Abstract
Leaflet movements in Samanea saman are driven by the shrinking and swelling of cells in opposing (extensor and flexor) regions of the motor organ (pulvinus). Changes in cell volume, in turn, depend upon large changes in motor cell content of K+, Cl− and other ions. We performed patch-clamp experiments on extensor and flexor protoplasts, to determine whether their plasma membranes contain channels capable of carrying the large K+ currents that flow during leaflet movement. Recordings in the “whole-cell” mode reveal depolarization-activated K+ currents in extensor and flexor cells that increase slowly (t½ = ca. 2 seconds) and remain active for minutes. Recordings from excised patches reveal a single channel conductance of ca. 20 picosiemens in both cell types. The magnitude of the K+ currents is adequate to account quantitatively for K+ loss, previously measured in vivo during cell shrinkage. The K+ channel blockers tetraethylammonium (5 millimolar) or quinine (1 millimolar) blocked channel opening and decreased light- and dark-promoted movements of excised leaflets. These results provide evidence for the role of potassium channels in leaflet movement.
Full text
PDF

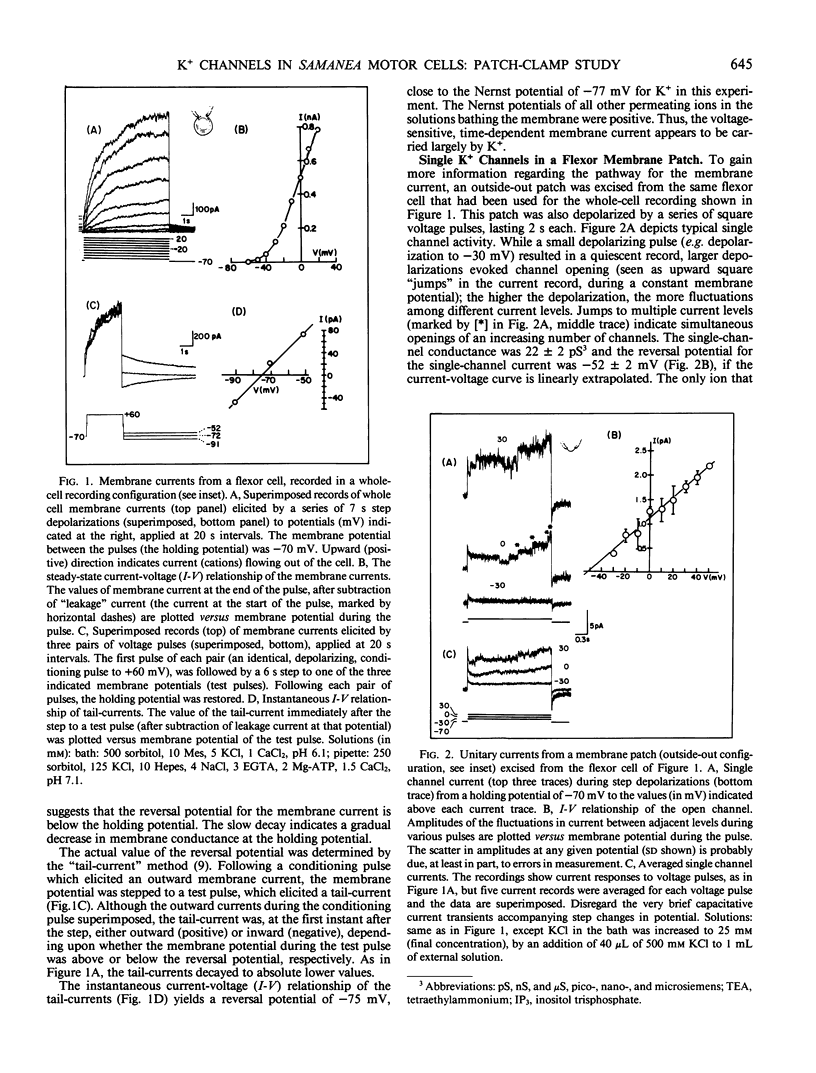
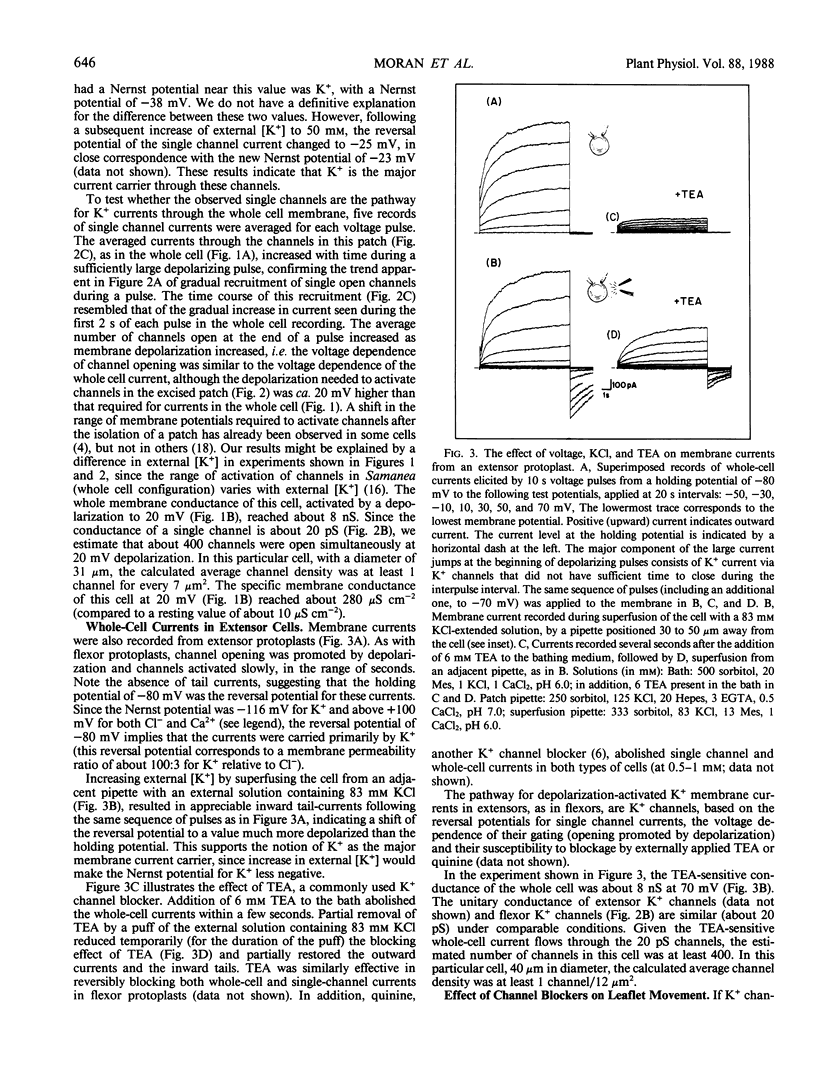
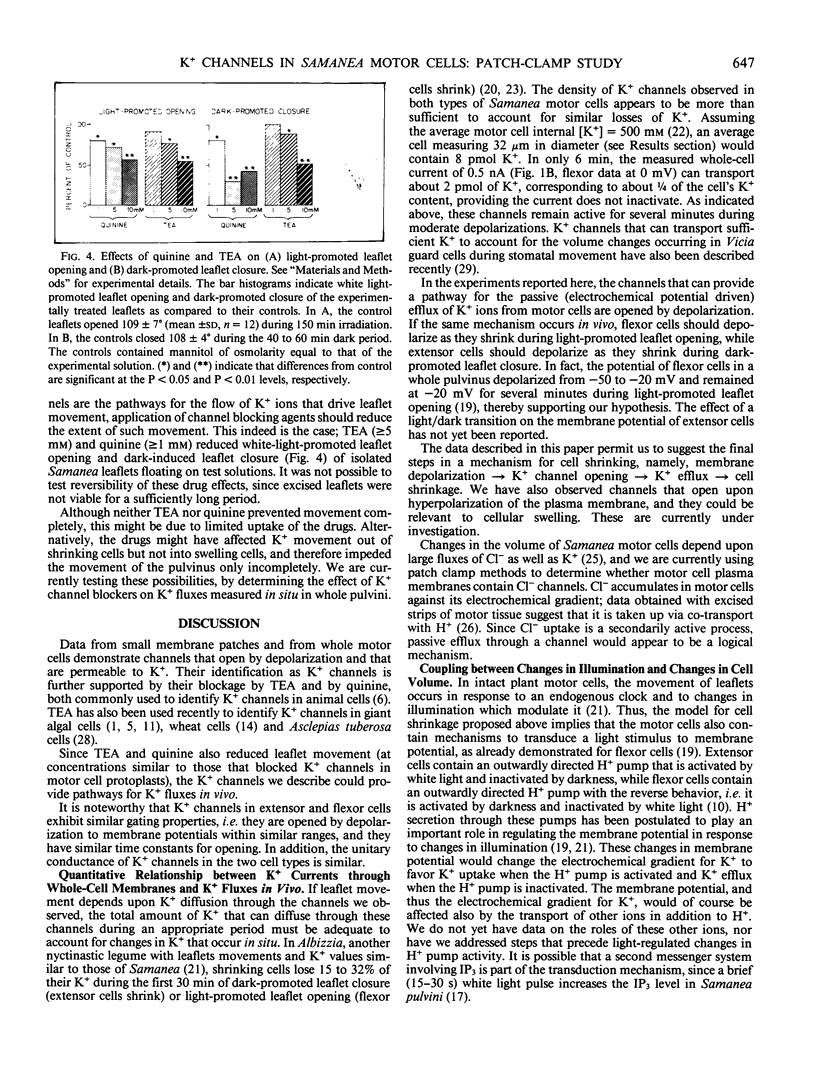

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards G. E., Robinson S. P., Tyler N. J., Walker D. A. Photosynthesis by isolated protoplasts, protoplast extracts, and chloroplasts of wheat: influence of orthophosphate, pyrophosphate, and adenylates. Plant Physiol. 1978 Aug;62(2):313–319. doi: 10.1104/pp.62.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman M. C., Spector I. Potassium current suppression by quinidine reveals additional calcium currents in neuroblastoma cells. