Abstract
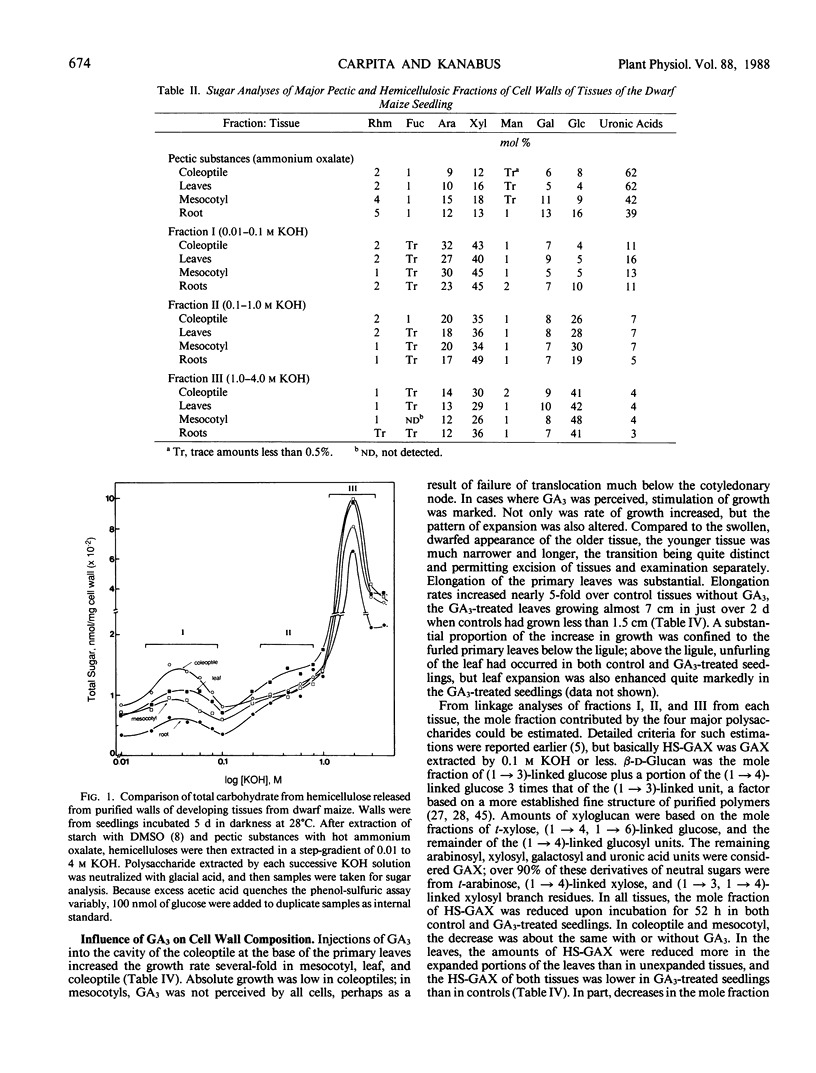
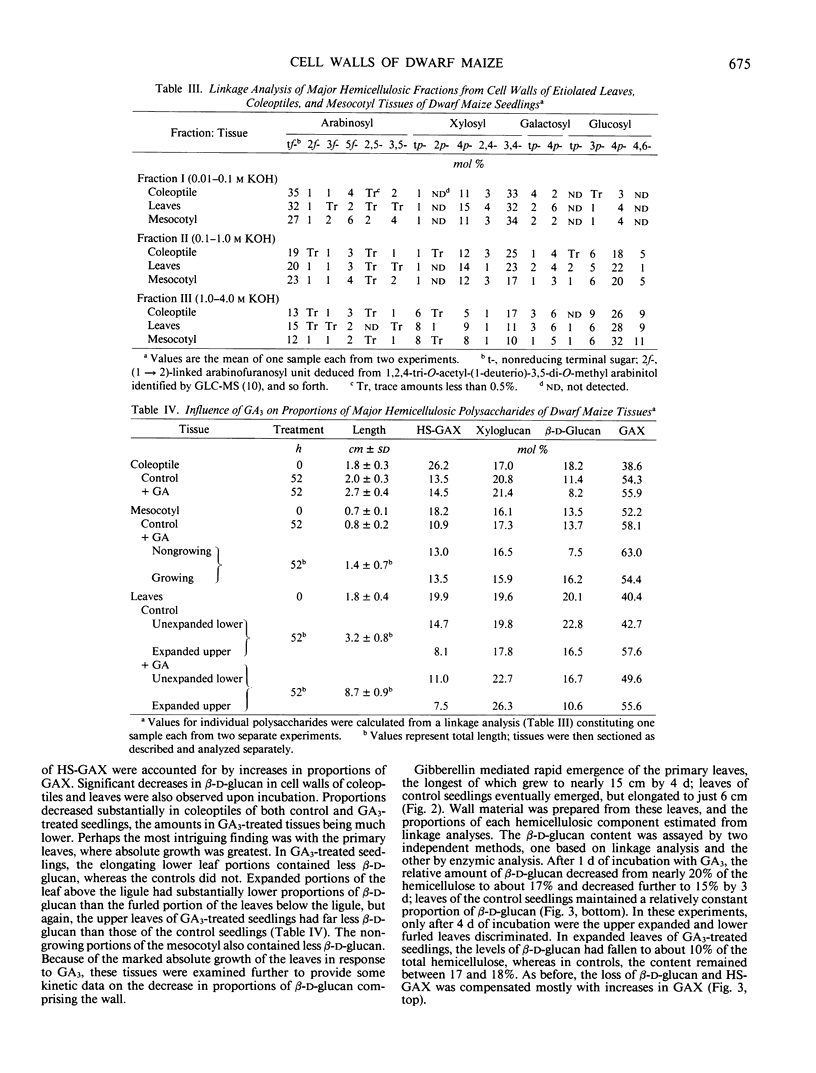
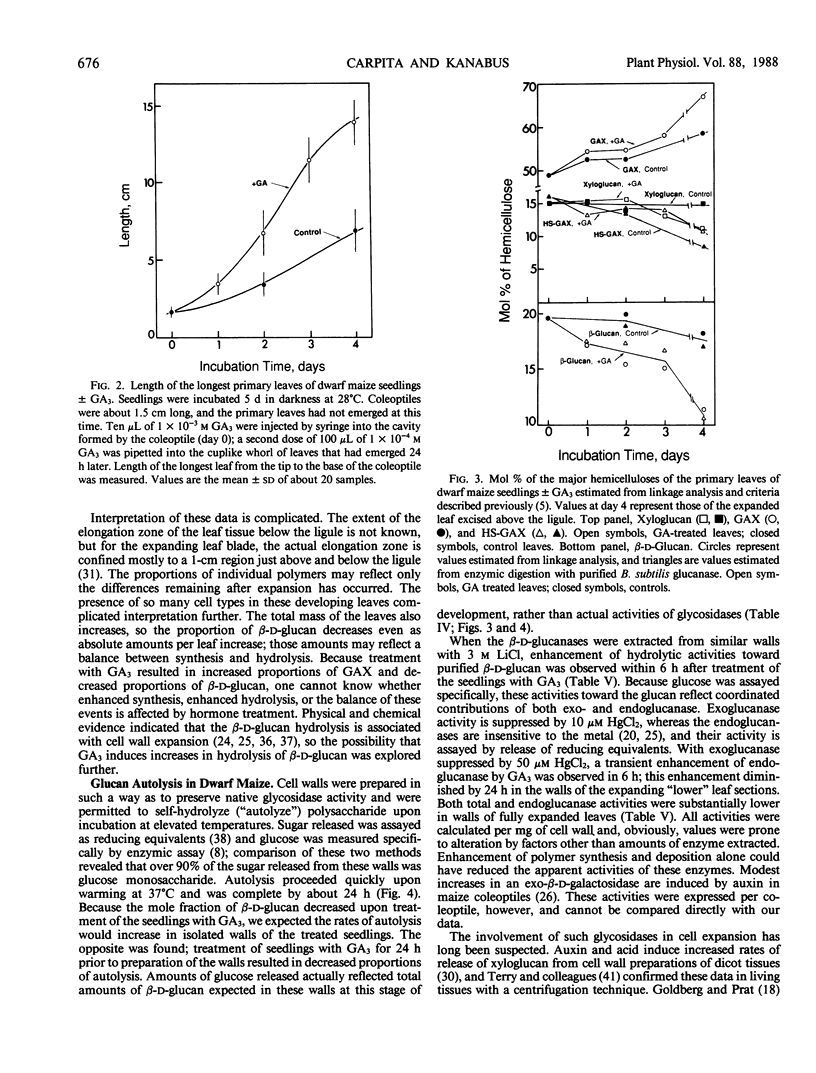
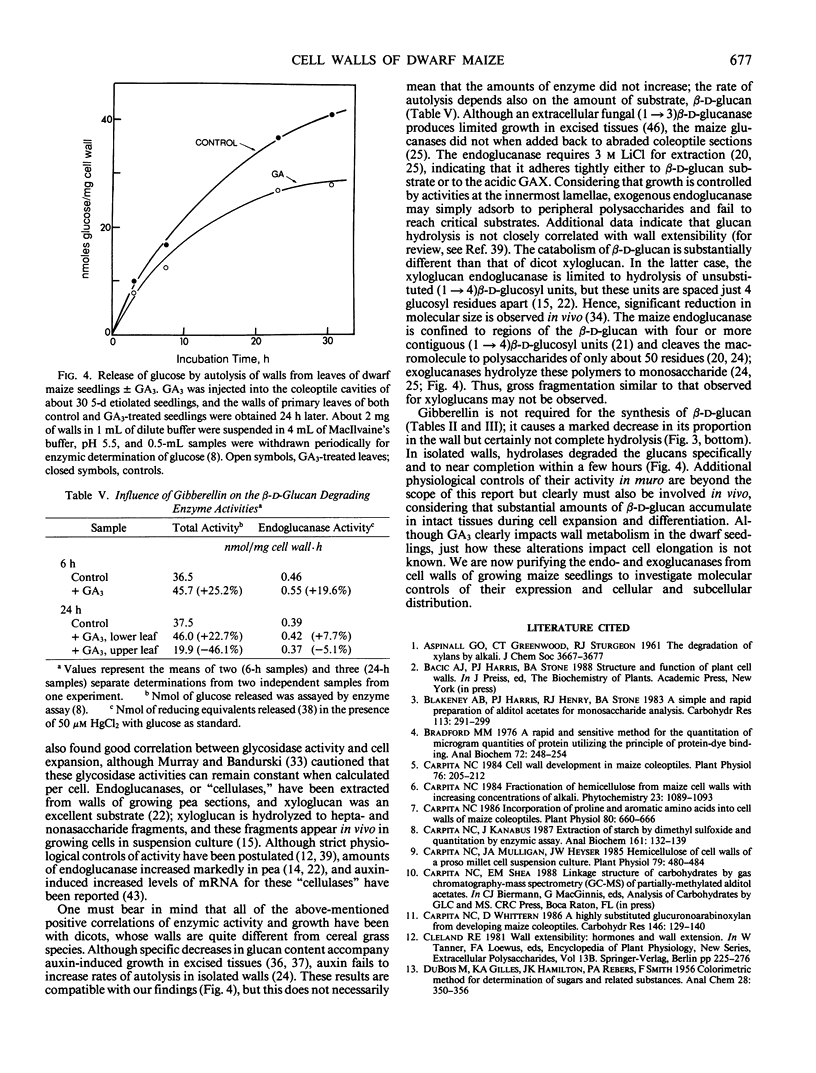
Dwarf maize (Zea mays L.), a mutant deficient in gibberellin synthesis, provides an excellent model to study the influence of gibberellin on biochemical processes related to plant development. Alterations in the chemical structure of the cell wall mediated by gibberellin were examined in seedlings of this mutant. The composition of the walls of roots, mesocotyl, coleoptile, and primary leaves of dwarf maize was similar to that of normal maize and other cereal grasses. Glucuronoarabinoxylans constituted the principal hemicelluloses, but walls also contained substantial amounts of xyloglucan and mixed-linkage β-d-glucan. Root growth in dwarf maize was essentially normal, but growth of mesocotyl and primary leaves was severely retarded. Injection of the gibberellin into the cavity of the coleoptile resulted in a marked increase in elongation of the primary leaves. This elongation was accompanied by increases in total wall mass, but the proportion of β-d-glucan decreased from 20% to 15% of the hemicellulosic polysaccharide. During leaf expansion, the proportion decreased further to only 10%. Through 4 days incubation, the proportion of β-d-glucan in leaves of control seedlings without gibberellin was nearly constant. Extraction of exo- and endo-β-d-glucan hydrolases from purified cell walls and assay against a purified oat bran β-d-glucan demonstrated that gibberellin increased the activity of the endo-β-d-glucan hydrolase. These and other data support the hypothesis that β-d-glucan metabolism is central to control of cell expansion in cereal grasses.