Abstract
Autism spectrum disorder (ASD) is a cluster of heterogeneous neurodevelopmental conditions with atypical social communication and repetitive sensory-motor behaviors. The formation of new neurons from neural precursors in the hippocampus has been unequivocally demonstrated in the dentate gyrus of rodents and non-human primates. Accumulating evidence sheds light on how the deficits in the hippocampal neurogenesis may underlie some of the abnormal behavioral phenotypes in ASD. In this review, we describe the current evidence concerning pre-clinical and clinical studies supporting the significant role of hippocampal neurogenesis in ASD pathogenesis, discuss the possibility of improving hippocampal neurogenesis as a new strategy for treating ASD, and highlight the prospect of emerging pro‐neurogenic therapies for ASD.
Keywords: Autism, hippocampus, dentate gyrus, neurogenesis, stem cell, neurodevelopment
1. INTRODUCTION
Autism spectrum disorder (ASD) is a cluster of heterogeneous neurodevelopmental disorders characterized by core features in two aspects: social communication deficits and repetitive sensory-motor behaviors [1]. The past years have witnessed a dramatic increase in the prevalence of ASD; nowadays, 1 in every 59 children has been diagnosed with ASD in the United States [2], with males being four times more likely to be diagnosed than females [3]. Due to the provision of support to patients who cannot function independently, which leads to higher costs in healthcare and school education, ASD has become a substantial social and economic burden. Behavior interventions and pharmacological treatments have been applied to ASD patients, yet they are currently limited to the treatment of co-occurring behaviors and not for ASD core symptoms. The benefits of therapeutic strategies for individuals with ASD are still limited. Therefore, there is an urgent need to develop new therapeutic options.
Both genetic and environmental factors could contribute to the occurrence of ASD [4]. Pre-clinical research works using different animal ASD models and clinical neuroimaging have also indicated several brain regions involved in the pathology of ASD, including but not limited to the hippocampus, amygdala, fusiform gyrus, inferior frontal gyrus, superior temporal sulcus, cerebellum, sensory cortices, and prefrontal region [5, 6]. Impairments in neurogenesis, excitatory/inhibitory balance, neurotransmitters, immune response, and functional connection in the above brain regions have been suggested to contribute to the ASD phenotype. Of note, the hippocampus, a region responsible mainly for memory and cognition, spatial reasoning, and pattern separation, has drawn increasing attention in ASD research [7-9]. Studies have indicated the hippocampus’s involvement in social behavior, a core aspect of the ASD phenotype [10, 11]. Abnormalities of hippocampal structure and function have been shown in several studies to contribute to the development of ASD [12]. Moreover, the hippocampus reaches major developmental milestones around two years of age, just as ASD symptoms typically become increasingly apparent [13]. Therefore, the Autism and Developmental Disabilities Monitoring network recommends that children with ASD be assessed at 36 months and start to receive community support and services by 48 months [14].
One of the typical features of hippocampal development is neurogenesis, a pool of neural progenitor cells (NPCs) enriched in the subgranular zone (SGZ) of the dentate gyrus (DG) that keeps generating new neurons. This process could last throughout the life span [15]. Emerging evidence has proved that deficits in hippocampal neurogenesis are involved in cognitive dysfunction and mood disorder and are closely related to several psychiatric disorders, such as depression, anxiety disorders, and ASD [16]. Accumulating evidence has indicated that promoting hippocampal neurogenesis could improve neurological conditions, such as depression, amyotrophic lateral sclerosis, Alzheimer’s disease, spinal cord injury, or Parkinson’s disease [17, 18]. It seems to be a promising option to remodel impaired and immature neurons forming pathological brain structures in ASD. Simultaneously, there is increasing evidence of the pro-neurogenic treatment of ASD [19, 20].
In this review, we will focus on current advances in the involvement of hippocampal neurogenesis in the pathogenic mechanism of ASD, discuss the possibility of improving hippocampal neurogenesis as a new strategy for treating ASD, and highlight the prospect of aiming at regulating DG neurogenesis as a potential therapeutic strategy.
2. HIPPOCAMPAL NEUROGENESIS
The hippocampus is a bilateral brain structure and plays a critical role in episodic memory, spatial reasoning, and social interaction [11, 21]. Postnatal and adult hippocampal neurogenesis have been unequivocally demonstrated in the DG of rodents and non-human primates, providing a particular type of structural plasticity to the brain [22, 23]. The SGZ is regarded as a secondary germinal zone expanding rapidly during the early 3rd trimester of human gestational development [24-26]. After birth, the granule cell layer (GCL) of the DG of the newborn infant continues to grow and expand during the 1st three months of postnatal life, and it peaks in size during the first week of postnatal development in mice. Neurogenesis during the fetal and early postnatal periods forms the DG and sustains activity-dependent continuous neurogenesis [27]. This ongoing generation of new neurons declines in adulthood and drops in old age [28].
A multi-step process schematizes the neurogenic niche (Fig. 1). The radial-glia (RG) like NPCs (also known as type 1 cells) are located in the SGZ expressing specific markers, such as glial fibrillary acidic protein (GFAP), Nestin, SRY-box transcription factor 2 (SOX2), and brain lipid binding protein (BLBP) [29]. Type 1 cells undergo self-renewal and generate intermediate progenitors (Type 2 cells), consisting of non-fate-determined (Type 2a) and fate-determined (Type 2b). The intermediate progenitors then produce neuroblasts (Type 3), which are neuronally restricted and characterized by the expression of doublecortin (DCX), NeuroD1, and polysialic acid-neural cell adhesion molecule (PSA-NCAM), but capable of mitosis [30, 31]. Only a fraction of the neuroblasts lose their ability to divide; they survive and differentiate into dentate granule neurons that express phenotypic markers of mature neurons (neuronal nuclear antigen (NeuN), Calbindin, and Prox1) and send their dendrites into the molecular layer and their axons through the hilus toward the CA3 region to be integrated into existing neuronal circuits [32]. It takes approximately 30 days for a mature neuron to be generated from a Type 2a NPC. After producing multiple generations of neurons, the Type 1 and Type 2a progenitors start to produce astrocytes.
Fig. (1).
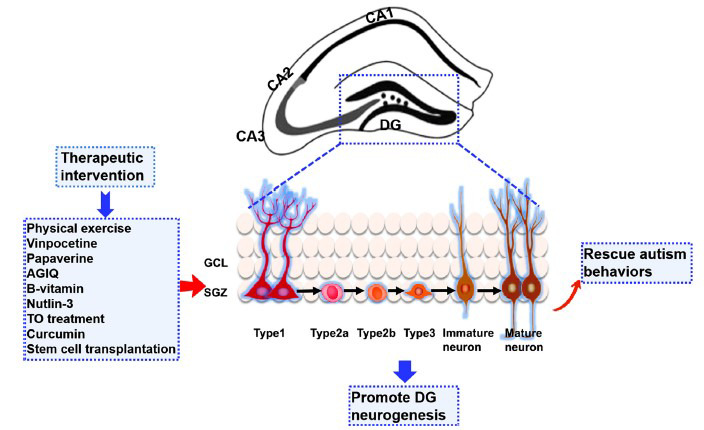
Multi-step processes involved in the DG neurogenesis and emerging pro‐neurogenic therapies for ASD.
It has been indicated that these produced new neurons play critical roles in hippocampal physiology and neuronal plasticity [33]. Importantly, the functional integration of newborn neurons generated in the postnatal and adult hippocampus links to essential functions in spatial processing and pattern separation, as well as cognitive flexibility and reversal learning [34]. Of note, extrinsic and intrinsic factors influence the highly dynamic process of adult neurogenesis. It has been confirmed that factors, such as brain-derived neurotrophic factor (BDNF) and DCX, play a critical role in neurogenesis, synapse formation, neuroplasticity, as well as neuronal survival, thus influencing repetitive behavior and stereotypy, as indicated by a clinical study on Korean males and animal study in the BTBR T+Itpr3tfJ (BTBR) mouse model of ASD [35, 36]. BDNF also plays a fundamental role in synaptic transmission and plasticity in the hippocampus, representing a key regulator for long-term potentiation (LTP), learning, and memory. Dysregulation of BDNF expression and signaling, inducing changes in neuronal maturation and plasticity, is a hallmark of several neurodevelopmental diseases, such as attention-deficit hyperactivity disorder (ADHD), Rett syndrome (RTT), and ASD, suggesting that neuronal malfunction present in these disorders is suspected to be related to excessive or reduced BDNF levels [37].
Several key signaling pathways, such as Notch, Sonic hedgehog (Shh), and Wnts, growth and neurotrophic factors, cytokines, transcription factors, as well as epigenetic modifications, have been indicated to exert a modulatory effect on the distinct steps of adult hippocampal neurogenesis [38-42]. In the postnatal and adult brains, the Notch signaling pathway, as a fundamental signaling system, together with the Wnt/β-catenin, bone morphogenetic proteins, and Shh molecular signaling pathways contribute to the hippocampal stem cell regulation and they also govern the plasticity of the neural stem cells (NSCs) or NPCs, which are dedicated to the neurogenesis process [38]. They also modulate cell fate determination, axon growth, dendrite pruning and retraction, innervation patterning, and the expression of proteins vital for normal neuronal function, including neurotransmitters and ion channels. In the mature nervous system, these proteins dynamically control synaptic function and plasticity, while continuing to regulate neuronal cell survival [43]. The development of the newly produced neuron is based on the fine-tuning temporal activity level of transcription factors, which requires the coordinated synthesis of the diverse stage-specific proteins [44]. Moreover, results showed that epigenetic changes in the GABAergic system are essential for adult hippocampal neurogenesis in prenatally stressed mice [45].
3. ALTERED HIPPOCAMPAL NEUROGENESIS IN ASD PATIENTS
The global volume of the hippocampus increases rapidly until two years of age and continues to grow slowly after that. Symptoms of ASD typically emerge from 12 to 24 months of age, a time window related to critical developmental events within the hippocampus. Of note, the period between 18-24 months of age is considered a developmental milestone of the hippocampus. During this stage, newborn neurons in the DG and CA3 matured sufficiently to connect with the cerebral cortex and acquire adult-like typical morphology [46]. Given this overlap and addressed altered structure and function in the hippocampus of ASD individuals, it is suspected that hippocampal formation alterations may contribute to the development of ASD symptoms.
Existing studies on older children and young adults with ASD have demonstrated abnormalities in the hippocampus [47]. For volumetric alteration in the hippocampus, the enlarged hippocampal volume has been reported in children and adolescents with ASD [48]. The enlarged hippocampal structures in ASD patients may be due to pathological development or experience-dependent structural and functional plasticity [49]. However, several studies yielded contradictory and confusing results; reduced hippocampus volume or no significant differences compared to typically developing (TD) children have also been found [50]. The hippocampal volume appears to present multiple atypical changes within ASD, which might be explained by different measurement approaches and sample characteristics across studies, such as age and cognitive impairment. Another key reason for this variability may be possibly due to the heterogeneity of ASD, compromising different etiologies. Moreover, the right-left asymmetry in hippocampal volume development has been compared in TD and ASD children. Schumann et al. [51] have found that children with autism show a larger right hippocampal volume than TD controls. However, another study has reported that the left hippocampus was enlarged in ASD individuals compared to that in TD children [52]. Growing evidence indicates functional connectivity alterations related to the hippocampus within the first year of life in high-risk (HR) infants. One study using resting-state fMRI (rsfMRI) found that functional connectivity of networks underlying neural processing of language is disrupted in HR infants, characterized by lower intrahemispheric connectivity between the auditory cortex and higher-order temporal regions as well as the hippocampus [53]. Different from HR infants, low-risk (LR) infants displayed early specialization for human voice processing in the right temporal and medial frontal regions. Meanwhile, LR infants manifested higher sensitivity than HR infants to sad vocalizations in the right fusiform gyrus and left hippocampus [54]. Similar to a previous report determining globally weak functional connectivity in ASD youths, typically lower connectivity in the left hippocampal networks was also confirmed in ASD youths [55]. Rudie et al. [56] showed that compared with the TD children, ASD children had reduced functional connectivity in the neural circuits involving the left hippocampus and the right parahippocampal gyrus. This suggests that impaired memory function in ASD patients may be a possible reason for causing social deficits. It is suspected that unusual lateralization development of the hippocampus might be related to ASD individuals’ disabilities in social interaction and communication and restricted and repetitive behavior.
The neuropathology of the hippocampus in ASD subjects was first reported in a postmortem study in 1980. Abnormally small and compactly packed cells were observed in the subiculum and CA1 of the hippocampus [57]. Histologic abnormalities in DG have been well documented. Focal thickening of hippocampal CA1 and irregularities in the appearance of the DG were identified in three adult men with Fragile X syndrome (FXS). The anomalous growth of the dentate nucleus and dysplasia of the GCL in the DG, found in this report, imply abnormal neurogenesis in the hippocampus [58]. Compared with healthy controls, MRI demonstrated that a marked anatomical abnormality was detected in autistic patients in the area of dentata (DG+CA4) [59]. Groen et al. [60] noticed that the abnormal enlargement of the hippocampus was presented in adolescents with autism ages 12-18 years compared to healthy adolescents [60]. Moreover, one autopsy study identified abnormal DG neurogenesis, neuronal migration, and maturation in the ASD brain, which may account for the heterogeneity of the patient’s symptoms [61]. Subtle brain dysplasia of the DG with a scattered distribution of granule cells within the molecular layer and distorted GCL has been detected in a 13-year-old autistic boy. Post-mortem analysis of pathologic neuroanatomical changes in ASD patients points to impairments in neurogenesis. Additional genetic studies conducted on human tissue have emphasized the critical roles of hippocampal neurogenesis in contributing to the development of ASD.
