Abstract
The antifungal activity of voriconazole (VCZ) was tested against Candida albicans in the absence or presence of polymorphonuclear neutrophils (PMN) or monocytes. In some experiments, VCZ was compared to fluconazole (FCZ). On a weight basis, VCZ was 10-fold more efficacious than FCZ against C. albicans Sh27. Against an FCZ-resistant isolate, VCZ at 1 μg/ml produced the same fungistasis as FCZ at 20 μg/ml. VCZ at 0.1 μg/ml collaborated with PMN for enhanced killing to the same extent as FCZ at 1.0 μg/ml. Granulocyte-colony-stimulating factor (G-CSF) enhanced the candidacidal activity of PMN, and it increased the collaboration of PMN with VCZ for killing. Granulocyte-macrophage (GM)-CSF also significantly enhanced both the killing by PMN and the collaboration of PMN with VCZ for killing. VCZ collaborated with monocytes for enhanced killing of C. albicans Sh27, and GM-CSF increased this collaboration. Taken together, these data show that VCZ is more potent than FCZ against C. albicans isolates, alone and in collaboration with PMN or monocytes for enhanced killing. In addition, G-CSF- or GM-CSF-activated PMN and monocytes have enhanced collaboration with VCZ compared to that of unstimulated phagocytes with VCZ.
Candidiasis is the most common fungal infection in acute leukemia, bone marrow, and liver transplant patients (3, 11, 12). Moreover, AIDS patients suffer frequent episodes of oral candidiasis, or thrush (7). Control of candidiasis in these situations can usually be successfully treated with antifungal agents.
Fluconazole (FCZ), an oral triazole with in vivo antifungal activity, has been a convenient and effective treatment for candidiasis (1). However, with the extensive use of FCZ, strains of Candida albicans resistant to FCZ have emerged (10). Recently, a new oral wide-spectrum triazole, voriconazole (VCZ), has been developed and has been suggested to be superior to FCZ in vitro (2).
Previously, we have reported a synergy of phagocytic cells with FCZ for enhanced killing of C. albicans and Candida species (4–6). Since this in vitro collaboration between phagocytic cells and FCZ reflects in vivo efficacy, we deemed it important to test VCZ in this system which simulates the in vivo conditions. If VCZ can collaborate with phagocytic cells for enhanced candidacidal activity, this would be a favorable condition with respect to the in vivo efficacy of VCZ.
Workers in our laboratory have shown that granulocyte colony-stimulating factor (G-CSF) activates neutrophils (8) and granulocyte-macrophage (GM)-CSF stimulates both neutrophils and monocytes (9) for enhanced candidacidal activity and synergy with FCZ. Here, we tested whether VCZ, like FCZ, can collaborate with activated polymorphonuclear neutrophils (PMN) or monocytes for significantly increased candidacidal activity.
MATERIALS AND METHODS
C. albicans.
C. albicans Sh27 (ATCC 56882), which is susceptible to FCZ (MIC, 0.5 μg/ml), and 94-179, which is resistant to FCZ (MIC, >64 μg/ml) (8), were used in these experiments. Yeast cells were grown on blood agar plates (BAP) at 35°C for 48 h. Yeast cells were washed in saline, diluted, counted and suspended in RPMI 1640 containing penicillin (100 U/ml), streptomycin (100 μg/ml), and 10% fresh frozen human serum (complete tissue culture medium [CTCM]). Dilutions of the suspension were plated on BAP in quadruplicate at time zero to determine the inoculum CFU.
Effector cells.
Polymorphonuclear neutrophils (PMN) and peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by 6% dextran 70 sedimentation followed by density gradient centrifugation on Histopaque 1077 (Sigma Chemical Co., St. Louis, Mo.). The pelleted cells (PMN plus some erythrocytes) were treated with 0.85% NH4Cl to lyse erythrocytes, washed with RPMI 1640, and counted. PMN were suspended to 2 × 106/ml of CTCM, and 0.1 ml was dispensed per microtest plate well. PBMC at 5 × 106/ml of CTCM were dispensed at 0.1 ml per microtest well (half-area wells; Corning no. 25870) and then were incubated for 2 h at 37°C in CO2–95% air (CO2 incubator). After incubation, nonadherent cells were aspirated, and the remaining cells were washed once with RPMI 1640.