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carpita N. C. Cell wall development in maize coleoptiles. Plant Physiol. 1984 Sep;76(1):205–212. doi: 10.1104/pp.76.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C. Incorporation of proline and aromatic amino acids into cell walls of maize coleoptiles. Plant Physiol. 1986 Mar;80(3):660–666. doi: 10.1104/pp.80.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpita N. C., Kanabus J. Extraction of starch by dimethyl sulfoxide and quantitation by enzymatic assay. Anal Biochem. 1987 Feb 15;161(1):132–139. doi: 10.1016/0003-2697(87)90662-2. [DOI] [PubMed] [Google Scholar]
- Carpita N. C., Mulligan J. A., Heyser J. W. Hemicelluloses of cell walls of a proso millet cell suspension culture. Plant Physiol. 1985 Oct;79(2):480–484. doi: 10.1104/pp.79.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. F., Maclachlan G. A. Massive synthesis of ribonucleic Acid and cellulase in the pea epicotyl in response to indoleacetic Acid, with and without concurrent cell division. Plant Physiol. 1967 Aug;42(8):1114–1122. doi: 10.1104/pp.42.8.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galambos J. T. The reaction of carbazole with carbohydrates. I. Effect of borate and sulfamate on the carbazole color of sugars. Anal Biochem. 