Moreover, it has been further confirmed that those factors present in the blood of autistic patients markedly affected pre-programmed neurogenesis, neuronal proliferation, migration, differentiation, and circuit organization. Mazur-Kolecka et al. [62] found that sera from autistic children markedly decreased the proliferation of NPCs but promoted neuronal migration, development of small neurons with processes, and synaptic formation. It has been further confirmed that autoantibodies against NPCs appeared in sera of autistic subjects. Meanwhile, the autoantibodies in the serum against differentiating NPCs from autistic children might alter neuronal maturation [62]. Remarkably, mild oxidative stress inhibited differentiating NPC proliferation when treated with sera from autistic children, suggesting that altered NPC response to increased oxidative stress may contribute to the development of autism [63].
4. UTILIZING MOUSE MODELS TO UNDERSTAND THE IMPACT OF ASD ON HIPPOCAMPAL NEUROGENESIS
Animal models are essential for understanding the altered hippocampal neurogenesis responsible for ASD. Based on different generation methods, in the ASD field, animal models of ASD mainly include environment-induced models, idiopathic models, and genetic models that have been generated based on human mutations and phenotypes in an attempt to recapitulate the disease.
4.1. Reduced Hippocampal Neurogenesis in the Idiopathic ASD Model of BTBR Mice
The BTBR mouse strain, originally bred for studies on insulin resistance, diabetes-induced nephropathy, and phenylketonuria, is now one of the most widely used idiopathic models of ASD [64]. These animals display distinct behavioral patterns that resemble the core deficits of human ASD, including impaired social behavior and communication, increased repetitive behavior, and cognitive rigidity. The BTBR mouse hippocampus showed a marked reduction in the thickness of the GCL of the DG and thinning of the hilus [65]. Histopathological studies of the BTBR strain have confirmed that reduced hippocampal neurogenesis is evidenced by the significant reductions in the number of DCX, PSA-NCAM, and NeuroD immunoreactive cells in the SGZ, as well as a marked decline in the number of BrdU positive progenitors [65]. Additionally, a robust decrease in neuronal differentiation was confirmed in BTBR mice, evidenced by a reduced percentage of BrdU-positive cells colocalized with NeuN and an increased percentage of BrdU-positive cells colocalized with GFAP [65]. Given the critical role of BDNF in promoting the survival, differentiation, and proliferation of NPCs, BDNF mRNA level has also been confirmed in the hippocampus of BTBR mice using in situ hybridization [65].
4.2. Abnormal Hippocampal Neurogenesis in Environment-induced ASD Mouse Models
Valproic acid (VPA), a well-known antiepileptic/anticonvulsant drug, is commonly prescribed during human pregnancy. Maternal exposure to VPA has long been considered a risk factor for ASD susceptibility in offspring from epidemiological studies in humans [66, 67]. Several studies have found that prenatal and neonatal VPA exposure to various mammalian species, such as mice, rats, common marmosets, and ferrets, can result in autistic behaviors, including stereotypic and repetitive behavior, decreased sociability, reduced nociceptive reactivity, and impaired communication [68-71]. Though not all children of mothers exposed to VPA had a diagnosis of ASD, the timing of VPA exposure during gestation impacts the effects of VPA on behaviors [72, 73]. A maternal challenge with VPA in experimental animals has nonetheless been used extensively as a model of ASD to increase our understanding of the neurobiology underlying autistic behaviors. Importantly, prenatal and neonatal VPA exposure can alter hippocampus development, such as DG neurogenesis [70]. It is confirmed that utero VPA-exposed male Albino Wistar rats displayed lowered cAMP activity as well as pCREB level, and thus may define the decreased levels of DCX and BDNF, an indication of reduced hippocampal neurogenesis [74]. Following VPA exposure during the critical developmental window of the hippocampus, an inconsistency in the GCL neuron number of adult DG between rat and mouse studies has been described. Watanabe et al. [68] have found that developmental exposure to VPA mainly targets interneurons, followed by late influences on NPC proliferation in the SGZ and consequently increased GCL neurons in rat hippocampus. However, in mice, there is a decrease in newly formed neurons during the adult stage. Yochum et al. [75] reported that at 12 and 24 hours after VPA exposure to P14, the number of granule cells in the DG of the hippocampus of BALB/c mice was decreased due to VPA-induced apoptosis. Similarly, using mice prenatally exposed to VPA, Juliandi et al. [76] found that postnatal cognitive dysfunction is possibly related to the untimely augmentation of embryonic neurogenesis, which induced exhaustion of the NPC pool and subsequent inhibition of adult hippocampal neurogenesis. A ferret is a small laboratory animal that has morphological characteristics of the brain similar to humans and is a good model candidate because of its social and playful traits. One recent study has indicated that VPA exposure to ferret infants induced disruption in social behavior. Meanwhile, it promoted the proliferation of neuron progenitors in the DG, and consequently brought extra NPCs into the DG subgranular layer [70].
Inflammation during pregnancy and perinatal infections are well-identified environmental risk factors, leading to ASD. Increased cytokines and activated microglia were found in the postmortem brain of ASD subjects. It is well-known that lipopolysaccharide (LPS), a cell wall component of gram-negative bacteria, leads to late-gestation maternal immune activation (MIA) that results in ASD-like behaviors in the offspring [77]. Similarly, neonatal LPS-treated rats at P3 induced acute pro-inflammatory responses in the brain, resulting in ASD-like behaviors and impaired hippocampal neurogenesis, serving as an ASD model [78]. Fractalkine signaling (CX3CL1/CX3CR1), a major neuron-to-microglia communication pathway, is involved in the pathogenesis of ASD induced by the immune challenge. An association of the variant of CX3CR1 with the ASD phenotype has been reported [79]. Meanwhile, CX3CR1 KO mice could model ASD-like behaviors, such as deficits in social interaction and increased repetitive behavior phenotypes [80]. Importantly, CX3CR1 KO mice displayed a reduction in neurogenesis in the DG via phagocytosis [81]. Loss of CX3CR1 in microglia could disrupt DG neurogenesis, reduce the number of dendritic spines as well as impair the synaptic integration of adult-born neurons [82]. This might explain a reduced CX3CR1 expression in MIA offspring involved in ASD-like behaviors with alerted synaptic pruning [83]. One recent study has reported that severe inflammatory pain in neonates is related to the development of ASD in juveniles. Meanwhile, reduced DG neurogenesis occurred in P21 juvenile rats subjected to inflammatory pain and related to IL-1β increase in the brain. These novel observations of Lee et al. [84] suggested that severe inflammatory pain in neonates and persistent inflammatory responses may reduce hippocampal neurogenesis, delay neuronal maturation, and ultimately contribute to psychiatric disorders, such as ASD. The alleviation of pain stimulus and inhibition of inflammation activities during development seem extremely important in blocking the possible progression of ASD [84].
Furthermore, epidemiological and experimental evidence document that gestational exposure to air pollution increases the risk of ASD. Cole et al. have demonstrated that gestational exposure to urban-derived nanosized particulate matter decreased neurogenesis in the DG, evidenced by a marked decrease in the number of EdU+/NeuN+ cells [85]. Another study reported that gestational exposure to PM2.5 caused autism-like behaviors, such as social communication deficits and stereotyped repetitive behavior, and inhibited hippocampal neurogenesis [86]. Consistent with these reports, we found that mice exposed to silica nanoparticles (SiO2-NPs) at higher doses during the first postnatal week resulted in a significant decrease of NPC proliferation in the DG-SGZ and exhaustion of the RGC population labeled with GFAP+/SOX2+ [87]. We further confirmed that mice exposed to 20 mg of SiO2-NPs exhibited defective social behavior deficits and slight anxiety-like behaviors in adulthood [87]. Thus, these potential environmental hazards have neurotoxic effects on DG neurogenesis and subsequently contribute to the development of autism-like behaviors in adults.
Perinatal medication exposures, such as selective serotonin reuptake inhibitor medications (SSRIs), the first lines of treatment for maternal affective disorders, are related to ASD risk in children [88]. Several meta-analyses indicated a significant positive association between in-utero exposure to SSRIs and ASD. Yet this association does not necessarily reflect a causal relationship since the results included in these meta-analyses are likely affected by other confounding factors, such as underlying maternal psychiatric disorders [89-92]. Besides, Ames et al. [93] found that maternal psychiatric conditions but not taking SSRIs during pregnancy were related to increased danger of neurodevelopmental disorders in offspring. On the other hand, emerging evidence from animal studies suggested that prenatal SSRI exposure might negatively affect social behavior. Zahra et al. observed abnormal behaviors, such as anxiety, altered locomotion, and disordered social interactions in 2-5 months old offspring with prenatal citalopram exposure [94]. Fluoxetine exposure throughout gestation and early lactation resulted in deficits in sociability and social novelty-seeking behavior in the juvenile offspring, which persisted into young adulthood [95]. One study has reported that perinatal fluoxetine treatment avoided effects induced by maternal stress on sibling play behavior, increased socially aggressive behavior, and reduced time grooming with a novel con-specific in males [96]. Qiu et al. [97] have confirmed that postpartum maternal fluoxetine treatment led to reduced proliferative DCX-positive cells in males and DCX-labeled post-mitotic cells in both sexes in the ventral DG.
4.3. Abnormal Hippocampal Neurogenesis in Genetic ASD Mouse Models
Advancement in human genetics has led to the discovery of many genes responsible for ‘monogenic’ forms of ASD. Studying these genes in pre-clinical models helps understand critical neurobiological pathways involved in ASD pathogenesis. Thus, over 1000 autism genetic mouse models and over 200 rat genetic models have been created. Some high-ranking candidate risk genes for ASD, such as calcium-dependent activator protein for secretion 2 (CAPS2), Engrailed-2 (EN2), Phosphatase and tensin homolog (Pten), Liver X receptor β (LXRβ), Topoisomerase 3β (Top3β), Methyl cytosine binding protein 2 (MECP2), Methyl-CpG binding protein 1 (MBD1), and Tbx1, are involved in the modulation of DG neurogenesis (Table 1).
Table 1.
Altered hippocampal neurogenesis in mouse models of ASD.
| Mouse Model | Hippocampus Neurogenesis | References |
|---|---|---|
| 8 to 10-week-old BTBR mice | Neurogenesis↓, DCX+↓, PSA-NCAM+↓, NeuroD+↓, BrdU+↓, BrdU+/NeuN+↓, BrdU+/GFAP+↑, BDNF mRNA↓ | [65] |
| VPA-exposed ferret infants | Neurogenesis↑, BrdU+↑, BrdU+/SOX2+↑ | [70] |
| Utero VPA-exposed male albino Wistar rats | Neurogenesis↓, DCX↓, BDNF↓, pCREB↓, synapsin-IIa↓ | [74] |
| Prenatal VPA-exposed mice | Embryonic neurogenesis↑, NPCs↓, adult neurogenesis↓, BrdU+↓, Ki67+↓, BrdU+/NeuN+↓, BrdU+/S100β+↓ | [76] |
| Rats exposed to VPA at P13 and P14 | Neurogenesis↓, DCX+↓, BrdU+↓ | [155] |
| Rats exposed to LPS at P3 | Neurogenesis↓, P21(NeuN+↓), P77(DCX+↓, TUBB3+↓, PCNA+↑) | [78] |
| Cx3cr1 KO mice | Neurogenesis↓, DCX+↓, dendritic complexity↓ | [81] |
| Perinatal SSRIs treated rats | Neurogenesis↓, males: proliferative DCX+↓; female: post-mitotic DCX+↓ | [97] |
| Adult Caps2 KO mice | Environmental enrichment-induced adult neurogenesis↓, BrdU+↓, BrdU+/calretinin+↓, BrdU+/NeuN+↓ | [102] |
| Caps2 KO mice | BDNF↓, GABAergic neurons↓, synapses↓ | [103] |
| En2-KO mice | NPC proliferation↑, BrdU+/Sox2+↑, BrdU+/Tbr2+↑; cell death↑, caspase-3+/DCX+↑ | [108] |
| Nestin-creERT2; Ptenloxp/loxp mice | Neurogenesis↑, RGL pool↓, Ki67+↑, BrdU+/NeuN+↓, BrdU+/S100β+↑, BrdU+↑ | [112] |
| SP-A-Cre; PTENflox/flox mice | NPC proliferation↑, BrdU+↑, Sox2+/GFAP-/BrdU+↑, Sox2+/GFAP+/BrdU+↓; neurogenesis↓, DCX+↓ BrdU+/NeuN+↓ | [113] |
| Fmr1 KO mice | BrdU+↑, BrdU+/NeuN+↓, BrdU+/S100β+↑ | [117] |
| Fmr1loxP/y: Nes-CreERT2 mice | Neurogenesis↓, RGC↑, DCX+↓, NeuN+↓S100β+/GFAP+↑ | [118] |
| Cyfip1 heterozygous KO mice | Neurogenesis↑, DCX+↑, caspase3+↓, BrdU+/NeuN+↑ | [123] |
| LXRβ KO mice | Neurogenesis↓, Sox2+/GFAP+↓, BLBP+/GFAP+↓, BrdU+↓, Sox2+↓, DCX+↓, Nestin+↓, Prox1+↓, Tbr2+↓ | [131] |
| Top3β KO mice | BrdU+↓, DCX+↓ | [133] |
| MECP2 transgenic mice | Neurogenesis↓, BrdU+↓, GFAP+/Nestin+/Ki67−↑ GFAP-/Nestin+/Ki67+↓, BrdU+/DCX+↑, | [142] |
| MBD1 KO mice | aNSCs↑, BrdU+↑, Tuj1+↓, GFAP+↓, NeuroD1 mRNA↓, Tuj1 mRNA↓, GFAP mRNA↓ | [144] |
| Nestin+↑, Nestin+/DCX+↑, Nestin-/DCX+↓ | [145] | |
| Tbx1-EGFP transfected mice | Migration and proliferation of adult neural stem/progenitor cells↓ | [149] |
Note: (↑ = increased, ↓ = decreased).
