderate somnolence, sedation, and altered mood. The median maximum concentrations of CBD after administration of the oil solution and wafer were 9.4 and 11.9 ng/mL, respectively. Maximum concentrations of CBD occurred 4 hours after administration, with an estimated terminal elimination half-life of 6 hours [72].
Pharmacokinetic, safety, and tolerability of oral CBD have been evaluated in healthy volunteers after the consumption of different meal compositions. A new lipid oral CBD formulation was administered at doses of 5, 10, and 20 mg/kg in 24 healthy volunteers randomized for each CBD dose, and placebo [73]. This RCT double-blind, placebo-controlled study demonstrated the new CBD formulation is well tolerated even at an escalating dose with no serious AEs reported. AEs of mild or moderate severity were reported by 42% of subjects; the most common being headache (17%) (CBD group 3/18 versus placebo group 1/6) and diarrhea (8%) (CBD group 3/18 versus placebo group 0/6). The CBD plasma exposure increased in a dose-proportional manner and declined to levels approaching the lower level of quantification by day 8. The terminal elimination half-life was approximately 70 h, suggesting that 2-3 weeks are needed to fully eliminate CBD [73]. Similar findings were found in an open-label crossover study using the highly purified formulation of CBD (Epidiolex). A single CBD dose of 750 mg was administered in thirty healthy subjects following a high-fat/calorie meal (n = 15), a low-fat/calorie meal (n = 14), whole milk (n = 15), or alcohol (n = 14), compared to the fasted state (n = 29). CBD plasma exposure increased under all test treatment conditions in this trial when compared to the fasted state. No serious AEs were recorded. Mild or moderate AEs were reported in 90% (27/30) of subjects. Only two (13%) subjects experienced moderate AEs after administration with alcohol. The most common AEs were headache in 13 (43%) subjects overall, and somnolence and diarrhea in five (17%) subjects overall. Many more subjects were affected by headaches while taking CBD with alcohol (eight (53%) subjects) versus the other treatment groups (7%-21% subjects affected). An increased incidence of dizziness was found in subjects while taking CBD with alcohol [71].
Finally, an RCT, double-blind, placebo-controlled trial assessed whether withdrawal symptoms occurred after the abrupt cessation of CBD after prolonged administration in 30 healthy volunteers. No evidence of withdrawal syndrome was found with abrupt discontinuation of short-term treatment with 750 mg CBD twice daily (b.i.d.) for 4 weeks (Part 1) followed by 2 weeks of 750 mg b.i.d. CBD (Part 2, Arm1) or matched placebo (Part 2, Arm2). No serious AEs were reported. Mild or moderate AEs reported were consistent with those reported by other clinical trials [74].
Interestingly, an open-label trial was conducted to assess the pharmacokinetics and safety of CBD in subjects with mild to severe hepatic impairment. The population group consisted of 30 subjects, aged 18-75 years. Alongside CBD, they consumed common concomitant medications, such as beta-blocking agents, diuretics, and drugs for acid-related disorders. The trial consisted of subjects being administered oral CBD 200 mg daily for 3 days. This resulted in common AEs, varying between groups with mild and moderate hepatic impairment. The subject group with mild hepatic impairment had one case of diarrhea and dizziness, while the subject group with moderate hepatic impairment had one case of diarrhea and a low platelet count. However, there was no case of AEs in the subject group of severe hepatic impairment. Neither was an increase in AEs severity or frequency noted with the elevating degree of hepatic impairment [78].
Patients who receive CBD may have co-existing renal disturbances. Therefore, it is important to understand whether dose adjustments are necessary to mitigate the risk of exposure-related toxicity. To gain a deeper insight into the pharmacokinetics, safety, and tolerability of CBD in subjects with mild to severe renal impairment, a phase I, open-label, parallel-group, a single-dose trial was conducted [79]. The subject group was selected to be 32 subjects, ages 18-75 with either mild (n = 8), moderate (n = 8), or severe (n = 8) renal impairment as well as some with normal renal function (n = 8). All groups equally of 8 subjects received 200 mg of oral CBD as a single dose and were followed up after 15 days. In addition to the CBD dose, they consumed concomitant medications including blood pressure-regulating agents (β-blockers, xanthine oxidase inhibitors, calcium channel blockers), thyroid hormones, and diuretics. None were considered to impact the safety or interpretation of the trial data. In the trial outcome, the subjects had defined all AEs as mild. Only five mild AEs were reported by two subjects, one in the mild renal impairment group and one in the normal renal function group. There was no increase in AE frequency or severity with an increasing degree of renal impairment.
3.4. Pain Management
CBD has been also evaluated for its analgesic and anti-inflammatory properties. Alaia et al. demonstrated that buccally absorbed CBD has good efficacy for pain control right after arthroscopic rotator cuff repair in an RCT, double-blind, placebo-controlled trial. Ninety-nine opioid-naive subjects, aged 18-75, undergoing arthroscopic rotator cuff repair were randomized in placebo (n = 47) and CBD groups (n = 54). For 14 days, subjects received 25 or 50 mg/kg/day CBD alongside opioids as their concomitant medication. One AE of increased transaminase level was experienced in both groups, 4/45 in the CBD group, and 4/47 in the placebo group [80]. Furthermore, a within-subject, randomized, placebo-controlled, double-blind study was carried out to present the analgesic effects, abuse liability, safety, and tolerability of acute CBD [22]. A group of 17 healthy non-cannabis-using subjects, aged 21-50 years were receiving a single dose of 200, 400, or 800 mg oral CBD in a randomized order. With no other concomitant medications reported, all AEs were mild to moderate. There were no differences in AEs between the placebo and CBD groups. Common AEs reported in both groups were lethargy and upset stomach. Subtle mood changes, frequent urination, and wooziness were also reported.