1967 Apr;19(1):119–132. doi: 10.1016/0003-2697(67)90141-8. [DOI] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Hatfield R. D., Nevins D. J. Hydrolytic Activity and Substrate Specificity of an Endoglucanase from Zea mays Seedling Cell Walls. Plant Physiol. 1987 Jan;83(1):203–207. doi: 10.1104/pp.83.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Wong Y. S., Maclachlan G. Pea Xyloglucan and Cellulose : II. Hydrolysis by Pea Endo-1,4-beta-Glucanases. Plant Physiol. 1984 Jul;75(3):605–610. doi: 10.1104/pp.75.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D. J., Nevins D. J. Preparation and Properties of a beta-d-Glucanase for the Specific Hydrolysis of beta-d-Glucans. Plant Physiol. 1977 Aug;60(2):300–304. doi: 10.1104/pp.60.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. D., Daniels D., Dowler M. J., Rayle D. L. Activation of Avena coleoptile cell wall glycosidases by hydrogen ions and auxin. Plant Physiol. 1974 Feb;53(2):224–228. doi: 10.1104/pp.53.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y., Nevins D. J. Enzymic Dissociation of Zea Shoot Cell Wall Polysaccharides : II. Dissociation of (1 --> 3),(1 --> 4)-beta-d-Glucan by Purified (1 --> 3),(1 --> 4)-beta-d-Glucan 4-Glucanohydrolase from Bacillus subtilis. Plant Physiol. 1984 Jul;75(3):745–752. doi: 10.1104/pp.75.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson C. A., Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968 Sep;24(3):470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- Labavitch J. M., Ray P. M. Relationship between Promotion of Xyloglucan Metabolism and Induction of Elongation by Indoleacetic Acid. Plant Physiol. 