Abstract
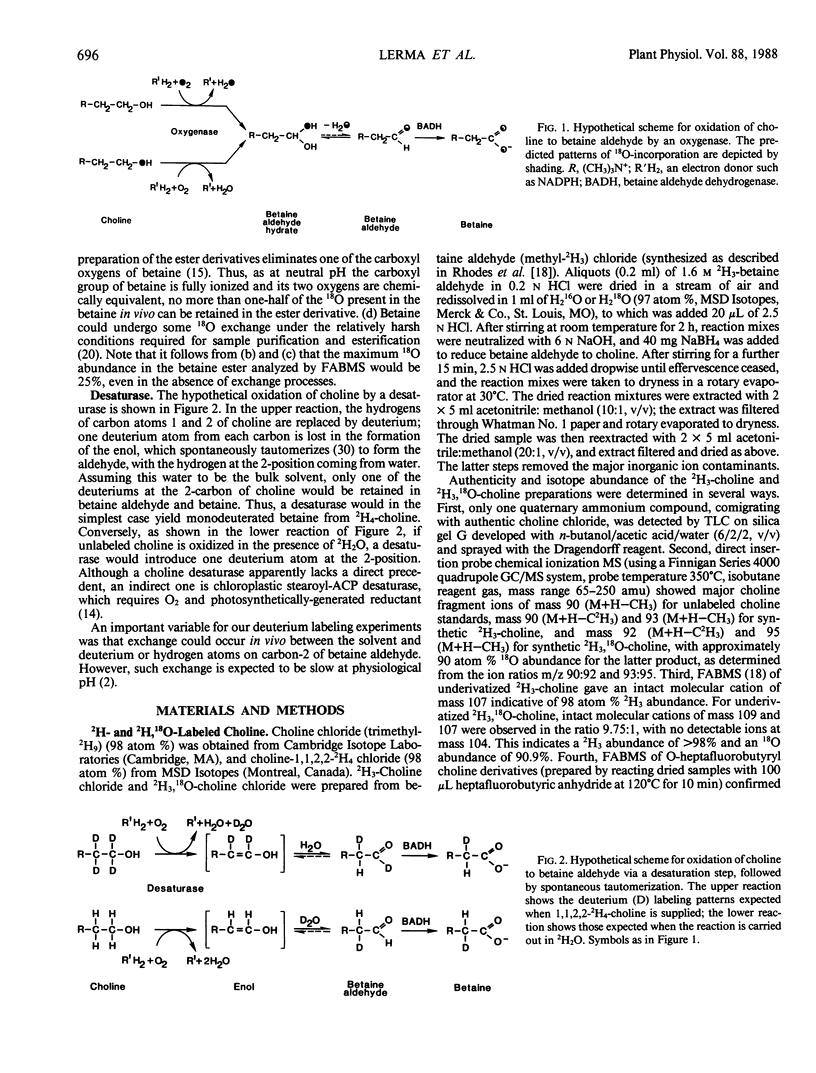
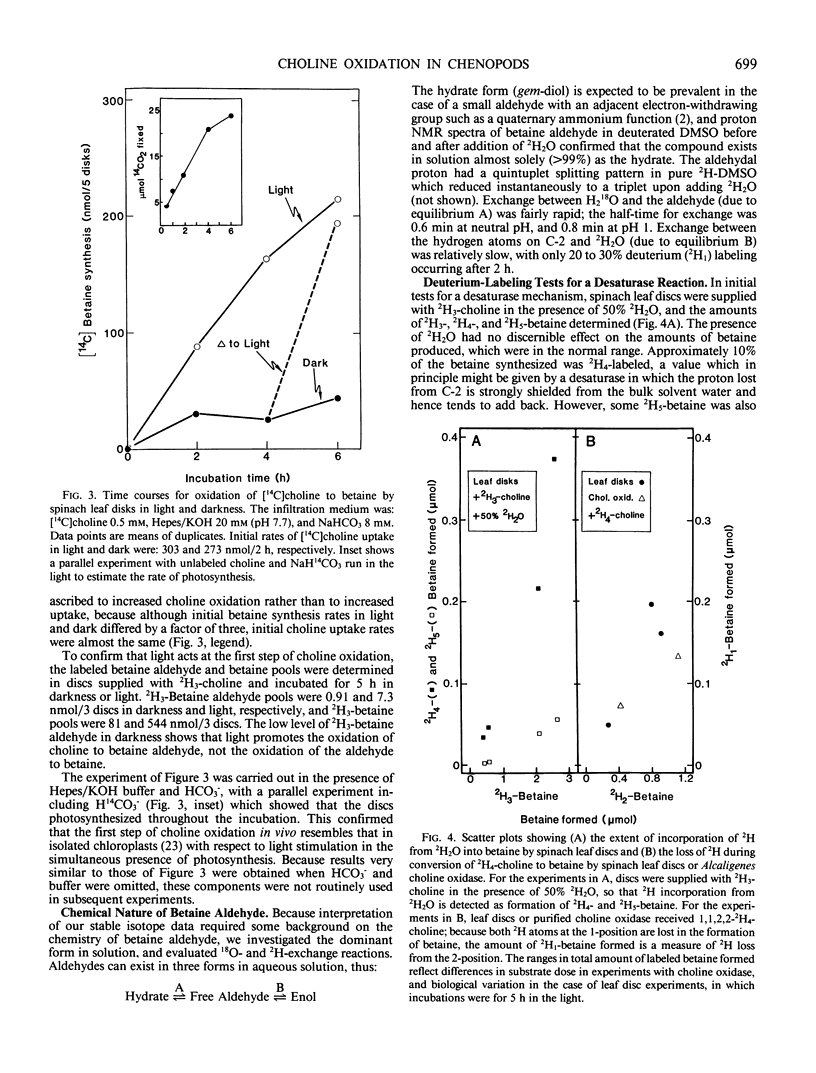
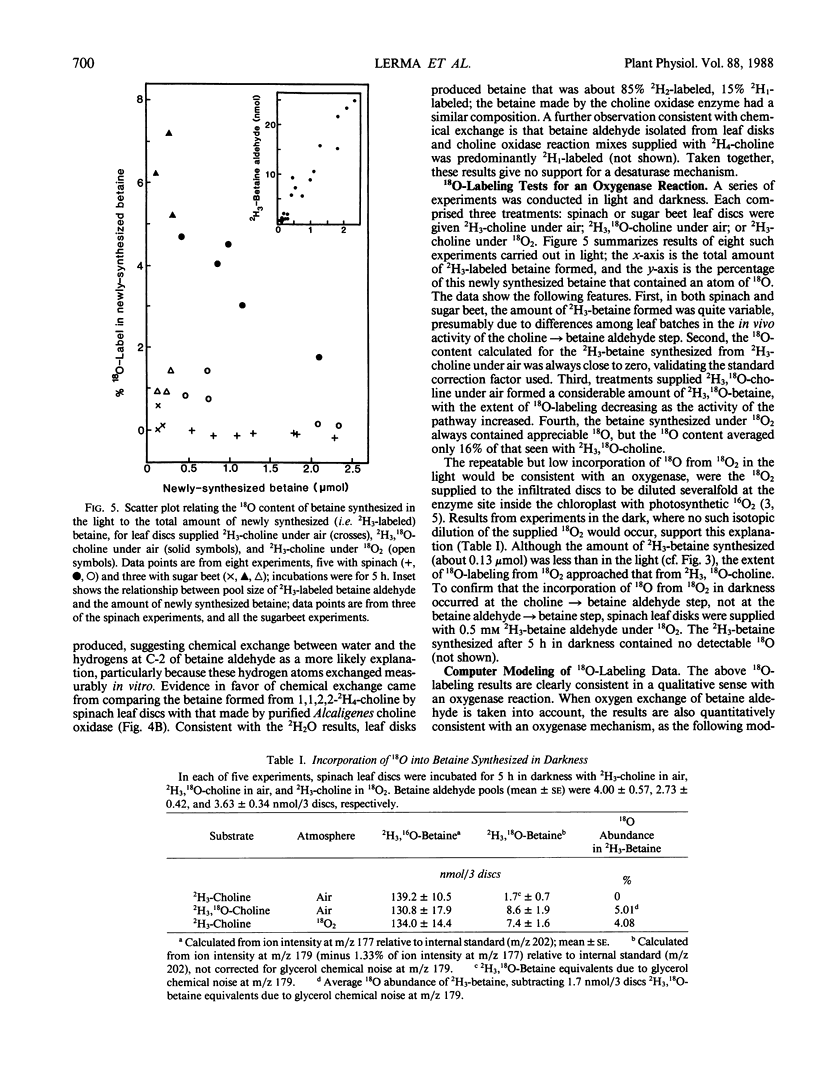
Chenopods synthesize betaine by a two-step oxidation of choline: choline → betaine aldehyde → betaine. The pathway is chloroplastic; the first step has been shown in isolated spinach (Spinacia oleracea L.) chloroplasts to be O2- and light-dependent, the role of light being to provide reducing power (P Weigel, EA Weretilnyk, AD Hanson 1988 Plant Physiol 86: 54-60). Here, we report use of in vivo18O- and 2H-labeling in conjunction with fast atom bombardment mass spectrometry to test for two hypothetical choline-oxidizing reactions that would explain the observed requirements for O2 and reductant: a desaturase or an oxygenase. Simple syntheses for 2H3-choline, 2H3, 18O-choline, and 2H3, 18O-betaine are given. A desaturase mechanism was sought by giving choline deuterated at the 2-carbon, or choline unlabeled at this position together with 2H2O and by analyzing newly synthesized betaine. About 15% of the 2H at C-2 was lost during oxidation of choline to betaine, and about 10% of the betaine made in the presence of 50% 2H2O was monodeuterated. These small effects are more consistent with chemical exchange than with a desaturase, because 10 to 15% losses of 2H from the C-2 position also occurred if choline was converted to betaine by a purified bacterial choline oxidase. To test for an oxygenase, the incorporation of 18O from 18O2 into newly synthesized betaine was compared with that from 18O-labeled choline, in light and darkness. Incorporation of 18O from 18O-choline was