Additionally, an RCT, double-blinded, placebo-controlled trial was performed to examine CBD as an add-on analgesic treatment option for subjects with hand osteoarthritis and psoriatic arthritis [81]. A group of 129 subjects was given synthetic CBD 20 to 30 mg or a placebo daily for 12 weeks. The CBD group comprised 70 subjects with a mean age of 62 years, while the placebo group entailed 66 subjects with a mean age of 61.50 years. The subject group received 10-20 mg/kg/day of either a CBD or placebo tablet orally. Alongside, they also consumed concomitant medication including paracetamol, non-steroidal anti-inflammatory drugs, antileptics, codeine, tramadol, and opioids. Four serious cases of AEs were reported including acute shoulder fracture, malignant hypertension, ductal carcinoma, and lipothymia that were not deemed to be CBD-related. Finally, the therapeutic potential of a topical CBD formulation with shea butter (6.2 mg/mL) has been evaluated for the treatment of pain associated with thumb basal joint arthritis [82]. Eighteen subjects were randomized in a crossover, double-blind, placebo-controlled study design and received topical CBD (6.2 mg/mL CBD with shea butter) or shea butter alone for two weeks. No adverse events were reported, and topical CBD resulted effective in pain control [82].
Since CBD may exert anti-inflammatory and antiviral properties, CBD has been investigated as a potential treatment option for coronavirus disease 2019 (COVID-19). An RCT, double-blind, placebo-controlled trial was carried out for two weeks. The trial involved 109 subjects with mild to moderate symptoms of COVID-19 related [62], subdivided into 49 subjects in the CBD group, who were administered oral CBD at a dosage of 300 mg daily, and 42 subjects in the placebo group. Subjects were taking concomitant medication such as paracetamol, acetaminophen, and dipyrone. Most AEs were mild to moderate. Somnolence, fatigue, decreased appetite, lethargy, weight loss, and diarrhea were commonly reported. These AEs were transient with no statistically significant differences between the placebo and CBD groups.
4. DISCUSSION
The therapeutical potential of drugs modulating the activity of endocannabinoid receptors has drawn substantial interest [23]. Since the approval of the highly purified formulation of CBD, Epidiolex, in 2018, a growing number of studies were conducted to investigate the efficacy and the safety of CBD in several medical conditions. We previously reviewed the literature regarding CBD pharmacokinetic properties, tolerability, and safety profile in both preclinical and clinical studies [28]. The main aim of the present work was to offer an updated review of the available evidence regarding the use of CBD in the clinical context.
Compared to our previously published article [28], new data have become available on the long-term efficacy and tolerability of CBD for the treatment of TREs suggesting that CBD has a good safety profile in both children and adults’ populations. Interestingly, in the last few years, a larger number of clinical trials have been conducted for multiple clinical indications, outside of LGS and DS, including psychosis, anxiety disorders, addictions, and pain management. Moreover, many ongoing clinical trials are evaluating the efficacy and the safety profiles of CBD in conditions such as Alzheimer’s disease, several forms of chronic pain, fibromyalgia, developmental and behavioral disorders, and liver and heart disorders in an effort to better understand CBD pharmacodynamics and pharmacokinetics, and its therapeutic potential. Overall, this updated systematic review provides new evidence confirming a well-tolerated safety profile and suggesting that CBD may represent a new therapeutic approach as a stand-alone or an add-on drug for several unmet clinical needs.
Across all included trials, most patients reported the occurrence of at least one AEs. Most AEs were mild or moderate in severity. The few serious AEs reported were frequent causes of treatment discontinuation or hospitalization with a full recovery after CBD interruption. CBD potently inhibits hepatic metabolism by acting on CYP3A4 and CYP2C19. These enzymes also metabolize several AEDs that are often taken concomitantly with CBD, such as clobazam, valproate, and stiripentol, in a cohort of patients enrolled in clinical trials focusing on TRE. Oral CBD, when prescribed at higher doses (10-20 mg/kg/day), was associated with an increased rate of serious AEs, among which elevation of liver transaminase, somnolence, sedation, and respiratory infections were most commonly reported. The majority of cases with increased liver transaminase were in children or adults with epilepsy who were also being treated with sodium valproate. The exact mechanism of this interaction is not fully understood. CBD and valproate co-administration does not significantly alter their plasma levels or of their metabolites [77]. However, CBD metabolite 7-COOH-CBD and valproate and its metabolite 4-ene-valproic acid may affect hepatic mitochondrial function. Similarly, concomitant CBD did not significantly affect clobazam plasma exposure in healthy subjects [77]. Given CBD complex and multimodal pharmacology with a potential increase of side effects when taken with clobazam, two metanalyses evaluated the efficacy and tolerability of CBD with or without concomitant clobazam, providing conflicting results. Indeed, a metanalysis of four phase III, RCTs, double-blind, placebo-controlled trials (GWPCARE1, 2, 3, 4) [83] found that the safety profile of oral highly purified CBD (10 or 20 mg/kg/day for a 14-week treatment) was affected by the concomitant administration of clobazam. Reported AEs and serious AEs were more frequent in patients taking both CBD and clobazam than in those taking CBD without clobazam and placebo. Somnolence was frequently reported by patients with concomitant clobazam and increased at higher CBD doses [83]. Conversely, Gunning et al. did not find an increased incidence of AEs in LGS and DS patients taking concomitant clobazam [84]. Somnolence was the most frequent AE reported in 22% of patients with LGS and 27% of patients with DS, of whom 67% and 84%, respectively, were taking concomitant clobazam, and was not considered dose related. Other common AEs, such as diarrhea, decreased appetite, and vomiting were not more common in patients on clobazam. Serious AEs related to CBD and their possible relation to DDIs were analyzed by a systematic review of RCTs involving the administration of oral CBD for at least one week in healthy subjects and in clinical populations, including patients with epilepsy, PD, social anxiety disorder, and psychosis [85]. A higher incidence of serious AEs was observed in epilepsy RCTs. Somnolence, sedation, the elevation of liver transaminase level, skin rash, and respiratory disturbances were the most commonly reported serious AEs. These effects seem to be related to DDIs, especially with valproate in the case of increased liver transaminase level and with clobazam in the case of sedation and somnolence. No significant serious AEs were found in studies conducted on healthy subjects or patients with PD, Huntington’s disease, type 2 diabetes, Crohn’s disorder, and social anxiety disorders, even considering the concomitant medications [85]. These findings were further confirmed by a systematic review and metanalysis of CBD AEs across all medical indications suggesting that CBD is well tolerated and induces few serious AEs, whose rate increases when combined with other drugs in childhood epilepsy populations [86]. Finally, the pharmacokinetic interaction between CBD and mTOR inhibitors has been evaluated only in one retrospective chart review. The plasma level of everolimus and sirolimus increased significantly when taken with CBD [44]. However, the overall severity and prevalence of AEs were similar to those identified in RCTs. Further research and clinical studies should evaluate the pharmacokinetic interactions of CBD with several classes of medications, as the clinical indication of CBD is expanding. New therapeutic drug monitoring methods should be developed to help clinicians in the clinical management of patients. Indeed, more accurate detection of CBD and its metabolites may help the dose adjustments and reduce the rate of AEs especially when CBD is co-administered with other drugs [87, 88].