4.3.1. CAPS2
The human CAPS2 gene is located on chromosome 7q31.32, within the autism susceptibility locus, in single alleles of autistic patients [98]. Sadakata et al. [99] showed decreased transcription of CAPS2 in autistic brains; CAPS2 was found to be highly expressed in the hippocampal DG region, related to BDNF secretion. Caps2 KO mice not only have deficits in neuronal development and survival but also display deficits in social interaction, social communication, and hyperactivity, which are reminiscent of the behaviors of autistic patients [100, 101]. It is revealed that the Caps2 gene deficit influences adult hippocampal neurogenesis and the maturation of newborn neurons induced by the environmental enrichment condition [102]. BDNF plays a critical role in the postnatal development of forebrain GABAergic neurons, including the development of inhibitory neurons and their circuits and the altered GABA signaling, which has been supposed to be involved in the symptoms of ASD. BDNF secretion in the hippocampus of Caps2-KO mice secretion is reduced, and GABAergic system function is impaired, as evidenced by decreased late-phase LTP at CA3-CA1 synapses, reduced theta oscillation frequency in the hippocampus, and increased anxiety-like behavior [103].
4.3.2. EN2
Genome-wide association studies revealed that EN2 is a homeobox transcription factor, functions as a patterning gene in early development, and is a candidate gene for ASD [104-106]. Mice lacking En2 display ASD-like behavioral traits, such as decreased sociability, spatial learning deficits, and increased seizure susceptibility [107]. The En2-KO mice displayed reductions in hippocampal volume and cell numbers due to aberrant neurogenesis characterized by excess proliferation in the early Sox2+/Tbr2+ progenitors, increased apoptosis in differentiating neuroblasts, and reduced newborn neuron survival [108]. Notably, En2 functions in hippocampal NPCs by inhibiting proliferation and promoting survival and differentiation in a cell-autonomous manner [109].
4.3.3. Pten
PTEN, located on chromosome 10 (10q23.3), is closely related to autism and accounts for 5-17% of cases of autism [110]. Mice with deleted Pten in the mature neurons of the cerebral cortex and hippocampus displayed deficits resembling certain features of human ASD [111]. Deleting Pten in adult NSCs leads to a higher proliferation rate and accelerated differentiation of the stem/progenitor cells, resulting in the depletion of the NSC pool and increased differentiation toward the astrocytic lineage at later stages [112]. Additionally, mice with conditional Pten deletion in adult NSCs have enlarged DG and exhibit impairments in social interactions and seizure activity resembling certain features of human ASD [112]. One recent study has further confirmed that cerebrovascular-specific deletion of Pten disrupts adult neurogenesis with accompanying lactate accumulation [113].
4.3.4. Fmr1
FXS is a monogenic syndrome caused by transcriptional silencing of the Fmr1 gene and a single-gene cause of autism, impairing the translation of Fragile X mental retardation protein (FMRP) [114]. The Fmr1-KO mouse has a behavioral phenotype that resembles ASD symptoms, such as social deficits, increased repetitive behavior, hyperactivity, and learning deficits [114-116]. Luo et al. [117] have confirmed specific mRNAs modulated by Fmrp in the proliferation and differentiation of stem cells, including glycogen synthase kinase 3β (GSK3β), which has been implicated in adult neurogenesis. This study reports a novel role for Fmrp in the regulation of adult neurogenesis and supplies a direct indication that altered adult neurogenesis could be involved in the pathogenesis of fragile X mental retardation [117]. Meanwhile, Guo et al. [118] found that deletion of Fmrp in adult NSCs by inducible gene recombination led to increased proliferation and altered fate specification of NSCs and significantly damaged hippocampus-dependent learning in mice. On the other hand, restoration of Fmrp expression typically in adult NSCs rescued learning dysfunctions in Fmrp KO mice [118]. Some studies have demonstrated that Fmrp plays a role in stem cells, including adult NSCs in the hippocampal DG. Eadie et al. [119] found that loss of Fmr1 in mice led to anxiety-related behaviors and produced alterations in adult hippocampal neurogenesis [119]. Besides, adult Fmr1 KO mice displayed an increased rate of progenitor cell proliferation, altered fate specification, and diminished medial perforant path-granule cell LTP [117, 118, 120].
4.3.5. CYFIP1
Copy number variation (CNV) at the 15q11.2 region has been identified as a significant risk locus for autism, including a gene coding for CYFIP1 (cytoplasmic FMR1 interacting protein 1) [121]. CYFIP1 haploinsufficiency in mice led to impairments in social behavior and motor learning and defects in dendritic spine morphogenesis [122]. One recent work found that CYFIP1 haploinsufficiency increased adult-born hippocampal neurons due to a specific deficit in microglia-induced apoptosis. Meanwhile, these newly produced neurons displayed migration deficits due to abnormal Arp2/3 activity and actin cytoskeleton remodeling [123].
4.3.6. LXRβ
LXRs, including LXRα and LXRβ, two isoforms, play critical roles in the nervous system, such as promoting NSC proliferation and neuronal differentiation, improving synaptic plasticity and function, preventing neurodegeneration, inhibiting inflammation as well as regulating cholesterol homeostasis in the brain [124-126]. Our previous studies have verified that LXRβ is essential for RGC development and laminated CNS structures [127-131]. We noticed that LXRβ KO mice displayed impaired DG development, including deficits in the formation of progenitor cells and granule cell differentiation [127]. Analysis of the proliferation and differentiation of cultured NPC revealed that T0901317 (TO), a potent LXR agonist, increased NPC proliferation and prompted NPC differentiation toward neurons [127, 131]. We also found that LXRβ deletion in mice led to autistic-like behaviors, including social interaction deficits, increased repetitive behavior, and impairment in reversal learning. These data revealed a central role for LXRβ in DG neurogenesis, explaining its association with the genesis of autism-related behaviors in LXRβ-deficient mice [131].
4.3.7. Top3β
Top3β, an RNA topoisomerase, biochemically and genetically interacts with FMRP and is involved in ASD pathogenesis [132]. Top3β mutant mice displayed increased latency before the first exploration with a target mouse, which suggested a deficit in social interactions, a hallmark of autism [133]. These mice also exhibited impaired hippocampal neurogenesis and synaptic plasticity [133]. Top3β mutation in flies and mice caused deficits in synapse formation, with similar findings in FMRP mutant flies and mice [134]. Top3β-KO mice were found to have greater generalized anxiety levels, and the impaired synaptic plasticity phenotype resembled those observed in patients. Top3β deletion in mouse impaired hippocampal neurogenesis and synaptic plasticity [135, 136].
4.3.8. MECP2
MECP2 is a structural chromosomal protein and is important for neuronal system development. Mutations in the gene encoding MECP2 were previously associated primarily with RTT [137], but a recent study by Wen et al. has identified genetic mutations of the MECP2 gene in autism patients [138]. Newly produced granule cells in adult DG resembled maturational defects observed at early postnatal ages. As a result, they might not integrate appropriately into the existing circuit of the adult hippocampus [139]. Duplications of MECP2-encompassing segments result in the MECP2 duplication syndrome, which is associated with severe autism [140]. Abnormal DG neurogenesis occurred in MECP2 transgenic mice, as evidenced by adult hippocampal quiescent NPCs, and significantly accumulated in transgenic mice compared to wild-type (WT) mice. The reduced NPCs and increased neuroblasts were also observed in the adult hippocampus of MECP2 transgenic mice [141, 142].
4.3.9. MBD1
MBD1 mediates gene expression through methylated DNA binding and epigenetic regulation [143]. Abnormal epigenetic pathways have been identified to be involved in several neurodevelopmental disorders, including autism. Deletion of MBD1 in mice results in core autism-related deficits and relevant behaviors, such as deficits in social interaction and learning, anxiety-like behavior, impaired sensory-motor gating, depression-like behavior, and aberrant brain serotonin activity [143]. Furthermore, epigenetic regulation contributes to keeping the self-renewal and multipotency of adult stem cells. MBD1 is expressed in the NSC of the adult DG, suggesting its role in hippocampal neurogenesis [144]. Deletion of MBD1 in mice was found to promote the accumulation of undifferentiated NSC and defective neuronal lineage differentiation. Transcriptome analysis of NSCs/NPCs dissected from the mouse DG has identified that gene sets associated with astrocyte lineage genes were up-regulated in cells from MBD1 mutant mice [144, 145].
4.3.10. Tbx1
Hemizygous deletion at human 22q11.2 is identified as related to ASD [146]. Tbx1 encodes a transcription factor and is a determining gene among 30-40 contiguous genes in a common deletion of a 22q11.2 hemizygous segment [147]. Tbx1 heterozygous mice display ASD-like behaviors, including deficits in reciprocal social interaction and communication, impaired working memory, increased repetitive behaviors, and heightened anxiety-related behavioral traits [148]. Tbx1 protein expression is found to be enriched in adult NPCs of the adolescent mouse brain [148]. Moreover, copy number elevations of catechol-O-methyl-transferase or Tbx1 reduced the proliferation of adult NSCs/NPCs in a cell-autonomous manner in vitro and the migration of their progenies in the hippocampus GCL in vivo [149].
5. HIPPOCAMPAL NEUROGENESIS-BASED REGENERATIVE THERAPY PROPOSED FOR THE TREATMENT OF ASD
The exceptional role of hippocampal neurogenesis involved in cellular and behavioral plasticity is confirmed by the evidence that impaired neurogenesis is tightly coupled to autistic deficits in social memory, cognitive flexibility, and repetitive behavior [17, 150-152]. Similarly, restoration of hippocampal neurogenesis through lifestyle or therapeutic interventions can rescue autistic behaviors [153, 154]. Restoring hippocampal neurogenesis has emerged as a promising therapeutic intervention in ASD [155].
It is well-documented that physical exercise alleviates memory dysfunction, rescues social deficits, and relieves hyperactivity in autism [156]. In a separate study, Seo et al. [157] found that treadmill exercise for four weeks, starting postnatal day 28, reduced aggressive tendency and prompted the correct decision in spatial learning and memory in the VPA-injected rats. Meanwhile, treadmill exercise increased DG neurogenesis in the VPA-injected rats, which might contribute to the alleviation of autism-like symptoms. Javadi et al. [158] found that young adult FMR1-deficient mice treated with Nutlin-3 for five injections produced a long-lasting therapeutic effect on hippocampal neurogenesis and cognitive function via modulating the adult NSC niche.
Vinpocetine, a specific inhibitor of the phosphodiesterase-1 enzyme, is known to exert neuroprotective properties [159]. One recent study has reported that adult male rats prenatally exposed to VPA administrated with vinpocetine displayed amelioration of hyperlocomotion, social deficits, stereotypy, anxiety, and nociceptive changes [36]. Furthermore, the increased level of DCX in DG by vinpocetine administration seemed to be involved in these behavioral alterations [36]. Vinpocetine may be a consideration for future investigation in the clinical setting. The PDE10A inhibitor, papaverine, has been confirmed to rescue social deficits and stereotypy in miR-137flox/+; Nestin-Cre mice and repair neuronal morphological alterations [160]. In addition, the administration of papaverine to Pre-VPA animals from P21 to P48 resulted in improvements in social behavior and corrected repetitive behavior, anxiety, locomotor, and nociceptive changes [161]. Moreover, papaverine treatment resulted in a significant increase in the levels of DCX of the Pre-VPA group, which was associated with the alleviation of the core behaviors of ASD [74].
LPS-exposed neonatal animals revealed detrimental effects in hippocampal neurogenesis from puberty to adulthood and showed the progression of ASD-like behaviors [162]. Alpha-glycosyl isoquercitrin (AGIQ) exerts chemo-preventive effects, such as antioxidant and anti-inflammatory effects. It has been further confirmed that continuous AGIQ treatment, starting during late gestation, ameliorated progressive autistic behavior deficits and suppressed DG neurogenesis by critically inhibiting both inflammatory and oxidative responses caused by LPS [78]. B-vitamin is an indispensable nutrient to the human body and possesses anti-inflammatory and antioxidative properties [163]. Consistent with this study, B-vitamin supplementation to pregnant mice exposed to PM2.5 alleviated the autistic-like behaviors of offspring mice by enhancing anti-inflammatory and antioxidant capacities as well as improving hippocampal neurogenesis [86]. It was found that B-vitamin supplementation exhibits long-lasting effects on mitigating neurodevelopmental abnormalities induced by gestational exposure to PM2.5 [86]. These studies implied that embryonic treatment results in lasting improvements in autistic phenotypes, potentially through structural repair.
It is well documented that the first 2 weeks after birth in mice, equivalent to the period from the last trimester of pregnancy to the first few years after birth in humans, is the critical period for DG development [164]. It has been indicated that early postnatal treatment potentially repairs the structure, which is associated with continuous symptom alleviation [165]. Our study has demonstrated that postnatal treatment with TO, typically activated endogenous LXRβ and its target genes in the hippocampus and rescued the social defects and stereotypical behaviors in adult BTBR and VPA-induced autism mouse models. Importantly, we further confirmed that early postnatal TO treatment could enhance hippocampal neurogenesis [155]. Curcumin, a natural herbal component, has been confirmed to exert potent anti-inflammatory, antioxidant, and antineoplastic effects in neurological and psychological disorders [166]. Remarkably, curcumin has been demonstrated to exert neuroprotective properties via enhanced hippocampal neurogenesis. We demonstrated that neonatal curcumin treatment from P6-P8 significantly alleviated social deficits, repetitive behavior, and cognitive impairments in BTBR mice [17]. We further confirmed that neonatal curcumin treatment efficiently rescued hippocampal neurogenesis by preventing the exhaustion of the NPC pool and boosting NPC differentiation in BTBR mice [17].