1974 Oct;54(4):499–502. doi: 10.1104/pp.54.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOSCATELLI E. A., HAM E. A., RICKES E. L. Enzymatic properties of a beta-glucanase from Bacillus subtilis. J Biol Chem. 1961 Nov;236:2858–2862. [PubMed] [Google Scholar]
- Michelena V. A., Boyer J. S. Complete turgor maintenance at low water potentials in the elongating region of maize leaves. Plant Physiol. 1982 May;69(5):1145–1149. doi: 10.1104/pp.69.5.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. K., Bandurski R. S. Correlative studies of cell wall enzymes and growth. Plant Physiol. 1975 Jul;56(1):143–147. doi: 10.1104/pp.56.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phinney B. O. GROWTH RESPONSE OF SINGLE-GENE DWARF MUTANTS IN MAIZE TO GIBBERELLIC ACID. Proc Natl Acad Sci U S A. 1956 Apr;42(4):185–189. doi: 10.1073/pnas.42.4.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Terry M. E., Jones R. L. Soluble Cell Wall Polysaccharides Released from Pea Stems by Centrifugation : I. EFFECT OF AUXIN. Plant Physiol. 1981 Sep;68(3):531–537. doi: 10.1104/pp.68.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Updegraff D. M. Semimicro determination of cellulose in biological materials. Anal Biochem. 1969 Dec;32(3):420–424. doi: 10.1016/s0003-2697(69)80009-6. [DOI] [PubMed] [Google Scholar]
- Verma D. P., Maclachlan G. A., Byrne H., Ewings D. Regulation and in vitro translation of messenger ribonucleic acid for cellulase from auxin-treated pea epicotyls. J Biol Chem. 1975 Feb 10;250(3):1019–1026. [PubMed] [Google Scholar]