Critical factors to evaluate CBD safety and tolerability are the dose, the route of administration, and the frequency of use. In the included trials, the CBD dose ranged from 2.5 to 50 mg/kg/day as a single or multiple administration. Importantly, compared to our previous review on CBD toxicity and AEs [28], more data are available on long-term CBD use, particularly in clinical trials in epileptic children and adults for up to 36 months. Moreover, clinical studies have focused on CBD antiseizure properties in other forms of refractory childhood epilepsy, expanding its therapeutic application.
Only three-open label studies aimed at evaluating CBD effects on cognition. Oral CBD, at the common dosages used in clinical trials, seems to not affect cognitive functions. These preliminary findings may pave the way for an expansion of CBD clinical indications. Interestingly, CBD has recently been under investigation as a potential add-on treatment for neurological disorders, such as PD and AD. In a study involving PD patients with rapid eye movement behavior disorder, although CBD was ineffective to reach the primary outcome, it showed a good safety profile with no significant AEs compared to a placebo. Similar findings were reported by studies investigating the therapeutic role of CBD in the adult cohort population with anxiety disorders, addictions, and chronic pain conditions.
Most of the included studies have used the highly purified formulation of CBD Epidiolex that is administered orally. Few well-designed clinical trials are still available on different formulations and routes of administration. In studies using a transdermal gel formulation, CBD dose ranged from 50 to 500 mg/day. Most patients experienced at least one AE of mild or moderate severity. No significant serious AEs were reported. The most common AEs were gastrointestinal and respiratory disturbances and somnolence. An increased rate of skin rash compared to studies using oral CBD was reported. Only single, open-label studies evaluated different formulations of CBD, including smoking CBD cigarettes, liquid spray, and sublingual wafers, reporting an acceptable safety profile. However, further well-designed and controlled trials are needed to support the therapeutic use of these CBD formulations for medical purposes.
A concerning issue in the CBD field is the availability of over-the-counter (OTC) CBD products, which have not been evaluated in clinical trials. OTC preparations may contain CBD at different doses along with other cannabinoids and/or other contaminants [89-91]. This issue requires more research, as the market of OTC CBD products is poorly regulated.
CONCLUSION
CBD has shown a well-tolerated safety profile with few AEs. However, its ability to inhibit the hepatic metabolism of other drugs, such as clobazam, valproate, and stiripentol, can potentially cause AEs. Indeed, an increased rate of AEs, serious AEs, and discontinuation of treatment has been demonstrated in several clinical trials focusing on TRE in children. Data from clinical studies involving adult subjects with disease conditions are still limited. Therefore, additional data on CBD tolerability and safety from clinical studies outside childhood epilepsy syndromes, studies involving subjects with different medical conditions, and studies of over-the-counter CBD products are needed.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s Disease
- AEs
Adverse Events
- AEDs
Antiepileptic Drugs
- CBD
Cannabidiol
- CYP
Cytochrome P450
- DEE
Developmental and Epileptic Encephalopathy
- DDI
Drug-drug Interaction
- DS
Dravet Syndrome
- EMA
European Medicine Agency
- ES
Epileptic Spasm
- FDA
US Food and Drug Administration
- LGS
Lennox-gastaut Syndrome
- PD
Parkinson’s Disease
- OLE
Open-label Extension
- OTC
Over-the-counter
- PPAR-γ
Peroxisome Proliferator-activated Receptor-gamma
- RBD
REM Behaviour Disorder
- RCT
Randomized Controlled Trial
- THC
Δ9-Tetrahydrocannabinol
- TRE
Treatment-resistant Epilepsy
- TSC
Tuberous Sclerosis Complex
- UGT
5'-diphosphoglucuronosyltransferase
CONSENT FOR PUBLICATION
Not applicable.
STANDARDS OF REPORTING
PRISMA guidelines and methodology were followed.
FUNDING
None.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
SUPPLEMENTARY MATERIAL
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.