Growing studies have indicated that gut dysbiosis might be involved in the increased vulnerability of ASD development [167, 168]. Liu et al. [168] revealed that antibiotic-induced gut microbial alteration in newborn mice resulted in ASD-like behavior impairment, which is related to a reduction in adult neurogenesis. Remarkably, the reconstruction of gut microbiota with healthy gut flora exerted therapeutic effects against adult neurogenesis and impaired behaviors in the dysbiosis mice [168]. This highlights the prospect of microbiome-mediated treatment for ASD individuals.
6. STEM CELL THERAPY IN THE TREATMENT OF ASD
A growing number of studies highlight that stem cell transplantation possesses a therapeutic potential for patients affected with incurable neurological disorders [169-171]. In the context of ASD, the evidence indicates that stem cell treatment markedly improves the ASD rating scale, abnormal behavior checklist scores, and clinical global impression evaluation, suggesting its potential benefit in treating children diagnosed with ASD [172, 173]. Up to now, no serious adverse events associated with stem cell therapy have been found, regardless of the cell source, dosage, and delivery routes [174-176]. It has been reported that 25 autistic children displayed behavioral improvements within 6 months of receiving umbilical cord blood-derived cell therapies, and adverse event assessments during the 12 months after injection showed that the treatment was safe and well tolerated [177]. Besides, Dawson et al. [178] found that autistic children without intellectual disability (ID), who received allogeneic cord blood infusion showed behavioral improvements, but a single infusion of cord blood was not associated with improved socialization skills or reduced autistic symptoms. It was also found that cord blood infusion was safe and well tolerated. Of note, this research is limited by a small sample size and restricted age and dosing ranges, and the safety of stem cell therapy certainly needs further investigation in larger cohorts.
Despite the gap between animal models and ASD patients, a growing number of preclinical studies showed that stem cell therapy could alleviate ASD symptoms by improving hippocampal neurogenesis.
Bone marrow-derived mesenchymal stem cells (MSCs) are multipotent stem cells typically differentiating into mesenchymal lineages, such as bone, cartilage, and fat. It is believed that MSC does have the potential to support and enhance endogenous neurogenesis and represent an attractive cell source for ASD treatment. In vitro, hippocampal neurogenesis has been dramatically increased in the presence of MSCs. Intracerebral transplantation of MSC can enhance hippocampal neurogenesis and improve hippocampal-related behaviors and function by induction of neurotrophic factors and immunomodulation [179]. Thus, MSC transplantation is speculated to be a potential therapy for ASD via enhanced hippocampal neurogenesis. Indeed, transplantation of MSCs into the cerebral lateral ventricles of BTBR mice resulted in the alleviation of ASD-like behaviors, such as a reduction of stereotypical behavior and impairments in cognitive flexibility and social behavior [180]. Meanwhile, enhanced hippocampal neurogenesis was identified in BTBR mice by MSC transplantation, as observed with increased Ki67-labeled NPCs and DCX-positive neurons in the DG [180]. It is well documented that the enhanced hippocampal neurogenesis by MSC transplantation is related to paracrine secretions, such as BDNF, IGF-1, NGF, and VEGF. As expected, an increased BDNF protein level in the hippocampus was also observed in MSC-transplanted mice [180]. Likewise, the intranasal delivery of human umbilical cord-MSC exosomes was found to efficiently alleviate the social deficit and repetitive, stereotyped behavior in the offspring of VPA-treated mice [179]. This is further supported by the finding that BTBR mouse transplantation with induced MSC that secreted high levels of neurotrophic factors had significant advantages over MSC transplantation in terms of improving communication skills and stereotypic behavior for as long as 6 months post-treatment [180]. Similarly, Gobshtis et al. [181] demonstrated that intracerebral transplantation of MSC efficiently rescued cognitive and social behavior deficits in VPA-exposed mice. Meanwhile, impaired hippocampal neurogenesis was also rescued by MSC transplantation. Furthermore, a statistically significant correlation between neuronal differentiation and behavioral scores suggested that increased hippocampal neurogenesis involved in improved ASD-like behaviors was due to MSC transplantation [182].
Noticeably, many investigations have found that human amniotic epithelial cells (hAECs) isolated from the layer closest to the fetus represent a unique class of stem cells [20]. Moreover, hAECs can exert neuroprotection in models of CNS disorders, such as stroke, perinatal hypoxic-ischemic brain injury, and fetal brain injury [183]. Our study has confirmed that intraventricular injection of hAECs into adult male BTBR mice could ameliorate social deficits significantly. In addition, hAEC transplantation restored the decline of neurogenesis, evidenced by increased BrdU-positive cells, DCX-positive neurons, Prox1-positive neurons, and GFAP+/Sox2+ double-stained NPCs in the hippocampus of BTBR mice. Increased levels of BDNF and TrkB in the hippocampus might be involved in the beneficial effects caused by hAEC transplantation [20].
A concerning issue is that the heterogeneity of ASD has implications for treatment efficiency. Current pro-neurogenic stem cell treatments were attempted in mouse models; however, whether they have the same impact on ASD patients remains to be investigated.
CONCLUSION
Based on pre-existing studies, impaired hippocampal neurogenesis in established ASD models may underlie some of the abnormal behavioral phenotypes seen in ASD, but only little is known about its potential significance in this context. Interestingly, interventions to regulate hippocampal neurogenesis and thereby reconstruct the hippocampal network in animal models present an effective neuroprotective strategy, evidenced by some ASD-like behavioral traits partially or completely rescued. The clinical application of pro‐neurogenic strategies in humans has yet to be demonstrated. Innate hippocampal neurogenesis is finite in type and scale, and pathologic factors involved in ASD might markedly influence the outcome of pro‐neurogenic treatments. The cellular and molecular pathways typically related to the neurogenic niche regulation mediating the pro‐neurogenic therapeutic effects on ASD need to be understood clearly. In the future, it is necessary to conduct additional, large-scale pre-clinical and clinical studies to illustrate the safety and efficiency of therapies targeting hippocampal neurogenesis for ASD.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ADHD
Attention-deficit Hyperactivity Disorder
- AGIQ
Alpha-glycosyl Isoquercitrin
- ASD
Autism Spectrum Disorder
- BDNF
Brain-derived Neurotrophic Factor
- BLBP
Brain Lipid Binding Protein
- DG
Dentate Gyrus
- FXS
Fragile X Syndrome
- GCL
Granule Cell Layer
- GFAP
Glial Fibrillary Acidic Protein
- hAECs
Human Amniotic Epithelial Cells
- ID
Intellectual Disability
- MSCs
Mesenchymal Stem Cells
- NPCs
Neural Progenitor Cells
- RTT
Rett Syndrome
- SGZ
Subgranular Zone
- TD
Typically Developing
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This study was supported by the National Key R&D Program of China (2021YFA1101203) and the National Nature Science Foundation of China (No. 82071544).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Güeita-Rodríguez J., Ogonowska-Slodownik A., Morgulec-Adamowicz N., Martín-Prades M.L., Cuenca-Zaldívar J.N., Palacios-Ceña D. Effects of aquatic therapy for children with autism spectrum disorder on social competence and quality of life: A mixed methods study. Int. J. Environ. Res. Public Health. 2021;18(6):3126. doi: 10.3390/ijerph18063126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman S.L., Levy S.E., Myers S.M., Kuo D.Z., Apkon S., Davidson L.F., Ellerbeck K.A., Foster J.E.A., Noritz G.H., Leppert M.O.C., Saunders B.S., Stille C., Yin L., Weitzman C.C., Childers D.O., Jr, Levine J.M., Peralta-Carcelen A.M., Poon J.K., Smith P.J., Blum N.J., Takayama J.I., Baum R., Voigt R.G., Bridgemohan C. Identification, evaluation, and management of children with autism spectrum disorder. Pediatrics. 2020;145(1):e20193447. doi: 10.1542/peds.2019-3447. [DOI] [PubMed] [Google Scholar]
- 3.Loomes R., Hull L., Mandy W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J. Am. Acad. Child Adolesc. Psychiatry. 2017;56(6):466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 4.Bai D., Yip B.H.K., Windham G.C., Sourander A., Francis R., Yoffe R., Glasson E., Mahjani B., Suominen A., Leonard H., Gissler M., Buxbaum J.D., Wong K., Schendel D., Kodesh A., Breshnahan M., Levine S.Z., Parner E.T., Hansen S.N., Hultman C., Reichenberg A., Sandin S. Association of genetic and environmental factors with autism in a 5-country cohort. JAMA Psychiatry. 2019;76(10):1035–1043. doi: 10.1001/jamapsychiatry.2019.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ecker C., Bookheimer S.Y., Murphy D.G.M. Neuroimaging in autism spectrum disorder: Brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121–1134. doi: 10.1016/S1474-4422(15)00050-2. [DOI] [PubMed] [Google Scholar]
- 6.Ecker C., Rocha-Rego V., Johnston P., Mourao-Miranda J., Marquand A., Daly E.M., Brammer M.J., Murphy C., Murphy D.G. Investigating the predictive value of whole-brain structural MR scans in autism: A pattern classification approach. Neuroimage. 2010;49(1):44–56. doi: 10.1016/j.neuroimage.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Makale M.T., McDonald C.R., Hattangadi-Gluth J.A., Kesari S. Mechanisms of radiotherapy-associated cognitive disability in patients with brain tumours. Nat. Rev. Neurol. 2017;13(1):52–64. doi: 10.1038/nrneurol.2016.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goel V., Makale M., Grafman J. The hippocampal system mediates logical reasoning about familiar spatial environments. J. Cogn. Neurosci. 2004;16(4):654–664. doi: 10.1162/089892904323057362. [DOI] [PubMed] [Google Scholar]
- 9.Yassa M.A., Stark C.E.L. Pattern separation in the hippocampus. Trends Neurosci. 2011;34(10):515–525. doi: 10.1016/j.tins.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zeidman P., Maguire E.A. Anterior hippocampus: The anatomy of perception, imagination and episodic memory. Nat. Rev. Neurosci. 2016;17(3):173–182. doi: 10.1038/nrn.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Felix-Ortiz A.C., Tye K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lavenex P., Banta Lavenex P., Favre G. What animals can teach clinicians about the hippocampus. Front Neurol. Neurosci. 2014;34:36–50. doi: 10.1159/000356418. [DOI] [PubMed] [Google Scholar]
- 13.Knickmeyer R.C., Gouttard S., Kang C., Evans D., Wilber K., Smith J.K., Hamer R.M., Lin W., Gerig G., Gilmore J.H. A structural MRI study of human brain development from birth to 2 years. J. Neurosci. 2008;28(47):12176–12182. doi: 10.1523/JNEUROSCI.3479-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen D.L., Baio J., Braun K.V.N., Bilder D., Charles J., Constantino J.N., Daniels J., Durkin M.S., Fitzgerald R.T., Kurzius-Spencer M., Lee L.C., Pettygrove S., Robinson C., Schulz E., Wells C., Wingate M.S., Zahorodny W., Yeargin-Allsopp M. Prevalence and characteristics of autism spectrum disorder among children aged 8 years--autism and developmental disabilities monitoring network, 11 sites, united states, 2012. MMWR Surveill. Summ. 2016;65(3):1–23. doi: 10.15585/mmwr.ss6503a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kempermann G., Gage F.H., Aigner L., Song H., Curtis M.A., Thuret S., Kuhn H.G., Jessberger S., Frankland P.W., Cameron H.A., Gould E., Hen R., Abrous D.N., Toni N., Schinder A.F., Zhao X., Lucassen P.J., Frisén J. Human adult neurogenesis: Evidence and remaining questions. Cell Stem Cell. 2018;23(1):25–30. doi: 10.1016/j.stem.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mellios N., Feldman D.A., Sheridan S.D., Ip J.P.K., Kwok S., Amoah S.K., Rosen B., Rodriguez B.A., Crawford B., Swaminathan R., Chou S., Li Y., Ziats M., Ernst C., Jaenisch R., Haggarty S.J., Sur M. MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol. Psychiatry. 2018;23(4):1051–1065. doi: 10.1038/mp.2017.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peng L., Bonaguidi M.A. Function and dysfunction of adult hippocampal neurogenesis in regeneration and disease. Am. J. Pathol. 2018;188(1):23–28. doi: 10.1016/j.ajpath.2017.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson A., Boekhoorn K., Van Dam A.M., Lucassen P.J. Changes in adult neurogenesis in neurodegenerative diseases: Cause or consequence? Genes Brain Behav. 2008;7(Suppl. 1):28–42. doi: 10.1111/j.1601-183X.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 19.Zhong H., Xiao R., Ruan R., Liu H., Li X., Cai Y., Zhao J., Fan X. Neonatal curcumin treatment restores hippocampal neurogenesis and improves autism-related behaviors in a mouse model of autism. Psychopharmacology (Berl.) 2020;237(12):3539–3552. doi: 10.1007/s00213-020-05634-5. [DOI] [PubMed] [Google Scholar]