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5245–5249. doi: 10.1073/pnas.78.8.5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton H. L., Satter R. L. Extensor and flexor protoplasts from samanea pulvini : I. Isolation and initial characterization. Plant Physiol. 1984 Nov;76(3):680–684. doi: 10.1104/pp.76.3.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iglesias A., Satter R. L. H fluxes in excised samanea motor tissue : I. Promotion by light. Plant Physiol. 1983 Jun;72(2):564–569. doi: 10.1104/pp.72.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer D. W., Lucas W. J. Potassium Channels in Chara corallina: CONTROL AND INTERACTION WITH THE ELECTROGENIC H PUMP. Plant Physiol. 1982 Apr;69(4):781–788. doi: 10.1104/pp.69.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran N., Ehrenstein G., Iwasa K., Bare C., Mischke C. Ion channels in plasmalemma of wheat protoplasts. Science. 1984 Nov 16;226(4676):835–838. doi: 10.1126/science.6093255. [DOI] [PubMed] [Google Scholar]
- Morse M. J., Crain R. C., Satter R. L. Light-stimulated inositolphospholipid turnover in Samanea saman leaf pulvini. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7075–7078. doi: 10.1073/pnas.84.20.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallotta B. S., Hepler J. R., Oglesby S. A., Harden T. K. A comparison of calcium-activated potassium channel currents in cell-attached and excised patches. J Gen Physiol. 1987 Jun;89(6):985–997. doi: 10.1085/jgp.89.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen R., Satter R. L. Rhythmic and phytochrome-regulated changes in transmembrane potential in samanea pulvini. Nature. 1975 May 29;255(5507):408–410. doi: 10.1038/255408a0. [DOI] [PubMed] [Google Scholar]