REFERENCES
- 1.Zuardi A.W., Crippa J.A.S., Hallak J.E.C., Moreira F.A., Guimarães F.S. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med. Biol. Res. 2006;39(4):421–429. doi: 10.1590/S0100-879X2006000400001. [DOI] [PubMed] [Google Scholar]
- 2.Crippa J.A., Guimarães F.S., Campos A.C., Zuardi A.W. Translational investigation of the therapeutic potential of cannabidiol (CBD): Toward a new age. Front. Immunol. 2018;9:2009. doi: 10.3389/fimmu.2018.02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol. Rev. 2016;96(4):1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 4.Lafaye G., Desterke C., Marulaz L., Benyamina A. Cannabidiol affects circadian clock core complex and its regulation in microglia cells. Addict. Biol. 2019;24(5):921–934. doi: 10.1111/adb.12660. [DOI] [PubMed] [Google Scholar]
- 5.Devane W.A., Hanuš L., Breuer A., Pertwee R.G., Stevenson L.A., Griffin G., Gibson D., Mandelbaum A., Etinger A., Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science (80-. ) 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Laprairie R.B., Bagher A.M., Kelly M.E.M., Denovan-Wright E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br. J. Pharmacol. 2015;172(20):4790–4805. doi: 10.1111/bph.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Straiker A., Dvorakova M., Zimmowitch A., Mackie K. Cannabidiol inhibits endocannabinoid signaling in autaptic hippocampal neurons. Mol. Pharmacol. 2018;94(1):743–748. doi: 10.1124/mol.118.111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tham M., Yilmaz O., Alaverdashvili M., Kelly M.E.M., Denovan-Wright E.M., Laprairie R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019;176(10):1455–1469. doi: 10.1111/bph.14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mechoulam R., Peters M., Murillo-Rodriguez E., Hanuš L.O. Cannabidiol-recent advances. Chem. Biodivers. 2007;4(8):1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- 10.Vallée A., Lecarpentier Y., Guillevin R., Vallée J.N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017;49(10):853–866. doi: 10.1093/abbs/gmx073. [DOI] [PubMed] [Google Scholar]
- 11.Franco R., Villa M., Morales P., Reyes-Resina I., Gutiérrez-Rodríguez A., Jiménez J., Jagerovic N., Martínez-Orgado J., Navarro G. Increased expression of cannabinoid CB2 and serotonin 5-HT1A heteroreceptor complexes in a model of newborn hypoxic-ischemic brain damage. Neuropharmacology. 2019;152:58–66. doi: 10.1016/j.neuropharm.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 12.Campos A.C., Guimarães F.S. Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2009;33(8):1517–1521. doi: 10.1016/j.pnpbp.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 13.Wise J. European drug agency approves cannabis-based medicine for severe forms of epilepsy. BMJ. 2019;366:l5708. doi: 10.1136/bmj.l5708. [DOI] [PubMed] [Google Scholar]
- 14.Jadoon K.A., Ratcliffe S.H., Barrett D.A., Thomas E.L., Stott C., Bell J.D., O’Sullivan S.E., Tan G.D. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with Type 2 Diabetes: A randomized, double-blind, placebo-controlled, parallel group pilot study. Diabetes Care. 2016;39(10):1777–1786. doi: 10.2337/dc16-0650. [DOI] [PubMed] [Google Scholar]
- 15.Study to Evaluate the Effect of GWP42003 on Liver Fat Levels in Participants With Fatty Liver Disease. NCT01284634. 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT01284634.
- 16.Suraev A., Grunstein R.R., Marshall N.S., D’Rozario A.L., Gordon C.J., Bartlett D.J., Wong K., Yee B.J., Vandrey R., Irwin C., Arnold J.C., McGregor I.S., Hoyos C.M. Cannabidiol (CBD) and Δ9-tetrahydrocannabinol (THC) for chronic insomnia disorder (‘CANSLEEP’ trial): protocol for a randomised, placebo-controlled, double-blinded, proof-of-concept trial. BMJ Open. 2020;10(5):e034421. doi: 10.1136/bmjopen-2019-034421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherner M. CBD for Sleep in People With HIV. NCT05097651. 2021. Available from: https://clinicaltrials.gov/ct2/show/NCT05097651.
- 18.McGuire P., Robson P., Jerzy Cubala W., Vasile D., Dugald Morrison P., Barron R., Taylor A., Wright S. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: A multicenter randomized controlled trial. Am J Psychiatry. 2018;175(3):225–231. doi: 10.1176/appi.ajp.2017.17030325. [DOI] [PubMed] [Google Scholar]
- 19.Köck P., Lang E., Trulley V.N., Dechent F., Mercer-Chalmers-Bender K., Frei P., Huber C., Borgwardt S. Cannabidiol cigarettes as adjunctive treatment for psychotic disorders - a randomized, open-label pilot-study. Front. Psychiatry. 2021;12:736822. doi: 10.3389/fpsyt.2021.736822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trauner D. Trial of Cannabidiol to Treat Severe Behavior Problems in Children With Autism. NCT04517799. 2022. Full Text View - ClinicalTrials Available from: https://clinicaltrials.gov/ct2/show/NCT04517799.
- 21.Tartaglia N.R., Sannar E. CASCADE: CAnnabidiol Study in Children With Autism Spectrum DisordEr. NCT04520685. 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT04520685.
- 22.Arout C.A., Haney M., Herrmann E.S., Bedi G., Cooper Z.D. A placebo‐controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br. J. Clin. Pharmacol. 2022;88(1):347–355. doi: 10.1111/bcp.14973. [DOI] [PubMed] [Google Scholar]
- 23.Zuardi A.W. Cannabidiol: from an inactive cannabinoid to a drug with wide spectrum of action. Rev. Bras. Psiquiatr. 2008;30(3):271–280. doi: 10.1590/S1516-44462008000300015. [DOI] [PubMed] [Google Scholar]
- 24.Almeida C.M.O., Brito M.M.C., Bosaipo N.B., Pimentel A.V., Tumas V., Zuardi A.W., Crippa J.A.S., Hallak J.E.C., Eckeli A.L. Cannabidiol for rapid eye movement sleep behavior disorder. Mov. Disord. 2021;36(7):1711–1715. doi: 10.1002/mds.28577. [DOI] [PubMed] [Google Scholar]
- 25.Pautex S., Bianchi F., Daali Y., Augsburger M., de Saussure C., Wampfler J., Curtin F., Desmeules J., Broers B. Cannabinoids for behavioral symptoms in severe dementia: Safety and feasibility in a long-term pilot observational study in nineteen patients. Front. Aging Neurosci. 2022;14:957665. doi: 10.3389/fnagi.2022.957665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ISRCTN - ISRCTN87895237: Multi-centre trial of cannabidiol (CBD) for the treatment of Parkinson’s disease psychosis. ISRCTN87895237. 2023. Available from: https://www.isrctn.com/ISRCTN87895237.