FCZ and VCZ.
FCZ and VCZ were supplied by Pfizer, Groton, Conn. FCZ powder was dissolved in distilled water at 2 mg/ml and stored at 4°C. A small amount of VCZ powder was first dissolved in dimethyl sulfoxide and then diluted with distilled water to a final concentration of 2 mg/ml. Desired dilutions were made for FCZ or VCZ stocks with RPMI 1640.
G-CSF and GM-CSF.
Recombinant methionyl human G-CSF (Filgrastim) was supplied by Amgen, Thousands Oaks, Calif. G-CSF at 108 U/mg of protein was supplied as a concentration of 0.3 mg/ml, and appropriate dilutions were made in RPMI 1640. Recombinant human GM-CSF (Leukine; Sargramostim) was produced and supplied by Immunex Corp., Seattle, Wash.). GM-CSF (0.5 mg/ml [1.5 × 108 IU/mg of protein]) was diluted to 7.5 × 105 IU/ml in RPMI 1640 and stored at −80°C.
PMN assays.
PMN cultures were challenged with a 0.1-ml suspension of yeast cells in CTCM. Some sets of quadruplicate cultures received 0.01 ml of a G-CSF or GM-CSF dilution to give 100 or 500 ng of G-CSF/ml or 50 to 500 IU of GM-CSF/ml. Other sets of quadruplicate cultures without PMN, with PMN, or with PMN plus G-CSF or GM-CSF received 0.01 ml of FCZ or VCZ to give the desired final concentrations of drugs. After 24 h of incubation at 37°C in a CO2 incubator, cultures were harvested with distilled water to lyse PMN and release yeast cells. Complete removal of well contents was monitored microscopically. Appropriate dilutions of harvested material were plated on BAP. After incubation of plated BAP for 48 h at 35°C, colony counts were made, and numbers of CFU per culture were calculated. Differences in CFU counts in experimental cultures could not be accounted for by clumping of fungal cells, because microscopic examination of harvested material did not reveal significant differences between control and experimental material.
Monocyte assays.
Monocyte monolayers were challenged with 0.1 ml of yeast cell suspensions in CTCM. To some sets of quadruplicate cultures, 0.01 ml of GM-CSF was added to give a desired final concentration. Other sets of quadruplicate cultures without monocytes, with monocytes, or containing monocytes plus GM-CSF received 0.01 ml of FCZ or VCZ to give the desired final concentrations. After cultures had been incubated for 24 h at 37°C in a CO2 incubator, they were harvested, and dilutions were plated and processed as described above for PMN.
Quantitative analysis.
Inhibition of growth, or percent fungistasis, was determined by the formula [1 − (experimental CFU/control CFU)] × 100. Fungicidal activity, or percent reduction of inoculum CFU, was calculated by the formula [1 − (experimental CFU/inoculum CFU)] × 100. When killing occurred, e.g., experimental CFU < inoculum CFU, fungistasis was defined as 100%. Student’s t test was used for statistical analysis of data, and significance was set at P < 0.05.
RESULTS
VCZ versus FCZ.
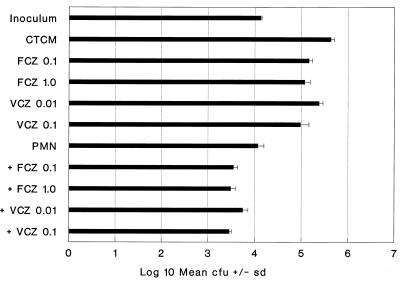
When VCZ was compared to FCZ for fungistatic activity against C. albicans Sh27, VCZ was 10-fold more potent. For example, VCZ at 0.1 μg/ml was as fungistatic (78%) as FCZ at 1.0 μg/ml (73%) (Fig. 1). Similarly, VCZ was 10-fold more effective than FCZ in collaborating with PMN for enhanced killing of C. albicans Sh27. For example, PMN plus VCZ at 0.1 μg/ml increased killing by PMN from 12% to 79%, and this was similar to increased killing by FCZ at 1.0 μg/ml plus PMN (Fig. 1). The data show that in both cases, i.e., fungistasis and collaboration for killing, VCZ was 10-fold more efficacious than FCZ. Similar results with PMN with or without VCZ are shown in Tables 1 and 2.