- 20.Zhang R., Cai Y., Xiao R., Zhong H., Li X., Guo L., Xu H., Fan X. Human amniotic epithelial cell transplantation promotes neurogenesis and ameliorates social deficits in BTBR mice. Stem Cell Res. Ther. 2019;10(1):153. doi: 10.1186/s13287-019-1267-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banker S.M., Gu X., Schiller D., Foss-Feig J.H. Hippocampal contributions to social and cognitive deficits in autism spectrum disorder. Trends Neurosci. 2021;44(10):793–807. doi: 10.1016/j.tins.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hochgerner H., Zeisel A., Lönnerberg P., Linnarsson S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 2018;21(2):290–299. doi: 10.1038/s41593-017-0056-2. [DOI] [PubMed] [Google Scholar]
- 23.Niklison-Chirou M.V., Agostini M., Amelio I., Melino G. Regulation of adult neurogenesis in mammalian brain. Int. J. Mol. Sci. 2020;21(14):4869. doi: 10.3390/ijms21144869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covey M.V., Loporchio D., Buono K.D., Levison S.W. Opposite effect of inflammation on subventricular zone versus hippocampal precursors in brain injury. Ann. Neurol. 2011;70(4):616–626. doi: 10.1002/ana.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumari E., Velloso F.J., Nasuhidehnavi A., Somasundaram A., Savanur V.H., Buono K.D., Levison S.W. Developmental il-6 exposure favors production of pdgf-responsive multipotential progenitors at the expense of neural stem cells and other progenitors. Stem Cell Reports. 2020;14(5):861–875. doi: 10.1016/j.stemcr.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Storer M.A., Gallagher D., Fatt M.P., Simonetta J.V., Kaplan D.R., Miller F.D. Interleukin-6 regulates adult neural stem cell numbers during normal and abnormal post-natal development. Stem Cell Reports. 2018;10(5):1464–1480. doi: 10.1016/j.stemcr.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicola Z., Fabel K., Kempermann G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015;9:53. doi: 10.3389/fnana.2015.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radic T., Frieß L., Vijikumar A., Jungenitz T., Deller T., Schwarzacher S.W. Differential postnatal expression of neuronal maturation markers in the dentate gyrus of mice and rats. Front. Neuroanat. 2017;11:104. doi: 10.3389/fnana.2017.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonaguidi M.A., Wheeler M.A., Shapiro J.S., Stadel R.P., Sun G.J., Ming G., Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145(7):1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao Z., Ure K., Ables J.L., Lagace D.C., Nave K.A., Goebbels S., Eisch A.J., Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lavado A., Lagutin O.V., Chow L.M.L., Baker S.J., Oliver G. Prox1 is required for granule cell maturation and intermediate progenitor maintenance during brain neurogenesis. PLoS Biol. 2010;8(8):e1000460. doi: 10.1371/journal.pbio.1000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao C., Teng E.M., Summers R.G., Jr, Ming G.L., Gage F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 2006;26(1):3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gros A., Veyrac A., Laroche S. Brain and memory: New neurons to remember. Biol. Aujourdhui. 2015;209(3):229–248. doi: 10.1051/jbio/2015028. [DOI] [PubMed] [Google Scholar]
- 34.Aimone J.B., Li Y., Lee S.W., Clemenson G.D., Deng W., Gage F.H. Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 2014;94(4):991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bath K.G., Jing D.Q., Dincheva I., Neeb C.C., Pattwell S.S., Chao M.V., Lee F.S., Ninan I. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology. 2012;37(5):1297–1304. doi: 10.1038/npp.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luhach K., Kulkarni G.T., Singh V.P., Sharma B. Vinpocetine amended prenatal valproic acid induced features of ASD possibly by altering markers of neuronal function, inflammation, and oxidative stress. Autism Res. 2021;14(11):2270–2286. doi: 10.1002/aur.2597. [DOI] [PubMed] [Google Scholar]
- 37.Camuso S., La Rosa P., Fiorenza M.T., Canterini S. Pleiotropic effects of BDNF on the cerebellum and hippocampus: Implications for neurodevelopmental disorders. Neurobiol. Dis. 2022;163:105606. doi: 10.1016/j.nbd.2021.105606. [DOI] [PubMed] [Google Scholar]
- 38.Bagheri-Mohammadi S. Adult neurogenesis and the molecular signalling pathways in brain: The role of stem cells in adult hippocampal neurogenesis. Int. J. Neurosci. 2022;132(12):1165–1177. doi: 10.1080/00207454.2020.1865953. [DOI] [PubMed] [Google Scholar]
- 39.Araki T., Ikegaya Y., Koyama R. The effects of microglia‐ and astrocyte‐derived factors on neurogenesis in health and disease. Eur. J. Neurosci. 2021;54(5):5880–5901. doi: 10.1111/ejn.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zonis S., Breunig J.J., Mamelak A., Wawrowsky K., Bresee C., Ginzburg N., Chesnokova V. Inflammation-induced Gro1 triggers senescence in neuronal progenitors: effects of estradiol. J. Neuroinflammation. 2018;15(1):260. doi: 10.1186/s12974-018-1298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Fan B., Chopp M., Zhang Z. Epigenetic mechanisms underlying adult post stroke neurogenesis. Int. J. Mol. Sci. 2020;21(17):6179. doi: 10.3390/ijms21176179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arredondo S.B., Guerrero F.G., Herrera-Soto A., Jensen-Flores J., Bustamante D.B., Oñate-Ponce A., Henny P., Varas-Godoy M., Inestrosa N.C., Varela-Nallar L. Wnt5a promotes differentiation and development of adult-born neurons in the hippocampus by noncanonical Wnt signaling. Stem Cells. 2020;38(3):422–436. doi: 10.1002/stem.3121. [DOI] [PubMed] [Google Scholar]
- 43.Huang E.J., Reichardt L.F. Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 2001;24(1):677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckervordersandforth R., Zhang C.L., Lie D.C. Transcription-factor-dependent control of adult hippocampal neurogenesis. Cold Spring Harb. Perspect. Biol. 2015;7(10):a018879. doi: 10.1101/cshperspect.a018879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhong H., Rong J., Zhu C., Liang M., Li Y., Zhou R. Epigenetic modifications of gabaergic interneurons contribute to deficits in adult hippocampus neurogenesis and depression-like behavior in prenatally stressed mice. Int. J. Neuropsychopharmacol. 2020;23(4):274–285. doi: 10.1093/ijnp/pyaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gómez R.L., Edgin J.O. The extended trajectory of hippocampal development: Implications for early memory development and disorder. Dev. Cogn. Neurosci. 2016;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aylward E.H., Minshew N.J., Goldstein G., Honeycutt N.A., Augustine A.M., Yates K.O., Barta P.E., Pearlson G.D. MRI volumes of amygdala and hippocampus in non-mentally retarded autistic adolescents and adults. Neurology. 1999;53(9):2145–2150. doi: 10.1212/WNL.53.9.2145. [DOI] [PubMed] [Google Scholar]
- 48.Maier S., Tebartz van Elst L., Beier D., Ebert D., Fangmeier T., Radtke M., Perlov E., Riedel A. Increased hippocampal volumes in adults with high functioning autism spectrum disorder and an IQ>100: A manual morphometric study. Psychiatry Res. Neuroimaging. 2015;234(1):152–155. doi: 10.1016/j.pscychresns.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Sussman D., Leung R.C., Vogan V.M., Lee W., Trelle S., Lin S., Cassel D.B., Chakravarty M.M., Lerch J.P., Anagnostou E., Taylor M.J. The autism puzzle: Diffuse but not pervasive neuroanatomical abnormalities in children with ASD. Neuroimage Clin. 2015;8:170–179. doi: 10.1016/j.nicl.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trontel H., Duffield T., Bigler E., Froehlich A., Prigge M., Nielsen J., Cooperrider J., Cariello A., Travers B., Anderson J., Zielinski B., Alexander A., Lange N., Lainhart J. Fusiform correlates of facial memory in autism. Behav. Sci. (Basel) 2013;3(3):348–371. doi: 10.3390/bs3030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schumann C.M., Hamstra J., Goodlin-Jones B.L., Lotspeich L.J., Kwon H., Buonocore M.H., Lammers C.R., Reiss A.L., Amaral D.G. The amygdala is enlarged in children but not adolescents with autism; the hippocampus is enlarged at all ages. J. Neurosci. 2004;24(28):6392–6401. doi: 10.1523/JNEUROSCI.1297-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richards R., Greimel E., Kliemann D., Koerte I.K., Schulte-Körne G., Reuter M., Wachinger C. Increased hippocampal shape asymmetry and volumetric ventricular asymmetry in autism spectrum disorder. Neuroimage Clin. 2020;26:102207. doi: 10.1016/j.nicl.2020.102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu J., Okada N.J., Cummings K.K., Jung J., Patterson G., Bookheimer S.Y., Jeste S.S., Dapretto M. Emerging atypicalities in functional connectivity of language-related networks in young infants at high familial risk for ASD. Dev. Cogn. Neurosci. 2020;45:100814. doi: 10.1016/j.dcn.2020.100814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blasi A., Lloyd-Fox S., Sethna V., Brammer M.J., Mercure E., Murray L., Williams S.C.R., Simmons A., Murphy D.G.M., Johnson M.H. Atypical processing of voice sounds in infants at risk for autism spectrum disorder. Cortex. 2015;71:122–133. doi: 10.1016/j.cortex.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gaigg S.B., Bowler D.M., Ecker C., Calvo-Merino B., Murphy D.G. Episodic recollection difficulties in asd result from atypical relational encoding: Behavioral and neural evidence. Autism Res. 2015;8(3):317–327. doi: 10.1002/aur.1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rudie J.D., Shehzad Z., Hernandez L.M., Colich N.L., Bookheimer S.Y., Iacoboni M., Dapretto M. Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cereb. Cortex. 2012;22(5):1025–1037. doi: 10.1093/cercor/bhr171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams R.S., Hauser S.L., Purpura D.P., DeLong G.R., Swisher C.N. Autism and mental retardation: Neuropathologic studies performed in four retarded persons with autistic behavior. Arch. Neurol. 1980;37(12):749–753. doi: 10.1001/archneur.1980.00500610029003. [DOI] [PubMed] [Google Scholar]
- 58.Greco C.M., Navarro C.S., Hunsaker M.R., Maezawa I., Shuler J.F., Tassone F., Delany M., Au J.W., Berman R.F., Jin L.W., Schumann C., Hagerman P.J., Hagerman R.J. Neuropathologic features in the hippocampus and cerebellum of three older men with fragile X syndrome. Mol. Autism. 2011;2(1):2. doi: 10.1186/2040-2392-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saitoh O., Karns C.M., Courchesne E. Development of the hippocampal formation from2 to 42 years: MRI evidence of smaller area dentata in autism. Brain. 2001;124(7):1317–1324. doi: 10.1093/brain/124.7.1317. [DOI] [PubMed] [Google Scholar]
- 60.Groen W., Teluij M., Buitelaar J., Tendolkar I. Amygdala and hippocampus enlargement during adolescence in autism. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(6):552–560. doi: 10.1016/j.jaac.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 61.Wegiel J., Kuchna I., Nowicki K., Imaki H., Wegiel J., Marchi E., Ma S.Y., Chauhan A., Chauhan V., Bobrowicz T.W., de Leon M., Louis L.A.S., Cohen I.L., London E., Brown W.T., Wisniewski T. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010;119(6):755–770. doi: 10.1007/s00401-010-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mazur-Kolecka B., Cohen I.L., Jenkins E.C., Kaczmarski W., Flory M., Frackowiak J. Altered development of neuronal progenitor cells after stimulation with autistic blood sera. Brain Res. 2007;1168:11–20. doi: 10.1016/j.brainres.2007.06.084. [DOI] [PubMed] [Google Scholar]
- 63.Mazur-Kolecka B., Cohen I.L., Jenkins E.C., Flory M., Merz G., Ted Brown W., Frackowiak J. Sera from children with autism alter proliferation of human neuronal progenitor cells exposed to oxidation. Neurotox. Res. 2009;16(1):87–95. doi: 10.1007/s12640-009-9052-y. [DOI] [PubMed] [Google Scholar]
- 64.Meyza K.Z., Blanchard D.C. The btbr mouse model of idiopathic autism - current view on mechanisms. Neurosci Biobehav Rev. 2017;76(Pt A):99–110. doi: 10.1016/j.neubiorev.2016.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stephenson D.T., O’Neill S.M., Narayan S., Tiwari A., Arnold E., Samaroo H.D., Du F., Ring R.H., Campbell B., Pletcher M., Vaidya V.A., Morton D. Histopathologic characterization of the BTBR mouse model of autistic-like behavior reveals selective changes in neurodevelopmental proteins and adult hippocampal neurogenesis. Mol. Autism. 2011;2(1):7. doi: 10.1186/2040-2392-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bjørk M.H., Zoega H., Leinonen M.K., Cohen J.M., Dreier J.W., Furu K., Gilhus N.E., Gissler M., Hálfdánarson Ó., Igland J., Sun Y., Tomson T., Alvestad S., Christensen J. Association of prenatal exposure to antiseizure medication with risk of autism and intellectual disability. JAMA Neurol. 2022;79(7):672–681. doi: 10.1001/jamaneurol.2022.1269. [DOI] [PubMed] [Google Scholar]