- 27.Freeman T.P., Hindocha C., Green S.F., Bloomfield M.A.P. Medicinal use of cannabis based products and cannabinoids. BMJ. 2019;365:l1141. doi: 10.1136/bmj.l1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huestis M.A., Solimini R., Pichini S., Pacifici R., Carlier J., Busardò F.P. Cannabidiol adverse effects and toxicity. Curr. Neuropharmacol. 2019;17(10):974–989. doi: 10.2174/1570159X17666190603171901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., Chou R., Glanville J., Grimshaw J.M., Hróbjartsson A., Lalu M.M., Li T., Loder E.W., Mayo-Wilson E., McDonald S., McGuinness L.A., Stewart L.A., Thomas J., Tricco A.C., Welch V.A., Whiting P., Moher D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Metternich B., Wagner K., Geiger M.J., Hirsch M., Schulze-Bonhage A., Klotz K.A. Cognitive and behavioral effects of cannabidiol in patients with treatment-resistant epilepsy. Epilepsy Behav. 2021;114(Pt A):107558. doi: 10.1016/j.yebeh.2020.107558. [DOI] [PubMed] [Google Scholar]
- 31.Laux L.C., Bebin E.M., Checketts D., Chez M., Flamini R., Marsh E.D., Miller I., Nichol K., Park Y., Segal E., Seltzer L., Szaflarski J.P., Thiele E.A., Weinstock A. Long-term safety and efficacy of cannabidiol in children and adults with treatment resistant Lennox-Gastaut syndrome or Dravet syndrome: Expanded access program results. Epilepsy Res. 2019;154:13–20. doi: 10.1016/j.eplepsyres.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Gaston T.E., Ampah S.B., Martina Bebin E., Grayson L.P., Cutter G.R., Hernando K., Szaflarski J.P. Long-term safety and efficacy of highly purified cannabidiol for treatment refractory epilepsy. Epilepsy Behav. 2021;117:107862. doi: 10.1016/j.yebeh.2021.107862. [DOI] [PubMed] [Google Scholar]
- 33.Martin R.C., Gaston T.E., Thompson M., Ampah S.B., Cutter G., Bebin E.M., Szaflarski J.P. Cognitive functioning following long-term cannabidiol use in adults with treatment-resistant epilepsy. Epilepsy Behav. 2019;97:105–110. doi: 10.1016/j.yebeh.2019.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Herlopian A., Hess E.J., Barnett J., Geffrey A.L., Pollack S.F., Skirvin L., Bruno P., Sourbron J., Thiele E.A. Cannabidiol in treatment of refractory epileptic spasms: An open-label study. Epilepsy Behav. 2020;106:106988. doi: 10.1016/j.yebeh.2020.106988. [DOI] [PubMed] [Google Scholar]
- 35.Nenert R., Allendorfer J.B., Bebin E.M., Gaston T.E., Grayson L.E., Houston J.T., Szaflarski J.P. Cannabidiol normalizes resting-state functional connectivity in treatment-resistant epilepsy. Epilepsy Behav. 2020;112:107297. doi: 10.1016/j.yebeh.2020.107297. [DOI] [PubMed] [Google Scholar]
- 36.Thompson M.D., Martin R.C., Grayson L.P., Ampah S.B., Cutter G., Szaflarski J.P., Bebin E.M. Cognitive function and adaptive skills after a one-year trial of cannabidiol (CBD) in a pediatric sample with treatment-resistant epilepsy. Epilepsy Behav. 2020;111:107299. doi: 10.1016/j.yebeh.2020.107299. [DOI] [PubMed] [Google Scholar]
- 37.Scheffer I.E., Hulihan J., Messenheimer J., Ali S., Keenan N., Griesser J., Gutterman D.L., Sebree T., Sadleir L.G. Safety and tolerability of transdermal cannabidiol gel in children with developmental and epileptic encephalopathies. JAMA Netw. Open. 2021;4(9):e2123930. doi: 10.1001/jamanetworkopen.2021.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pietrafusa N., Ferretti A., Trivisano M., de Palma L., Calabrese C., Carfì Pavia G., Tondo I., Cappelletti S., Vigevano F., Specchio N. Purified cannabidiol for treatment of refractory epilepsies in pediatric patients with developmental and epileptic encephalopathy. P ediatr. Drugs. 2019;214(21):283–290. doi: 10.1007/s40272-019-00341-x. [DOI] [PubMed] [Google Scholar]
- 39.Caraballo R., Demirdjian G., Reyes G., Huaman M., Gutierrez R. Effectiveness of cannabidiol in a prospective cohort of children with drug-resistant epileptic encephalopathy in Argentina. Seizure. 2020;80:75–80. doi: 10.1016/j.seizure.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 40.Patel A.D., Mazurkiewicz-Bełdzińska M., Chin R.F., Gil-Nagel A., Gunning B., Halford J.J., Mitchell W., Scott Perry M., Thiele E.A., Weinstock A., Dunayevich E., Checketts D., Devinsky O. Long‐term safety and efficacy of add‐on cannabidiol in patients with Lennox-Gastaut syndrome: Results of a long‐term open‐label extension trial. Epilepsia. 2021;62(9):2228–2239. doi: 10.1111/epi.17000. [DOI] [PubMed] [Google Scholar]
- 41.Thiele E., Marsh E., Mazurkiewicz-Beldzinska M., Halford J.J., Gunning B., Devinsky O., Checketts D., Roberts C. Cannabidiol in patients with Lennox-Gastaut syndrome: Interim analysis of an open-label extension study. Epilepsia. 2019;60(3):419–428. doi: 10.1111/epi.14670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park Y.D., Linder D.F., Pope J., Flamini J.R., Moretz K., Diamond M.P., Long S.A. Long-term efficacy and safety of cannabidiol (CBD) in children with treatment-resistant epilepsy: Results from a state-based expanded access program. Epilepsy Behav. 2020;112:107474. doi: 10.1016/j.yebeh.2020.107474. [DOI] [PubMed] [Google Scholar]