FIG. 1.
Collaboration of FCZ and VCZ with PMN for enhanced killing of Sh27. Complete tissue culture medium (CTCM) was RPMI 1640 plus human serum. FCZ and VCZ concentrations are in micrograms per milliliter. Standard deviations (sd) from the mean of quadruplicate 24-h cultures are shown at the tops of bars in all figures.
TABLE 1.
Effect of GM-CSF on PMN activity against C. albicans Sh27: synergy with VCZ
| Condition | VCZ concn (μg/ml) | Mean CFU ± SDa | % Fungistasis | % Killing |
P vs reference conditionb
|
||
|---|---|---|---|---|---|---|---|
| PMN alone | PMN + 0.01 μg of VCZ/ml | PMN + 0.10 μg of VCZ/ml | |||||
| Inoculum | 28,800 ± 1,058 | ||||||
| CTCM | 0 | 783,300 ± 162,500 | 0 | 0 | |||
| 0.01 | 200,000 ± 28,300 | 75 | 0 | ||||
| 0.10 | 71,250 ± 26,550 | 91 | 0 | ||||
| PMN | 0 | 15,300 ± 2,270 | 100 | 47 | — | ||
| 0.01 | 5,200 ± 655 | 100 | 82 | — | |||
| 0.10 | 3,666 ± 550 | 100 | 88 | — | |||
| PMN + GM-CSF (100 U/ml) | |||||||
| 0 | 11,466 ± 1,500 | 100 | 61 | <0.05 | |||
| 0.01 | 2,233 ± 40 | 100 | 93 | <0.01 | |||
| 0.10 | 4,350 ± 173 | 100 | 85 | NS | |||
Values represent mean CFU ± standard deviation (SD) of quadruplicate 24-h cultures.
—, row of data serving as reference for P value in column. NS, not significant (P > 0.05).
TABLE 2.
Effect of G-CSF on PMN activity against C. albicans Sh27: synergy with VCZ
| Condition | VCZ concn (μg/ml) | Mean CFU ± SD | % Fungistasis | % Killing |
P vs reference conditiona
|
||
|---|---|---|---|---|---|---|---|
| Inoculum | PMN alone | PMN + 0.01 μg of VCZ/ml | |||||
| Inoculum | 39,250 ± 6,200 | — | |||||
| CTCM | 0 | 470,000 ± 12,500 | 0 | 0 | |||
| 0.01 | 205,000 ± 22,500 | 57 | 0 | ||||
| PMN | 0 | 29,000 ± 280 | 100 | 26 | <0.05 | — | |
| 0.01 | 3,025 ± 920 | 100 | 93 | <0.01 | — | ||
| PMN + G-CSF (ng/ml) | |||||||
| 100 | 0 | 2,930 ± 602 | 100 | 93 | <0.01 | <0.01 | |
| 0.01 | 2,400 ± 100 | 100 | 94 | <0.01 | NS | ||
| 500 | 0 | 2,500 ± 800 | 100 | 94 | <0.01 | <0.01 | |
| 0.01 | 1,415 ± 200 | 100 | 96 | <0.01 | <0.05 | ||
—, row of data serving as reference for P values in column. NS, not significant (P > 0.05).
Effect of GM-CSF on PMN.
GM-CSF incubated with PMN plus C. albicans Sh27 significantly (P < 0.05) enhanced their candidacidal activity compared to that of control PMN (Table 1). PMN plus VCZ (0.01 μg/ml) had enhanced killing compared to that of PMN alone, and this synergy was significantly enhanced from 82% killing to 93% killing when GM-CSF (100 U/ml, an optimal concentration determined from prior work [9]) was present in cultures (Table 1).
Effect of G-CSF on PMN.