- 67.Christensen J., Grønborg T.K., Sørensen M.J., Schendel D., Parner E.T., Pedersen L.H., Vestergaard M. Prenatal valproate exposure and risk of autism spectrum disorders and childhood autism. JAMA. 2013;309(16):1696–1703. doi: 10.1001/jama.2013.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watanabe Y., Murakami T., Kawashima M., Hasegawa-Baba Y., Mizukami S., Imatanaka N., Akahori Y., Yoshida T., Shibutani M. Maternal exposure to valproic acid primarily targets interneurons followed by late effects on neurogenesis in the hippocampal dentate gyrus in rat offspring. Neurotox. Res. 2017;31(1):46–62. doi: 10.1007/s12640-016-9660-2. [DOI] [PubMed] [Google Scholar]
- 69.Mimura K., Oga T., Sasaki T., Nakagaki K., Sato C., Sumida K., Hoshino K., Saito K., Miyawaki I., Suhara T., Aoki I., Minamimoto T., Ichinohe N. Abnormal axon guidance signals and reduced interhemispheric connection via anterior commissure in neonates of marmoset ASD model. Neuroimage. 2019;195:243–251. doi: 10.1016/j.neuroimage.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 70.Sawada K., Kamiya S., Aoki I. The proliferation of dentate gyrus progenitors in the ferret hippocampus by neonatal exposure to valproic acid. Front. Neurosci. 2021;15:736313. doi: 10.3389/fnins.2021.736313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner G.C., Reuhl K.R., Cheh M., McRae P., Halladay A.K. A new neurobehavioral model of autism in mice: pre- and postnatal exposure to sodium valproate. J. Autism Dev. Disord. 2006;36(6):779–793. doi: 10.1007/s10803-006-0117-y. [DOI] [PubMed] [Google Scholar]
- 72.Kataoka S., Takuma K., Hara Y., Maeda Y., Ago Y., Matsuda T. Autism-like behaviours with transient histone hyperacetylation in mice treated prenatally with valproic acid. Int. J. Neuropsychopharmacol. 2013;16(1):91–103. doi: 10.1017/S1461145711001714. [DOI] [PubMed] [Google Scholar]
- 73.Kim K.C., Kim P., Go H.S., Choi C.S., Yang S.I., Cheong J.H., Shin C.Y., Ko K.H. The critical period of valproate exposure to induce autistic symptoms in Sprague–Dawley rats. Toxicol. Lett. 2011;201(2):137–142. doi: 10.1016/j.toxlet.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 74.Luhach K., Kulkarni G.T., Singh V.P., Sharma B. Attenuation of neurobehavioural abnormalities by papaverine in prenatal valproic acid rat model of ASD. Eur. J. Pharmacol. 2021;890:173663. doi: 10.1016/j.ejphar.2020.173663. [DOI] [PubMed] [Google Scholar]
- 75.Yochum C.L., Dowling P., Reuhl K.R., Wagner G.C., Ming X. VPA-induced apoptosis and behavioral deficits in neonatal mice. Brain Res. 2008;1203:126–132. doi: 10.1016/j.brainres.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 76.Juliandi B., Tanemura K., Igarashi K., Tominaga T., Furukawa Y., Otsuka M., Moriyama N., Ikegami D., Abematsu M., Sanosaka T., Tsujimura K., Narita M., Kanno J., Nakashima K. Reduced adult hippocampal neurogenesis and cognitive impairments following prenatal treatment of the antiepileptic drug valproic acid. Stem Cell Reports. 2015;5(6):996–1009. doi: 10.1016/j.stemcr.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee G.A., Lin Y.K., Lai J.H., Lo Y.C., Yang Y.C.S.H., Ye S.Y., Lee C.J., Wang C.C., Chiang Y.H., Tseng S.H. Maternal immune activation causes social behavior deficits and hypomyelination in male rat offspring with an autism-like microbiota profile. Brain Sci. 2021;11(8):1085. doi: 10.3390/brainsci11081085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Okano H., Takashima K., Takahashi Y., Ojiro R., Tang Q., Ozawa S., Ogawa B., Koyanagi M., Maronpot R.R., Yoshida T., Shibutani M. Ameliorating effect of continuous alpha-glycosyl isoquercitrin treatment starting from late gestation in a rat autism model induced by postnatal injection of lipopolysaccharides. Chem. Biol. Interact. 2022;351:109767. doi: 10.1016/j.cbi.2021.109767. [DOI] [PubMed] [Google Scholar]
- 79.Ishizuka K., Fujita Y., Kawabata T., Kimura H., Iwayama Y., Inada T., Okahisa Y., Egawa J., Usami M., Kushima I., Uno Y., Okada T., Ikeda M., Aleksic B., Mori D., Someya T., Yoshikawa T., Iwata N., Nakamura H., Yamashita T., Ozaki N. Rare genetic variants in CX3CR1 and their contribution to the increased risk of schizophrenia and autism spectrum disorders. Transl. Psychiatry. 2017;7(8):e1184. doi: 10.1038/tp.2017.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhan Y., Paolicelli R.C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., Vyssotski A.L., Bifone A., Gozzi A., Ragozzino D., Gross C.T. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014;17(3):400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- 81.Jiang X., Xiao F., Xu J. CX3 chemokine receptor 1 defciency leads to reduced dendritic complexity and delayed maturation of newborn neurons in the adult mouse hippocampus. Neural Regen. Res. 2015;10(5):772–777. doi: 10.4103/1673-5374.156979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolós M., Perea J.R., Terreros-Roncal J., Pallas-Bazarra N., Jurado-Arjona J., Ávila J., Llorens-Martín M. Absence of microglial CX3CR1 impairs the synaptic integration of adult-born hippocampal granule neurons. Brain Behav. Immun. 2018;68:76–89. doi: 10.1016/j.bbi.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 83.Fernández de Cossío L., Guzmán A., van der Veldt S., Luheshi G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017;63:88–98. doi: 10.1016/j.bbi.2016.09.028. [DOI] [PubMed] [Google Scholar]
- 84.Lee J.H., Espinera A.R., Chen D., Choi K.E., Caslin A.Y., Won S., Pecoraro V., Xu G.Y., Wei L., Yu S.P. Neonatal inflammatory pain and systemic inflammatory responses as possible environmental factors in the development of autism spectrum disorder of juvenile rats. J. Neuroinflammation. 2016;13(1):109. doi: 10.1186/s12974-016-0575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cole T.B., Chang Y.C., Dao K., Daza R., Hevner R., Costa L.G. Developmental exposure to diesel exhaust upregulates transcription factor expression, decreases hippocampal neurogenesis, and alters cortical lamina organization: relevance to neurodevelopmental disorders. J. Neurodev. Disord. 2020;12(1):41. doi: 10.1186/s11689-020-09340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang T., Zhang T., Sun L., Li W., Zhang C., Yu L., Guan Y. Gestational B-vitamin supplementation alleviates PM2.5-induced autism-like behavior and hippocampal neurodevelopmental impairment in mice offspring. Ecotoxicol. Environ. Saf. 2019;185:109686. doi: 10.1016/j.ecoenv.2019.109686. [DOI] [PubMed] [Google Scholar]
- 87.Fu J., Gao J., Gong L., Ma Y., Xu H., Gu Z., Zhu J., Fan X. Silica nanoparticle exposure during the neonatal period impairs hippocampal precursor proliferation and social behavior later in life. Int. J. Nanomedicine. 2018;13:3593–3608. doi: 10.2147/IJN.S160828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boukhris T., Sheehy O., Mottron L., Bérard A. Antidepressant use during pregnancy and the risk of autism spectrum disorder in children. JAMA Pediatr. 2016;170(2):117–124. doi: 10.1001/jamapediatrics.2015.3356. [DOI] [PubMed] [Google Scholar]
- 89.Dragioti E., Solmi M., Favaro A., Fusar-Poli P., Dazzan P., Thompson T., Stubbs B., Firth J., Fornaro M., Tsartsalis D., Carvalho A.F., Vieta E., McGuire P., Young A.H., Shin J.I., Correll C.U., Evangelou E. Association of antidepressant use with adverse health outcomes: A systematic umbrella review. JAMA Psychiatry. 2019;76(12):1241–1255. doi: 10.1001/jamapsychiatry.2019.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Leshem R., Bar-Oz B., Diav-Citrin O., Gbaly S., Soliman J., Renoux C., Matok I. Selective serotonin reuptake inhibitors (ssris) and serotonin norepinephrine reuptake inhibitors (snris) during pregnancy and the risk for autism spectrum disorder (asd) and attention deficit hyperactivity disorder (adhd) in the offspring: A true effect or a bias? A systematic review & meta-analysis. Curr. Neuropharmacol. 2021;19(6):896–906. doi: 10.2174/1570159X19666210303121059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim J.Y., Son M.J., Son C.Y., Radua J., Eisenhut M., Gressier F., Koyanagi A., Carvalho A.F., Stubbs B., Solmi M., Rais T.B., Lee K.H., Kronbichler A., Dragioti E., Shin J.I., Fusar-Poli P. Environmental risk factors and biomarkers for autism spectrum disorder: an umbrella review of the evidence. Lancet Psychiatry. 2019;6(7):590–600. doi: 10.1016/S2215-0366(19)30181-6. [DOI] [PubMed] [Google Scholar]
- 92.Mezzacappa A., Lasica P.A., Gianfagna F., Cazas O., Hardy P., Falissard B., Sutter-Dallay A.L., Gressier F. Risk for autism spectrum disorders according to period of prenatal antidepressant exposure: A systematic review and meta-analysis. JAMA Pediatr. 2017;171(6):555–563. doi: 10.1001/jamapediatrics.2017.0124. [DOI] [PubMed] [Google Scholar]
- 93.Ames J.L., Ladd-Acosta C., Fallin M.D., Qian Y., Schieve L.A., DiGuiseppi C., Lee L.C., Kasten E.P., Zhou G., Pinto-Martin J., Howerton E.M., Eaton C.L., Croen L.A. Maternal psychiatric conditions, treatment with selective serotonin reuptake inhibitors, and neurodevelopmental disorders. Biol. Psychiatry. 2021;90(4):253–262. doi: 10.1016/j.biopsych.2021.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zahra A., Jiang J., Chen Y., Long C., Yang L. Memantine rescues prenatal citalopram exposure-induced striatal and social abnormalities in mice. Exp. Neurol. 2018;307:145–154. doi: 10.1016/j.expneurol.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 95.Bond C.M., Johnson J.C., Chaudhary V., McCarthy E.M., McWhorter M.L., Woehrle N.S. Perinatal fluoxetine exposure results in social deficits and reduced monoamine oxidase gene expression in mice. Brain Res. 2020;1727:146282. doi: 10.1016/j.brainres.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 96.Gemmel M., Hazlett M., Bögi E., De Lacalle S., Hill L.A., Kokras N., Hammond G.L., Dalla C., Charlier T.D., Pawluski J.L. Perinatal fluoxetine effects on social play, the HPA system, and hippocampal plasticity in pre-adolescent male and female rats: Interactions with pre-gestational maternal stress. Psychoneuroendocrinology. 2017;84:159–171. doi: 10.1016/j.psyneuen.2017.07.480. [DOI] [PubMed] [Google Scholar]
- 97.Qiu W., Go K.A., Wen Y., Duarte-Guterman P., Eid R.S., Galea L.A.M. Maternal fluoxetine reduces hippocampal inflammation and neurogenesis in adult offspring with sex-specific effects of periadolescent oxytocin. Brain Behav. Immun. 2021;97:394–409. doi: 10.1016/j.bbi.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 98.Bonora E., Graziano C., Minopoli F., Bacchelli E., Magini P., Diquigiovanni C., Lomartire S., Bianco F., Vargiolu M., Parchi P., Marasco E., Mantovani V., Rampoldi L., Trudu M., Parmeggiani A., Battaglia A., Mazzone L., Tortora G., Maestrini E., Seri M., Romeo G. Maternally inherited genetic variants ofCADPS 2 are present in Autism Spectrum Disorders and Intellectual Disability patients. EMBO Mol. Med. 2014;6(6):795–809. doi: 10.1002/emmm.201303235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadakata T., Washida M., Iwayama Y., Shoji S., Sato Y., Ohkura T., Katoh-Semba R., Nakajima M., Sekine Y., Tanaka M., Nakamura K., Iwata Y., Tsuchiya K.J., Mori N., Detera-Wadleigh S.D., Ichikawa H., Itohara S., Yoshikawa T., Furuichi T. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J. Clin. Invest. 2007;117(4):931–943. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fujima S., Yamaga R., Minami H., Mizuno S., Shinoda Y., Sadakata T., Abe M., Sakimura K., Sano Y., Furuichi T. Caps2 deficiency impairs the release of the social peptide oxytocin, as well as oxytocin-associated social behavior. J. Neurosci. 2021;41(20):4524–4535. doi: 10.1523/JNEUROSCI.3240-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sadakata T., Furuichi T. Ca2+-dependent activator protein for secretion 2 and autistic-like phenotypes. Neurosci. Res. 2010;67(3):197–202. doi: 10.1016/j.neures.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 102.Yagishita K., Suzuki R., Mizuno S., Katoh-Semba R., Sadakata T., Sano Y., Furuichi T., Shinoda Y. CAPS2 deficiency affects environmental enrichment-induced adult neurogenesis and differentiation/survival of newborn neurons in the hippocampal dentate gyrus. Neurosci. Lett. 2017;661:121–125. doi: 10.1016/j.neulet.2017.09.047. [DOI] [PubMed] [Google Scholar]