- 43.Thiele E.A., Bebin E.M., Filloux F., Kwan P., Loftus R., Sahebkar F., Sparagana S., Wheless J. Long‐term cannabidiol treatment for seizures in patients with tuberous sclerosis complex: An open‐label extension trial. Epilepsia. 2022;63(2):426–439. doi: 10.1111/epi.17150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ebrahimi-Fakhari D., Agricola K.D., Tudor C., Krueger D., Franz D.N. Cannabidiol elevates mechanistic target of rapamycin inhibitor levels in patients with tuberous sclerosis complex. Pediatr. Neurol. 2020;105:59–61. doi: 10.1016/j.pediatrneurol.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Koo C.M., Kim S.H., Lee J.S., Park B.J., Lee H.K., Kim H.D., Kang H.C. Cannabidiol for treating lennox-gastaut syndrome and dravet syndrome in Korea. J. Korean Med. Sci. 2020;35(50):e427. doi: 10.3346/jkms.2020.35.e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thiele E.A., Bebin E.M., Bhathal H., Jansen F.E., Kotulska K., Lawson J.A., O’Callaghan F.J., Wong M., Sahebkar F., Checketts D., Knappertz V., Archer J., Arndt D.H., Barron T., Bebin E.M., Bhathal H., Cantarín-Extremera V., Sanchez-Carpintero R., Ciliberto M.A., Cock H., De Wit M-C.Y., Devinsky O., Falip M., Filloux F.M., Fountain N.B., Gawlowicz J., Greenwood R.S., Hamandi K., Jansen F.E., Joshi C., Józwiak S., Klein P., Kotulska K., Kwan P., Lawson J.A., Lisewski P., Miller I.O., Morse R.P., Mostajelean A.S., Nolan D.A., O’Brien T.J., O’Callaghan F.J., Paredes F., Perry M.S., Ramos F.J., Reutens D., Roberts C.M., Saneto R.P., Sharp G.B., Saxena A., Sparagana S.P., Tatachar P., Thiele E.A., Wheless J.W., Wirrell E.C., Wong M.H., Wong M., Wu J.Y., Zolnowska M. Add-on cannabidiol treatment for drug-resistant seizures in tuberous sclerosis complex. JAMA Neurol. 2021;78(3):285–292. doi: 10.1001/jamaneurol.2020.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devinsky O., Nabbout R., Miller I., Laux L., Zolnowska M., Wright S., Roberts C. Long‐term cannabidiol treatment in patients with Dravet syndrome: An open‐label extension trial. Epilepsia. 2019;60(2):294–302. doi: 10.1111/epi.14628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Brien T.J., Berkovic S.F., French J.A., Messenheimer J.A., Sebree T.B., Bonn-Miller M.O., Gutterman D.L., Wijayath M., Patrikios P., Reutens D., Frasca J., Seneviratne U., D’Souza W., Bergin P., Anderson T., Rosemergy I., Nikpour A., Kwan P., Asztely F., Somerville E. Adjunctive transdermal cannabidiol for adults with focal epilepsy. JAMA Netw. Open. 2022;5(7):e2220189. doi: 10.1001/jamanetworkopen.2022.20189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.VanLandingham K.E., Crockett J., Taylor L., Morrison G. A phase 2, double‐blind, placebo‐controlled trial to investigate potential drug‐drug interactions between cannabidiol and clobazam. J. Clin. Pharmacol. 2020;60(10):1304–1313. doi: 10.1002/jcph.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ben-Menachem E., Gunning B., Arenas Cabrera C.M., Van-Landingham K., Crockett J., Critchley D., Wray L., Tayo B., Morrison G., Toledo M. A phase II randomized trial to explore the potential for pharmacokinetic drug-drug interactions with stiripentol or valproate when combined with cannabidiol in patients with epilepsy. CNS Drugs. 2020;34(6):661–672. doi: 10.1007/s40263-020-00726-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miller I., Scheffer I.E., Gunning B., Sanchez-Carpintero R., Gil-Nagel A., Perry M.S., Saneto R.P., Checketts D., Dunayevich E., Knappertz V. Dose-ranging effect of adjunctive oral cannabidiol vs. placebo on convulsive seizure frequency in dravet syndrome. JAMA Neurol. 2020;77(5):613–621. doi: 10.1001/jamaneurol.2020.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scheffer I.E., Halford J.J., Miller I., Nabbout R., Sanchez-Carpintero R., Shiloh-Malawsky Y., Wong M., Zolnowska M., Checketts D., Dunayevich E., Devinsky O. Add‐on cannabidiol in patients with Dravet syndrome: Results of a long‐term open‐label extension trial. Epilepsia. 2021;62(10):2505–2517. doi: 10.1111/epi.17036. [DOI] [PubMed] [Google Scholar]
- 53.Caraballo R., Valenzuela G.R. Cannabidiol-enriched medical cannabis as add-on therapy in children with treatment-resistant West syndrome: A study of eight patients. Seizure. 2021;92:238–243. doi: 10.1016/j.seizure.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 54.Heussler H., Cohen J., Silove N., Tich N., Bonn-Miller M.O., Du W., O’Neill C., Sebree T. A phase 1/2, open-label assessment of the safety, tolerability, and efficacy of transdermal cannabidiol (ZYN002) for the treatment of pediatric fragile X syndrome. J. Neurodev. Disord. 2019;11(1):16. doi: 10.1186/s11689-019-9277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuchenbuch M., D’Onofrio G., Chemaly N., Barcia G., Teng T., Nabbout R. Add‐on cannabidiol significantly decreases seizures in 3 patients with SYNGAP1 developmental and epileptic encephalopathy. Epilepsia Open. 2020;5(3):496–500. doi: 10.1002/epi4.12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheless J.W., Dlugos D., Miller I. Pharmacokinetics and tolerability of multiple doses of pharmaceutical-grade synthetic cannabidiol in pediatric patients with treatment-resistant epilepsy. CNS Drugs. 123AD:33. doi: 10.1007/s40263-019-00624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Freeman T.P., Hindocha C., Baio G., Shaban N.D.C., Thomas E.M., Astbury D., Freeman A.M., Lees R., Craft S., Morrison P.D., Bloomfield M.A.P., O’Ryan D., Kinghorn J., Morgan C.J.A., Mofeez A., Curran H.V. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double-blind, placebo-controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7(10):865–874. doi: 10.1016/S2215-0366(20)30290-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kwee C.M.B., Baas J.M.P., van der Flier F.E., Groenink L., Duits P., Eikelenboom M., van der Veen D.C., Moerbeek M., Batelaan N.M., van Balkom A.J.L.M., Cath D.C. Cannabidiol enhancement of exposure therapy in treatment refractory patients with social anxiety disorder and panic disorder with agoraphobia: A randomised controlled trial. Eur. Neuropsychopharmacol. 2022;59:58–67. doi: 10.1016/j.euroneuro.2022.04.003. [DOI] [PubMed] [Google Scholar]