readily detectable and varied from about 15 to 50% of the theoretical maximum value; the 18O losses were attributable to exchange of the intermediate betaine aldehyde with water. In darkness, incorporation of 18O from 18O2 approached that from 18O-choline, but in the light was severalfold lower, presumably due to isotopic dilution by photosynthetic 16O2. These data indicate that the chloroplast choline-oxidizing enzyme is an oxygenase.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa K., Takabe T., Sugiyama T., Akazawa T. Purification of betaine-aldehyde dehydrogenase from spinach leaves and preparation of its antibody. J Biochem. 1987 Jun;101(6):1485–1488. doi: 10.1093/oxfordjournals.jbchem.a122019. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodley F. H., Blair A. H. Substrate characteristics of human liver aldehyde dehydrogenase. Can J Biochem. 1971 Jan;49(1):1–5. doi: 10.1139/o71-001. [DOI] [PubMed] [Google Scholar]
- GOOD N. E., BROWN A. H. The contribution of endogenous oxygen to the respiration of photosynthesizing Chlorella cells. Biochim Biophys Acta. 1961 Jul 8;50:544–554. doi: 10.1016/0006-3002(61)90014-2. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., May A. M., Grumet R., Bode J., Jamieson G. C., Rhodes D. Betaine synthesis in chenopods: Localization in chloroplasts. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3678–3682. doi: 10.1073/pnas.82.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuta S., Imamura S., Misaki H., Horiuti Y. Purification and characterization of choline oxidase from Arthrobacter globiformis. J Biochem. 