PMN collaborated with VCZ (0.01 μg/ml) by increasing killing from 26% to 93% (Table 2). G-CSF treatment at 100 or 500 ng/ml increased (P < 0.01) the fungicidal activity of PMN for C. albicans from 26% to 93 and 94%, respectively (Table 2). Moreover, the synergistic killing by PMN plus VCZ (0.01 μg/ml) was significantly (P < 0.05) enhanced in the presence of G-CSF at 500 ng/ml (Table 2). Similar results were obtained in two other experiments in which G-CSF enhanced PMN activity and enhanced PMN activity with VCZ at 0.01 and 0.10 μg/ml over a G-CSF concentration range of 25 to 500 ng/ml.
Collaboration of VCZ and monocytes.
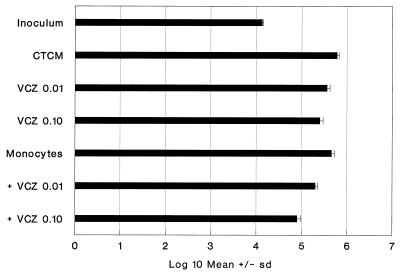
When monocyte monolayers were challenged with a high dose of C. albicans Sh27, they did not reduce the inoculum CFU, but were fungistatic (24%) (Fig. 2). The effect of combining fungistatic monocytes with fungistatic VCZ was additive. For example, fungistasis by VCZ at 0.01 μg/ml (40%) and fungistasis by monocytes alone (24%) added up to the amount of fungistasis (66%) obtained with the cultures containing VCZ and monocytes (Fig. 2). This was also the case for VCZ at 0.10 μg/ml.
FIG. 2.
Collaboration of VCZ and monocytes for enhanced fungistasis for Sh27. The format is same as that in Fig. 1. Monocyte activity was significant (P < 0.05), as were the activities of both VCZ concentrations with or without monocytes (P < 0.01 for all four comparisons). Monocytes plus 0.10 μg of VCZ per ml were significantly more active than monocytes or 0.10 μg of VCZ per ml alone (P < 0.01).
Effect of GM-CSF on monocytes.
In another experiment, VCZ alone was fungistatic (98%) but not fungicidal (Table 3). Monocytes alone were fungicidal (11%) when a low challenge dose was used. VCZ collaborated with monocytes for significantly (P < 0.01) increased killing of C. albicans (e.g., 11% versus 59 and 82%) (Table 3). The monocyte collaboration with VCZ was seen in two other experiments with low (1,022 ± 170 and 2,217 ± 285) inocula; monocytes plus 0, 0.01, and 0.10 μg of VCZ per ml killed 6, 40, and 58% and 10, 54, and 65%, respectively (P < 0.01 for monocytes plus either VCZ concentration in both additional experiments). When GM-CSF (500 U/ml) was present (Table 3) during the incubation period, killing by treated monocytes was significantly increased compared to that by untreated monocytes, rising from 11% to 51%. GM-CSF-treated monocytes at all GM-CSF concentrations tested synergized with VCZ (0.01 μg/ml) for significantly increased killing compared to untreated monocytes plus VCZ at 0.01 μg/ml. Due to the high degree of killing by monocytes plus VCZ at 0.10 μg/ml (82%), significantly increased killing (91%) was found only with the combination of GM-CSF (100 U/ml) and VCZ at 0.10 μg/ml (Table 3).
TABLE 3.
Effect of GM-CSF on monocyte activity against C. albicans Sh27: synergy with VCZ
| Condition | VCZ concn (μg/ml) | Mean CFU ± SD | % Fungistasis | % Killing |
P vs reference conditiona
|
|||
|---|---|---|---|---|---|---|---|---|
| Monocytes alone | Monocytes alone | Monocytes + 0.01 μg of VCZ/ml | Monocytes + 0.10 μg of VCZ/ml | |||||
| Inoculum | 825 ± 77 | |||||||
| CTCM | 0 | 106,330 ± 5,600 | 0 | 0 | ||||
| 0.01 | 2,837 ± 228 | 98 | 0 | |||||
| 0.10 | 2,162 ± 221 | 98 | 0 | |||||
| Monocytes | 0 | 740 ± 198 | 100 | 11 | — | — | ||
| 0.01 | 340 ± 86 | 100 | 59 | <0.01 | — | |||
| 0.10 | 153 ± 3 | 100 | 82 | <0.01 | — | |||
| Monocytes + GM-CSF (U/ml) | ||||||||
| 50 | 0 | 465 ± 216 | 100 | 44 | NS | |||
| 0.01 | 153 ± 28 | 100 | 82 | <0.01 | ||||
| 0.10 | 180 ± 42 | 100 | 79 | NS | ||||
| 100 | 0 | 513 ± 23 | 100 | 38 | NS | |||
| 0.01 | 210 ± 65 | 100 | 75 | <0.05 | ||||
| 0.10 | 80 ± 40 | 100 | 91 | <0.05 | ||||
| 500 | 0 | 406 ± 155 | 100 | 51 | <0.05 | |||
| 0.01 | 105 ± 19 | 100 | 88 | <0.01 | ||||
| 0.10 | 120 ± 40 | 100 | 84 | NS | ||||
—, row of data serving as reference for P values in column. NS, not significant (P > 0.05).