- 59.Efron D., Freeman J.L., Cranswick N., Payne J.M., Mulraney M., Prakash C., Lee K.J., Taylor K., Williams K. A pilot randomised placebo‐controlled trial of cannabidiol to reduce severe behavioural problems in children and adolescents with intellectual disability. Br. J. Clin. Pharmacol. 2021;87(2):436–446. doi: 10.1111/bcp.14399. [DOI] [PubMed] [Google Scholar]
- 60.Hurd Y.L., Spriggs S., Alishayev J., Winkel G., Gurgov K., Kudrich C., Oprescu A.M., Salsitz E. Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: A double-blind randomized placebo-controlled trial. Am. J. Psychiatry. 2019;176(11):911–922. doi: 10.1176/appi.ajp.2019.18101191. [DOI] [PubMed] [Google Scholar]
- 61.Meneses-Gaya C., Crippa J.A., Hallak J.E., Miguel A.Q., Laranjeira R., Bressan R.A., Zuardi A.W., Lacerda A.L. Cannabidiol for the treatment of crack-cocaine craving: An exploratory double-blind study. Br. J. Psychiatry. 2021;43(5):467–476. doi: 10.1590/1516-4446-2020-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crippa J.A.S., Pacheco J.C., Zuardi A.W., Guimarães F.S., Campos A.C., Osório F.L., Loureiro S.R., dos Santos R.G., Souza J.D.S., Ushirohira J.M., Ferreira R.R., Mancini Costa K.C., Scomparin D.S., Scarante F.F., Pires-Dos-Santos I., Mechoulam R., Kapczinski F., Fonseca B.A.L., Esposito D.L.A., Passos A.D.C., Dal Fabbro A.L., Bellissimo-Rodrigues F., Arruda E., Scarpelini S., Andraus M.H., Nather Junior, J.C., Wada D.T., Koenigkam-Santos M., Santos A.C., Busatto Filho G., Hallak J.E.C. Cannabidiol for COVID-19 patients with mild to moderate symptoms (CANDIDATE Study): A randomized, double-blind, placebo-controlled clinical trial. Cannabis Cannabinoid Res. 2022;7(5):658–669. doi: 10.1089/can.2021.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger M., Li E., Rice S., Davey C.G., Ratheesh A., Adams S., Jackson H., Hetrick S., Parker A., Spelman T., Kevin R., McGregor I.S., McGorry P., Amminger G.P. Cannabidiol for treatment-resistant anxiety disorders in young people. J. Clin. Psychiatry. 2022;83(5):21m14130. doi: 10.4088/JCP.21m14130. [DOI] [PubMed] [Google Scholar]
- 64.Suzuki J., Martin B., Prostko S., Chai P.R., Weiss R.D. Cannabidiol effect on cue-induced craving for individuals with opioid use disorder treated with buprenorphine: A small proof-of-concept open-label study. Integr. Med. Res. 2022;1(1):157–163. doi: 10.1089/imr.2022.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elms L., Shannon S., Hughes S., Lewis N. Cannabidiol in the treatment of post-traumatic stress disorder: A case series. J. Altern. Complement. Med. 2019;25(4):392–397. doi: 10.1089/acm.2018.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Crippa J.A.S., Zuardi A.W., Guimarães F.S., Campos A.C., de Lima Osório F., Loureiro S.R., dos Santos R.G., Souza J.D.S., Ushirohira J.M., Pacheco J.C., Ferreira R.R., Mancini Costa K.C., Scomparin D.S., Scarante F.F., Pires-Dos-Santos I., Mechoulam R., Kapczinski F., Fonseca B.A.L., Esposito D.L.A., Pereira-Lima K., Sen S., Andraus M.H., Hallak J.E.C., Litcanov D.C., Rodrigues L., Alves T.F., Coutinho B.M. Efficacy and safety of cannabidiol plus standard care vs. standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID-19 pandemic. JAMA Netw. Open. 2021;4(8):e2120603. doi: 10.1001/jamanetworkopen.2021.20603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paton W.D.M., Pertwee R.G. Effect of cannabis and certain of its constituents on pentobarbitone sleeping time and phenazone metabolism. Br. J. Pharmacol. 1972;44(2):250–261. doi: 10.1111/j.1476-5381.1972.tb07261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Devinsky O., Cilio M.R., Cross H., Fernandez-Ruiz J., French J., Hill C., Katz R., Di Marzo V., Jutras-Aswad D., Notcutt W.G., Martinez-Orgado J., Robson P.J., Rohrback B.G., Thiele E., Whalley B., Friedman D. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia. 2014;55(6):791–802. doi: 10.1111/epi.12631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pertwee R.G. Cannabinoid pharmacology: the first 66 years. Br. J. Pharmacol. 2006;147(S1) Suppl. 1:S163–S171. doi: 10.1038/sj.bjp.0706406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Abbotts K.S.S., Ewell T.R., Butterklee H.M., Bomar M.C., Akagi N., Dooley G.P., Bell C. Cannabidiol and cannabidiol metabolites: Pharmacokinetics, interaction with food, and influence on liver function. Nutrients. 2022;14(10):2152. doi: 10.3390/nu14102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Crockett J., Critchley D., Tayo B., Berwaerts J., Morrison G. A phase 1, randomized, pharmacokinetic trial of the effect of different meal compositions, whole milk, and alcohol on cannabidiol exposure and safety in healthy subjects. Epilepsia. 2020;61(2):267–277. doi: 10.1111/epi.16419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hosseini A., McLachlan A.J., Lickliter J.D. A phase I trial of the safety, tolerability and pharmacokinetics of cannabidiol administered as single‐dose oil solution and single and multiple doses of a sublingual wafer in healthy volunteers. Br. J. Clin. Pharmacol. 2021;87(4):2070–2077. doi: 10.1111/bcp.14617. [DOI] [PubMed] [Google Scholar]
- 73.Perkins D., Butler J., Ong K., Nguyen T.H., Cox S., Francis B., Mcintosh M., Lilley B. A Phase 1, randomised, placebo-controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. Eur. J. Drug Metab. Pharmacokinet. 2020;45(5):575–586. doi: 10.1007/s13318-020-00624-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taylor L., Crockett J., Tayo B., Checketts D., Sommerville K. Abrupt withdrawal of cannabidiol (CBD): A randomized trial. Epilepsy Behav. 2020;104(Pt A):106938. doi: 10.1016/j.yebeh.2020.106938. [DOI] [PubMed] [Google Scholar]