- Satter R. L., Galston A. W. Phytochrome-controlled Nyctinasty in Albizzia julibrissin: III. Interactions between an Endogenous Rhythm and Phytochrome in Control of Potassium Flux and Leaflet Movement. Plant Physiol. 1971 Dec;48(6):740–746. doi: 10.1104/pp.48.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Geballe G. T., Applewhite P. B., Galston A. W. Potassium flux and leaf movement in Samanea saman. I. Rhythmic movement. J Gen Physiol. 1974 Oct;64(4):413–430. doi: 10.1085/jgp.64.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Schrempf M., Chaudhri J., Galston A. W. Phytochrome and Circadian Clocks in Samanea: Rhythmic Redistribution of Potassium and Chloride within the Pulvinus during Long Dark Periods. Plant Physiol. 1977 Feb;59(2):231–235. doi: 10.1104/pp.59.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satter R. L., Xu Y., Depass A. Effects of Temperature on H Secretion and Uptake by Excised Flexor Cells during Dark-Induced Closure of Samanea Leaflets. Plant Physiol. 1987 Nov;85(3):850–855. doi: 10.1104/pp.85.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauf C. L., Wilson K. J. Effects of abscisic acid on K+ channels in Vicia faba guard cell protoplasts. Biochem Biophys Res Commun. 1987 May 29;145(1):284–290. doi: 10.1016/0006-291x(87)91318-0. [DOI] [PubMed] [Google Scholar]
- Schauf C. L., Wilson K. J. Properties of Single K and Cl Channels in Asclepias tuberosa Protoplasts. Plant Physiol. 1987 Oct;85(2):413–418. doi: 10.1104/pp.85.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J. I., Raschke K., Neher E. Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4108–4112. doi: 10.1073/pnas.84.12.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. I., Gulline H. F. Membrane changes in a circadian system. Nature. 1975 Mar 6;254(5495):69–70. doi: 10.1038/254069a0. [DOI] [PubMed] [Google Scholar]