- 103.Shinoda Y., Sadakata T., Nakao K., Katoh-Semba R., Kinameri E., Furuya A., Yanagawa Y., Hirase H., Furuichi T. Calcium-dependent activator protein for secretion 2 (CAPS2) promotes BDNF secretion and is critical for the development of GABAergic interneuron network. Proc. Natl. Acad. Sci. USA. 2011;108(1):373–378. doi: 10.1073/pnas.1012220108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gharani N., Benayed R., Mancuso V., Brzustowicz L.M., Millonig J.H. Association of the homeobox transcription factor, ENGRAILED 2, 3, with autism spectrum disorder. Mol. Psychiatry. 2004;9(5):474–484. doi: 10.1038/sj.mp.4001498. [DOI] [PubMed] [Google Scholar]
- 105.Benayed R., Gharani N., Rossman I., Mancuso V., Lazar G., Kamdar S., Bruse S.E., Tischfield S., Smith B.J., Zimmerman R.A., DiCicco-Bloom E., Brzustowicz L.M., Millonig J.H. Support for the homeobox transcription factor gene ENGRAILED 2 as an autism spectrum disorder susceptibility locus. Am. J. Hum. Genet. 2005;77(5):851–868. doi: 10.1086/497705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Benayed R., Choi J., Matteson P.G., Gharani N., Kamdar S., Brzustowicz L.M., Millonig J.H. Autism-associated haplotype affects the regulation of the homeobox gene, ENGRAILED 2. Biol. Psychiatry. 2009;66(10):911–917. doi: 10.1016/j.biopsych.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cheh M.A., Millonig J.H., Roselli L.M., Ming X., Jacobsen E., Kamdar S., Wagner G.C. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Res. 2006;1116(1):166–176. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- 108.Genestine M., Lin L., Durens M., Yan Y., Jiang Y., Prem S., Bailoor K., Kelly B., Sonsalla P.K., Matteson P.G., Silverman J., Crawley J.N., Millonig J.H., DiCicco-Bloom E. Engrailed-2 (En2) deletion produces multiple neurodevelopmental defects in monoamine systems, forebrain structures and neurogenesis and behavior. Hum. Mol. Genet. 2015;24(20):5805–5827. doi: 10.1093/hmg/ddv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Durens M., Soliman M., Millonig J., DiCicco-Bloom E. Engrailed‐2 is a cell autonomous regulator of neurogenesis in cultured hippocampal neural stem cells. Dev. Neurobiol. 2021;81(5):724–735. doi: 10.1002/dneu.22824. [DOI] [PubMed] [Google Scholar]
- 110.Feng C., Chen Y., Zhang Y., Yan Y., Yang M., Gui H., Wang M. Pten regulates mitochondrial biogenesis via the akt/gsk-3β/pgc-1α pathway in autism. Neuroscience. 2021;465:85–94. doi: 10.1016/j.neuroscience.2021.04.010. [DOI] [PubMed] [Google Scholar]
- 111.Sgadò P., Genovesi S., Kalinovsky A., Zunino G., Macchi F., Allegra M., Murenu E., Provenzano G., Tripathi P.P., Casarosa S., Joyner A.L., Bozzi Y. Loss of GABAergic neurons in the hippocampus and cerebral cortex of Engrailed-2 null mutant mice: Implications for autism spectrum disorders. Exp. Neurol. 2013;247:496–505. doi: 10.1016/j.expneurol.2013.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amiri A., Cho W., Zhou J., Birnbaum S.G., Sinton C.M., McKay R.M., Parada L.F. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J. Neurosci. 2012;32(17):5880–5890. doi: 10.1523/JNEUROSCI.5462-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang J., Cui Y., Yu Z., Wang W., Cheng X., Ji W., Guo S., Zhou Q., Wu N., Chen Y., Chen Y., Song X., Jiang H., Wang Y., Lan Y., Zhou B., Mao L., Li J., Yang H., Guo W., Yang X. Brain endothelial cells maintain lactate homeostasis and control adult hippocampal neurogenesis. Cell Stem Cell. 2019;25(6):754–767.e9. doi: 10.1016/j.stem.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 114.Pinar C., Yau S., Sharp Z., Shamei A., Fontaine C.J., Meconi A.L., Lottenberg C.P., Christie B.R. Effects of voluntary exercise on cell proliferation and neurogenesis in the dentate gyrus of adult fmr1 knockout mice. Brain Plast. 2018;4(2):185–195. doi: 10.3233/BPL-170052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pieretti M., Zhang F., Fu Y.H., Warren S.T., Oostra B.A., Caskey C.T., Nelson D.L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991;66(4):817–822. doi: 10.1016/0092-8674(91)90125-I. [DOI] [PubMed] [Google Scholar]
- 116.Wang T., Bray S.M., Warren S.T. New perspectives on the biology of fragile X syndrome. Curr. Opin. Genet. Dev. 2012;22(3):256–263. doi: 10.1016/j.gde.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luo Y., Shan G., Guo W., Smrt R.D., Johnson E.B., Li X., Pfeiffer R.L., Szulwach K.E., Duan R., Barkho B.Z., Li W., Liu C., Jin P., Zhao X. Fragile x mental retardation protein regulates proliferation and differentiation of adult neural stem/progenitor cells. PLoS Genet. 2010;6(4):e1000898. doi: 10.1371/journal.pgen.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo W., Allan A.M., Zong R., Zhang L., Johnson E.B., Schaller E.G., Murthy A.C., Goggin S.L., Eisch A.J., Oostra B.A., Nelson D.L., Jin P., Zhao X. Ablation of Fmrp in adult neural stem cells disrupts hippocampus-dependent learning. Nat. Med. 2011;17(5):559–565. doi: 10.1038/nm.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Eadie B.D., Zhang W.N., Boehme F., Gil-Mohapel J., Kainer L., Simpson J.M., Christie B.R. Fmr1 knockout mice show reduced anxiety and alterations in neurogenesis that are specific to the ventral dentate gyrus. Neurobiol. Dis. 2009;36(2):361–373. doi: 10.1016/j.nbd.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 120.Franklin A.V., King M.K., Palomo V., Martinez A., McMahon L.L., Jope R.S. Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol. Psychiatry. 2014;75(3):198–206. doi: 10.1016/j.biopsych.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pathania M., Davenport E.C., Muir J., Sheehan D.F., López-Doménech G., Kittler J.T. The autism and schizophrenia associated gene CYFIP1 is critical for the maintenance of dendritic complexity and the stabilization of mature spines. Transl. Psychiatry. 2014;4(3):e374. doi: 10.1038/tp.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cox D., Butler M. The 15q11.2 BP1-BP2 microdeletion syndrome: A review. Int. J. Mol. Sci. 2015;16(2):4068–4082. doi: 10.3390/ijms16024068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Haan N., Westacott L.J., Carter J., Owen M.J., Gray W.P., Hall J., Wilkinson L.S. Haploinsufficiency of the schizophrenia and autism risk gene Cyfip1 causes abnormal postnatal hippocampal neurogenesis through microglial and Arp2/3 mediated actin dependent mechanisms. Transl. Psychiatry. 2021;11(1):313. doi: 10.1038/s41398-021-01415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peng Z., Deng B., Jia J., Hou W., Hu S., Deng J., Lin W., Hou L., Sang H. Liver X receptor β in the hippocampus: A potential novel target for the treatment of major depressive disorder? Neuropharmacology. 2018;135:514–528. doi: 10.1016/j.neuropharm.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 125.Xu P., Li D., Tang X., Bao X., Huang J., Tang Y., Yang Y., Xu H., Fan X. LXR agonists: new potential therapeutic drug for neurodegenerative diseases. Mol. Neurobiol. 2013;48(3):715–728. doi: 10.1007/s12035-013-8461-3. [DOI] [PubMed] [Google Scholar]
- 126.Li X., Zhong H., Wang Z., Xiao R., Antonson P., Liu T., Wu C., Zou J., Wang L., Nalvarte I., Xu H., Warner M., Gustafsson J.A., Fan X. Loss of liver X receptor β in astrocytes leads to anxiety-like behaviors via regulating synaptic transmission in the medial prefrontal cortex in mice. Mol. Psychiatry. 2021;26(11):6380–6393. doi: 10.1038/s41380-021-01139-5. [DOI] [PubMed] [Google Scholar]
- 127.Fan X., Kim H.J., Bouton D., Warner M., Gustafsson J.Å. Expression of liver X receptor β is essential for formation of superficial cortical layers and migration of later-born neurons. Proc. Natl. Acad. Sci. USA. 2008;105(36):13445–13450. doi: 10.1073/pnas.0806974105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xing Y., Fan X., Ying D. Liver X receptor agonist treatment promotes the migration of granule neurons during cerebellar development. J. Neurochem. 2010;115(6):1486–1494. doi: 10.1111/j.1471-4159.2010.07053.x. [DOI] [PubMed] [Google Scholar]
- 129.Xu P., Xu H., Tang X., Xu L., Wang Y., Guo L., Yang Z., Xing Y., Wu Y., Warner M., Gustafsson J-A., Fan X. Liver X receptor β is essential for the differentiation of radial glial cells to oligodendrocytes in the dorsal cortex. Mol. Psychiatry. 2014;19(8):947–957. doi: 10.1038/mp.2014.60. [DOI] [PubMed] [Google Scholar]
- 130.Guo L., Xu P., Tang X., Wu Q., Xing Y., Gustafsson J.A., Xu H., Fan X. Liver X receptor β delays transformation of radial glial cells into astrocytes during mouse cerebral cortical development. Neurochem. Int. 2014;71:8–16. doi: 10.1016/j.neuint.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 131.Cai Y., Tang X., Chen X., Li X., Wang Y., Bao X., Wang L., Sun D., Zhao J., Xing Y., Warner M., Xu H., Gustafsson J.Å., Fan X. Liver X receptor β regulates the development of the dentate gyrus and autistic-like behavior in the mouse. Proc. Natl. Acad. Sci. USA. 2018;115(12):E2725–E2733. doi: 10.1073/pnas.1800184115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stoll G., Pietiläinen O.P.H., Linder B., Suvisaari J., Brosi C., Hennah W., Leppä V., Torniainen M., Ripatti S., Ala-Mello S., Plöttner O., Rehnström K., Tuulio-Henriksson A., Varilo T., Tallila J., Kristiansson K., Isohanni M., Kaprio J., Eriksson J.G., Raitakari O.T., Lehtimäki T., Jarvelin M.R., Salomaa V., Hurles M., Stefansson H., Peltonen L., Sullivan P.F., Paunio T., Lönnqvist J., Daly M.J., Fischer U., Freimer N.B., Palotie A. Deletion of TOP3β a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat. Neurosci. 2013;16(9):1228–1237. doi: 10.1038/nn.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Joo Y., Xue Y., Wang Y., McDevitt R.A., Sah N., Bossi S., Su S., Lee S.K., Peng W., Xie A., Zhang Y., Ding Y., Ku W.L., Ghosh S., Fishbein K., Shen W., Spencer R., Becker K., Zhao K., Mattson M.P., van Praag H., Sharov A., Wang W. Topoisomerase 3β knockout mice show transcriptional and behavioural impairments associated with neurogenesis and synaptic plasticity. Nat. Commun. 2020;11(1):3143. doi: 10.1038/s41467-020-16884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xu D., Shen W., Guo R., Xue Y., Peng W., Sima J., Yang J., Sharov A., Srikantan S., Yang J., Fox D., III, Qian Y., Martindale J.L., Piao Y., Machamer J., Joshi S.R., Mohanty S., Shaw A.C., Lloyd T.E., Brown G.W., Ko M.S.H., Gorospe M., Zou S., Wang W. Top3β is an RNA topoisomerase that works with fragile X syndrome protein to promote synapse formation. Nat. Neurosci. 2013;16(9):1238–1247. doi: 10.1038/nn.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ahmad M., Shen W., Li W., Xue Y., Zou S., Xu D., Wang W. Topoisomerase 3β is the major topoisomerase for mRNAs and linked to neurodevelopment and mental dysfunction. Nucleic Acids Res. 2017;45(5):2704–2713. doi: 10.1093/nar/gkw1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rahman F.U., Kim Y.R., Kim E.K., Kim H., Cho S.M., Lee C.S., Kim S.J., Araki K., Yamamura K., Lee M.N., Park S.G., Yoon W.K., Lee K., Won Y.S., Kim H.C., Lee Y., Lee H.Y., Nam K.H. Topoisomerase iiiβ deficiency induces neuro-behavioral changes and brain connectivity alterations in mice. Int. J. Mol. Sci. 2021;22(23):12806. doi: 10.3390/ijms222312806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plummer J.T., Evgrafov O.V., Bergman M.Y., Friez M., Haiman C.A., Levitt P., Aldinger K.A. Transcriptional regulation of the MET receptor tyrosine kinase gene by MeCP2 and sex-specific expression in autism and Rett syndrome. Transl. Psychiatry. 2013;3(10):e316. doi: 10.1038/tp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wen Z., Cheng T.L., Li G., Sun S.B., Yu S.Y., Zhang Y., Du Y.S., Qiu Z. Identification of autism-related MECP2 mutations by whole-exome sequencing and functional validation. Mol. Autism. 2017;8(1):43. doi: 10.1186/s13229-017-0157-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bertoldi M.L., Zalosnik M.I., Fabio M.C., Aja S., Roth G.A., Ronnett G.V., Degano A.L. Mecp2 deficiency disrupts kainate-induced presynaptic plasticity in the mossy fiber projections in the hippocampus. Front. Cell. Neurosci. 2019;13:286. doi: 10.3389/fncel.2019.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu Z., Li X., Zhang J.T., Cai Y.J., Cheng T.L., Cheng C., Wang Y., Zhang C.C., Nie Y.H., Chen Z.F., Bian W.J., Zhang L., Xiao J., Lu B., Zhang Y.F., Zhang X.D., Sang X., Wu J.J., Xu X., Xiong Z.Q., Zhang F., Yu X., Gong N., Zhou W.H., Sun Q., Qiu Z. Autism-like behaviours and germline transmission in transgenic monkeys overexpressing MeCP2. Nature. 2016;530(7588):98–102. doi: 10.1038/nature16533. [DOI] [PubMed] [Google Scholar]