- 75.Thai C., Tayo B., Critchley D. A phase 1 open‐label, fixed‐sequence pharmacokinetic drug interaction trial to investigate the effect of cannabidiol on the cyp1a2 probe caffeine in healthy subjects. Clin. Pharmacol. Drug Dev. 2021;10(11):1279–1289. doi: 10.1002/cpdd.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Watkins P.B., Church R.J., Li J., Knappertz V. Cannabidiol and abnormal liver chemistries in healthy adults: Results of a phase i clinical trial. Clin. Pharmacol. Ther. 2021;109(5):1224–1231. doi: 10.1002/cpt.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Morrison G., Crockett J., Blakey G., Sommerville K. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin. Pharmacol. Drug Dev. 2019;8(8):1009–1031. doi: 10.1002/cpdd.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor L., Crockett J., Tayo B., Morrison G. A phase 1, open‐label, parallel‐group, single‐dose trial of the pharmacokinetics and safety of Cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J. Clin. Pharmacol. 2019;59(8):1110–1119. doi: 10.1002/jcph.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tayo B., Taylor L., Sahebkar F., Morrison G. A phase i, open-label, parallel-group, single-dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin. Pharmacokinet. 2020;59(6):747–755. doi: 10.1007/s40262-019-00841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alaia M.J., Hurley E.T., Vasavada K., Markus D.H., Britton B., Gonzalez-Lomas G., Rokito A.S., Jazrawi L.M., Kaplan K. Buccally absorbed cannabidiol shows significantly superior pain control and improved satisfaction immediately after arthroscopic rotator cuff repair: A placebo-controlled, double-blinded, randomized trial. Am. J. Sports Med. 2022;50(11):3056–3063. doi: 10.1177/03635465221109573. [DOI] [PubMed] [Google Scholar]
- 81.Vela J., Dreyer L., Petersen K.K., Arendt-Nielsen L., Duch K.S., Kristensen S. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: A randomized, double-blind, placebo-controlled trial. Pain. 2022;163(6):1206–1214. doi: 10.1097/j.pain.0000000000002466. [DOI] [PubMed] [Google Scholar]
- 82.Heineman J.T., Forster G.L., Stephens K.L., Cottler P.S., Timko M.P., DeGeorge B.R., Jr A randomized controlled trial of topical cannabidiol for the treatment of thumb basal joint arthritis. J. Hand Surg. Am. 2022;47(7):611–620. doi: 10.1016/j.jhsa.2022.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Devinsky O., Thiele E.A., Wright S., Checketts D., Morrison G., Dunayevich E., Knappertz V. Cannabidiol efficacy independent of clobazam: Meta‐analysis of four randomized controlled trials. Acta Neurol. Scand. 2020;142(6):531–540. doi: 10.1111/ane.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gunning B., Mazurkiewicz-Bełdzińska M., Chin R.F.M., Bhathal H., Nortvedt C., Dunayevich E., Checketts D. Cannabidiol in conjunction with clobazam: Analysis of four randomized controlled trials. Acta Neurol. Scand. 2021;143(2):154–163. doi: 10.1111/ane.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dos Santos R.G., Guimarães F.S., Crippa J.A.S., Hallak J.E.C., Rossi G.N., Rocha J.M., Zuardi A.W. Serious adverse effects of cannabidiol (CBD): A review of randomized controlled trials. Expert Opin. Drug Metab. Toxicol. 2020;16(6):517–526. doi: 10.1080/17425255.2020.1754793. [DOI] [PubMed] [Google Scholar]
- 86.Chesney E., Oliver D., Green A., Sovi S., Wilson J., Englund A., Freeman T.P., McGuire P. Adverse effects of cannabidiol: A systematic review and meta-analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799–1806. doi: 10.1038/s41386-020-0667-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Malaca S., Gottardi M., Pigliasco F., Barco S., Cafaro A., Amadori E., Riva A., Marcenaro M., Striano P., Cangemi G., Pacifici R., Pichini S., Busardò F.P. UHPLC-MS/MS Analysis of cannabidiol and its metabolites in serum of patients with resistant epilepsy treated with CBD formulations. Pharmaceuticals. 2021;14(7):630. doi: 10.3390/ph14070630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pérez-Acevedo A., Busardò F., Pacifici R., Mannocchi G., Gottardi M., Poyatos L., Papaseit E., Pérez-Mañá C., Martin S., Di Trana A., Pichini S., Farré M. Disposition of cannabidiol metabolites in serum and urine from healthy individuals treated with pharmaceutical preparations of medical cannabis. Pharmaceuticals. 2020;13(12):459. doi: 10.3390/ph13120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brunetti P., Pichini S., Pacifici R., Busardò F.P., del Rio A. Herbal preparations of medical cannabis: A vademecum for prescribing doctors. Medicina. 2020;56(5):237. doi: 10.3390/medicina56050237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marchei E., Tittarelli R., Pellegrini M., Rotolo M.C., Pacifici R., Pichini S. Is “light cannabis” really light? Determination of cannabinoids content in commercial products. Clin. Chem. Lab. Med. (CCLM) 2020;58(9):e175–e177. doi: 10.1515/cclm-2020-0040. [DOI] [PubMed] [Google Scholar]
- 91.Pichini S., Mannocchi G., Berretta P., Zaami S., Pirani F., Pacifici R., Busardò F.P. Δ9-Tetrahydrocannabinol and cannabidiol time courses in the sera of “Light Cannabis” smokers: Discriminating light cannabis use from illegal and medical cannabis use. Ther. Drug Monit. 2020;42(1):151–156. doi: 10.1097/FTD.0000000000000683. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA checklist is available as supplementary material on the publisher’s website along with the published article.