1977 Dec;82(6):1741–1749. doi: 10.1093/oxfordjournals.jbchem.a131872. [DOI] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoh T., Watanabe J., Takahashi E. Sodium, Potassium, Chloride, and Betaine Concentrations in Isolated Vacuoles from Salt-Grown Atriplex gmelini Leaves. Plant Physiol. 1987 May;84(1):173–177. doi: 10.1104/pp.84.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeon T. A., Stumpf P. K. Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. J Biol Chem. 1982 Oct 25;257(20):12141–12147. [PubMed] [Google Scholar]
- Naylor J. F., 3rd, Fridovich I. The form of the aldehyde susceptible to enzymic oxidation. J Biol Chem. 1968 Jan 25;243(2):341–345. [PubMed] [Google Scholar]
- Rhodes D., Rich P. J., Myers A. C., Reuter C. C., Jamieson G. C. Determination of Betaines by Fast Atom Bombardment Mass Spectrometry : Identification of Glycine Betaine Deficient Genotypes of Zea mays. Plant Physiol. 1987 Jul;84(3):781–788. doi: 10.1104/pp.84.3.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge H., Nakano Y., Onishi H., Futamura Y., Ohashi K. A novel purification and some properties of rat liver mitochondrial choline dehydrogenase. Biochim Biophys Acta. 1980 Aug 7;614(2):274–284. doi: 10.1016/0005-2744(80)90217-x. [DOI] [PubMed] [Google Scholar]
- Weigel P., Lerma C., Hanson A. D. Choline oxidation by intact spinach chloroplasts. Plant Physiol. 1988 Jan;86(1):54–60. doi: 10.1104/pp.86.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P., Weretilnyk E. A., Hanson A. D. Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiol. 1986 Nov;82(3):753–759. doi: 10.1104/pp.82.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]