- 141.Qiu Z. Deciphering MECP2 -associated disorders: Disrupted circuits and the hope for repair. Curr. Opin. Neurobiol. 2018;48:30–36. doi: 10.1016/j.conb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 142.Chen Z., Li X., Zhou J., Yuan B., Yu B., Tong D., Cheng C., Shao Y., Xia S., Zhang R., Lyu J., Yu X., Dong C., Zhou W.H., Qiu Z. Accumulated quiescent neural stem cells in adult hippocampus of the mouse model for the MECP2 duplication syndrome. Sci. Rep. 2017;7(1):41701. doi: 10.1038/srep41701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Allan A.M., Liang X., Luo Y., Pak C., Li X., Szulwach K.E., Chen D., Jin P., Zhao X. The loss of methyl-CpG binding protein 1 leads to autism-like behavioral deficits. Hum. Mol. Genet. 2008;17(13):2047–2057. doi: 10.1093/hmg/ddn102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liu C., Teng Z.Q., Santistevan N.J., Szulwach K.E., Guo W., Jin P., Zhao X. Epigenetic regulation of miR-184 by MBD1 governs neural stem cell proliferation and differentiation. Cell Stem Cell. 2010;6(5):433–444. doi: 10.1016/j.stem.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jobe E.M., Gao Y., Eisinger B.E., Mladucky J.K., Giuliani C.C., Kelnhofer L.E., Zhao X. Methyl-cpg-binding protein mbd1 regulates neuronal lineage commitment through maintaining adult neural stem cell identity. J. Neurosci. 2017;37(3):523–536. doi: 10.1523/JNEUROSCI.1075-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jalal R., Nair A., Lin A., Eckfeld A., Kushan L., Zinberg J., Karlsgodt K.H., Cannon T.D., Bearden C.E. Social cognition in 22q11.2 deletion syndrome and idiopathic developmental neuropsychiatric disorders. J. Neurodev. Disord. 2021;13(1):15. doi: 10.1186/s11689-021-09363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baldini A. The 22q11.2 deletion syndrome: a gene dosage perspective. Sci. World J. 2006;6:1881–1887. doi: 10.1100/tsw.2006.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hiramoto T., Kang G., Suzuki G., Satoh Y., Kucherlapati R., Watanabe Y., Hiroi N. Tbx1: identification of a 22q11.2 gene as a risk factor for autism spectrum disorder in a mouse model. Hum. Mol. Genet. 2011;20(24):4775–4785. doi: 10.1093/hmg/ddr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Boku S., Izumi T., Abe S., Takahashi T., Nishi A., Nomaru H., Naka Y., Kang G., Nagashima M., Hishimoto A., Enomoto S., Duran-Torres G., Tanigaki K., Zhang J., Ye K., Kato S., Männistö P.T., Kobayashi K., Hiroi N. Copy number elevation of 22q11.2 genes arrests the developmental maturation of working memory capacity and adult hippocampal neurogenesis. Mol. Psychiatry. 2018;23(4):985–992. doi: 10.1038/mp.2017.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gradari S., Herrera A., Tezanos P., Fontán-Lozano Á., Pons S., Trejo J.L. The role of smad2 in adult neuroplasticity as seen through hippocampal-dependent spatial learning/memory and neurogenesis. J. Neurosci. 2021;41(32):6836–6849. doi: 10.1523/JNEUROSCI.2619-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sippel D., Schwabedal J., Snyder J.C., Oyanedel C.N., Bernas S.N., Garthe A., Tröndle A., Storch A., Kempermann G., Brandt M.D. Disruption of NREM sleep and sleep-related spatial memory consolidation in mice lacking adult hippocampal neurogenesis. Sci. Rep. 2020;10(1):16467. doi: 10.1038/s41598-020-72362-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Walgrave H., Balusu S., Snoeck S., Vanden Eynden E., Craessaerts K., Thrupp N., Wolfs L., Horré K., Fourne Y., Ronisz A., Silajdžić E., Penning A., Tosoni G., Callaerts-Vegh Z., D'Hooge R., Thal D.R., Zetterberg H., Thuret S., Fiers M., Frigerio C.S., De Strooper B., Salta E. Restoring mir-132 expression rescues adult hippocampal neurogenesis and memory deficits in alzheimer's disease. Cell Stem Cell. 2021;28(10):1805–1821. doi: 10.1016/j.stem.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 153.Tokatly Latzer I., Leitner Y., Karnieli-Miller O. Core experiences of parents of children with autism during the COVID-19 pandemic lockdown. Autism. 2021;25(4):1047–1059. doi: 10.1177/1362361320984317. [DOI] [PubMed] [Google Scholar]
- 154.Steiner H., Kertesz Z. Effects of therapeutic horse riding on gait cycle parameters and some aspects of behavior of children with autism. Acta Physiol. Hung. 2015;102(3):324–335. doi: 10.1556/036.102.2015.3.10. [DOI] [PubMed] [Google Scholar]
- 155.Cai Y., Zhong H., Li X., Xiao R., Wang L., Fan X. The liver x receptor agonist to901317 ameliorates behavioral deficits in two mouse models of autism. Front. Cell. Neurosci. 2019;13:213. doi: 10.3389/fncel.2019.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cai K.L., Wang J.G., Liu Z.M., Zhu L.N., Xiong X., Klich S., Maszczyk A., Chen A.G. Mini-basketball training program improves physical fitness and social communication in preschool children with autism spectrum disorders. J. Hum. Kinet. 2020;73(1):267–278. doi: 10.2478/hukin-2020-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Seo T.B., Cho H.S., Shin M.S., Kim C.J., Ji E.S., Baek S.S. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J. Exerc. Rehabil. 2013;9(2):220–229. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Javadi S., Li Y., Sheng J., Zhao L., Fu Y., Wang D., Zhao X. Sustained correction of hippocampal neurogenic and cognitive deficits after a brief treatment by Nutlin-3 in a mouse model of fragile X syndrome. BMC Med. 2022;20(1):163. doi: 10.1186/s12916-022-02370-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luhach K., Kulkarni G.T., Singh V.P., Sharma B. Vinpocetine ameliorates developmental hyperserotonemia induced behavioral and biochemical changes: role of neuronal function, inflammation, and oxidative stress. Acta Neurobiol. Exp. (Warsz.) 2022;82(1):35–51. doi: 10.55782/ane-2022-004. [DOI] [PubMed] [Google Scholar]
- 160.Cheng Y., Wang Z.M., Tan W., Wang X., Li Y., Bai B., Li Y., Zhang S.F., Yan H.L., Chen Z.L., Liu C.M., Mi T.W., Xia S., Zhou Z., Liu A., Tang G.B., Liu C., Dai Z.J., Wang Y.Y., Wang H., Wang X., Kang Y., Lin L., Chen Z., Xie N., Sun Q., Xie W., Peng J., Chen D., Teng Z.Q., Jin P. Partial loss of psychiatric risk gene Mir137 in mice causes repetitive behavior and impairs sociability and learning via increased Pde10a. Nat. Neurosci. 2018;21(12):1689–1703. doi: 10.1038/s41593-018-0261-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Luhach K., Kulkarni G.T., Singh V.P., Sharma B. Effect of papaverine on developmental hyperserotonemia induced autism spectrum disorder related behavioural phenotypes by altering markers of neuronal function, inflammation, and oxidative stress in rats. Clin. Exp. Pharmacol. Physiol. 2021;48(4):614–625. doi: 10.1111/1440-1681.13459. [DOI] [PubMed] [Google Scholar]
- 162.Fernández de Cossío L., Lacabanne C., Bordeleau M., Castino G., Kyriakakis P., Tremblay M.È. Lipopolysaccharide-induced maternal immune activation modulates microglial CX3CR1 protein expression and morphological phenotype in the hippocampus and dentate gyrus, resulting in cognitive inflexibility during late adolescence. Brain Behav. Immun. 2021;97:440–454. doi: 10.1016/j.bbi.2021.07.025. [DOI] [PubMed] [Google Scholar]
- 163.Chen C., Whitsel E.A., Espeland M.A., Snetselaar L., Hayden K.M., Lamichhane A.P., Serre M.L., Vizuete W., Kaufman J.D., Wang X., Chui H.C., D’Alton M.E., Chen J.C., Kahe K. B vitamin intakes modify the association between particulate air pollutants and incidence of all‐cause dementia: Findings from the Women’s Health Initiative Memory Study. Alzheimers Dement. 2022;18(11):2188–2198. doi: 10.1002/alz.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Philippot G., Hellsten S.V., Viberg H., Fredriksson R. Evaluation of the dentate gyrus in adult mice exposed to acetaminophen (paracetamol) on postnatal day 10. Int. J. Dev. Neurosci. 2021;81(1):91–97. doi: 10.1002/jdn.10079. [DOI] [PubMed] [Google Scholar]
- 165.Otellin V.A., Khozhaĭ L.I., Vataeva L.A. Effect of hypoxia in early perinatal ontogenesis on behavior and structural characteristics of the rat brain. Zh. Evol. Biokhim. Fiziol. 2012;48(5):467–473. [PubMed] [Google Scholar]
- 166.Bhat A., Mahalakshmi A.M., Ray B., Tuladhar S., Hediyal T.A., Manthiannem E., Padamati J., Chandra R., Chidambaram S.B., Sakharkar M.K. Benefits of curcumin in brain disorders. Biofactors. 2019;45(5):666–689. doi: 10.1002/biof.1533. [DOI] [PubMed] [Google Scholar]
- 167.Li J., Wang H., Qing W., Liu F., Zeng N., Wu F., Shi Y., Gao X., Cheng M., Li H., Shen W., Meng F., He Y., Chen M., Li Z., Zhou H., Wang Q. Congenitally underdeveloped intestine drives autism-related gut microbiota and behavior. Brain Behav. Immun. 2022;105:15–26. doi: 10.1016/j.bbi.2022.06.006. [DOI] [PubMed] [Google Scholar]
- 168.Liu G., Yu Q., Tan B., Ke X., Zhang C., Li H., Zhang T., Lu Y. Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microbes. 2022;14(1):2104089. doi: 10.1080/19490976.2022.2104089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.De Gioia R., Biella F., Citterio G., Rizzo F., Abati E., Nizzardo M., Bresolin N., Comi G.P., Corti S. Neural stem cell transplantation for neurodegenerative diseases. Int. J. Mol. Sci. 2020;21(9):3103. doi: 10.3390/ijms21093103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Chang B.L., Chang K.H. Stem cell therapy in treating epilepsy. Front. Neurosci. 2022;16:934507. doi: 10.3389/fnins.2022.934507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat. Rev. Neurosci. 2020;21(2):103–115. doi: 10.1038/s41583-019-0257-7. [DOI] [PubMed] [Google Scholar]
- 172.Villarreal-Martínez L., González-Martínez G., Sáenz-Flores M., Bautista-Gómez A.J., González-Martínez A., Ortiz-Castillo M., Robles-Sáenz D.A., Garza-López E. Stem cell therapy in the treatment of patients with autism spectrum disorder: A systematic review and meta-analysis. Stem Cell Rev. Rep. 2022;18(1):155–164. doi: 10.1007/s12015-021-10257-0. [DOI] [PubMed] [Google Scholar]
- 173.Qu J., Liu Z., Li L., Zou Z., He Z., Zhou L., Luo Y., Zhang M., Ye J. Efficacy and safety of stem cell therapy in children with autism spectrum disorders: A systematic review and meta-analysis. Front Pediatr. 2022;10:897398. doi: 10.3389/fped.2022.897398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kobinia G.S., Zaknun J.J., Pabinger C., Laky B. Case report: Autologous bone marrow derived intrathecal stem cell transplant for autistic children - a report of four cases and literature review. Front Pediatr. 2021;9:620188. doi: 10.3389/fped.2021.620188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maltman D.J., Hardy S.A., Przyborski S.A. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem. Int. 2011;59(3):347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 176.Segal-Gavish H., Karvat G., Barak N., Barzilay R., Ganz J., Edry L., Aharony I., Offen D., Kimchi T. Mesenchymal stem cell transplantation promotes neurogenesis and ameliorates autism related behaviors in btbr mice. Autism Res. 2016;9(1):17–32. doi: 10.1002/aur.1530. [DOI] [PubMed] [Google Scholar]
- 177.Dawson G., Sun J.M., Baker J., Carpenter K., Compton S., Deaver M., Franz L., Heilbron N., Herold B., Horrigan J., Howard J., Kosinski A., Major S., Murias M., Page K., Prasad V.K., Sabatos-DeVito M., Sanfilippo F., Sikich L., Simmons R., Song A., Vermeer S., Waters-Pick B., Troy J., Kurtzberg J. A phase ii randomized clinical trial of the safety and efficacy of intravenous umbilical cord blood infusion for treatment of children with autism spectrum disorder. J. Pediatr. 2020;222:164–173.e5. doi: 10.1016/j.jpeds.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 178.Dawson G., Sun J.M., Davlantis K.S., Murias M., Franz L., Troy J., Simmons R., Sabatos-DeVito M., Durham R., Kurtzberg J. Autologous cord blood infusions are safe and feasible in young children with autism spectrum disorder: Results of a single-center phase i open-label trial. Stem Cells Transl. Med. 2017;6(5):1332–1339. doi: 10.1002/sctm.16-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Liang Y., Duan L., Xu X., Li X., Liu M., Chen H., Lu J., Xia J. Mesenchymal stem cell-derived exosomes for treatment of autism spectrum disorder. ACS Appl. Bio Mater. 2020;3(9):6384–6393. doi: 10.1021/acsabm.0c00831. [DOI] [PubMed] [Google Scholar]
- 180.Perets N., Segal-Gavish H., Gothelf Y., Barzilay R., Barhum Y., Abramov N., Hertz S., Morozov D., London M., Offen D. Long term beneficial effect of neurotrophic factors-secreting mesenchymal stem cells transplantation in the BTBR mouse model of autism. Behav. Brain Res. 2017;331:254–260. doi: 10.1016/j.bbr.2017.03.047. [DOI] [PubMed] [Google Scholar]
- 181.Gobshtis N., Tfilin M., Wolfson M., Fraifeld V.E., Turgeman G. Transplantation of mesenchymal stem cells reverses behavioural deficits and impaired neurogenesis caused by prenatal exposure to valproic acid. Oncotarget. 2017;8(11):17443–17452. doi: 10.18632/oncotarget.15245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Yang P., Yuan W., Liu J., Li J., Tan B., Qiu C., Zhu X., Qiu C., Lai D., Guo L., Yu L. Biological characterization of human amniotic epithelial cells in a serum-free system and their safety evaluation. Acta Pharmacol. Sin. 2018;39(8):1305–1316. doi: 10.1038/aps.2018.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xu H., Zhang J., Tsang K.S., Yang H., Gao W.Q. Therapeutic potential of human amniotic epithelial cells on injuries and disorders in the central nervous system. Stem Cells Int. 2019;2019:1–11. doi: 10.1155/2019/5432301. [DOI] [PMC free article] [PubMed] [Google Scholar]