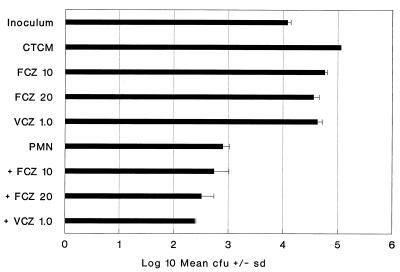
VCZ versus FCZ against an FCZ-resistant isolate.
VCZ was about 20-fold more effective than FCZ in inhibiting the growth of isolate 94-179. FCZ at 20 μg/ml was 68% fungistatic, and VCZ at 1.0 μg/ml inhibited growth by 62% (Fig. 3). This isolate was highly sensitive to killing by PMN (93%) compared to killing of C. albicans Sh27. Nevertheless, collaboration of PMN with FCZ and VCZ could be demonstrated. VCZ was 20-fold more effective than FCZ. VCZ at 1.0 μg/ml collaborated with PMN for killing (98%) to the same extent as FCZ at 20 μg/ml plus PMN did (98%) (Fig. 3).
FIG. 3.
Comparison of VCZ and FCZ with or without PMN against an FCZ-resistant isolate of C. albicans. The format is the same as that in Fig. 1. PMN plus FCZ (20 μg/ml) and PMN plus VCZ (1.0 μg/ml) were both superior to PMN alone (P < 0.01 for both).
DISCUSSION
By using a quantitative culture system method, we have corroborated previous reports, with a broth dilution system, that VCZ is more potent than FCZ against C. albicans in vitro (2). This was also true for an FCZ-resistant isolate of C. albicans, 94-179.
Previously we have reported a collaboration of phagocytic cells with FCZ for enhanced killing of C. albicans and Candida species (4, 5, 6). We report here for the first time that VCZ, like FCZ, can collaborate with human PMN or monocytes for enhanced killing of C. albicans. Moreover, we found that VCZ is 10-fold more potent than FCZ for collaboration with PMN or monocytes in killing C. albicans.
In previous work, we found that both G-CSF and GM-CSF can activate PMN in a 24-h assay system for enhanced killing of C. albicans (8, 9). Here we have confirmed those results. In that work, PMN activated with G-CSF or GM-CSF collaborated with FCZ for enhanced killing. Here we found that PMN activated with G-CSF or GM-CSF could also collaborate with VCZ for enhanced killing.
Monocytes collaborated with VCZ for increased killing of C. albicans, and GM-CSF treatment of monocytes significantly increases this collaboration. These results extend previous findings in which FCZ collaborated with GM-CSF-treated monocytes for increased killing (9).
The data presented here suggest VCZ would have good efficacy in the treatment of candidiasis in humans and may help explain previous clinical results. Moreover, VCZ would have additional efficacy in clinical settings in which G-CSF or GM-CSF is used.
REFERENCES
- 1.Ansari A M, Gould I M, Douglas J G. High dose oral fluconazole for oropharyngeal candidiasis in AIDS. J Antimicrob Chemother. 1990;25:720–721. doi: 10.1093/jac/25.4.720. [DOI] [PubMed] [Google Scholar]
- 2.Barry A L, Brown S D. In vitro studies of two triazole antifungal agents (voriconazole [UK-109,496] and fluconazole) against Candida species. Antimicrob Agents Chemother. 1996;40:1948–1949. doi: 10.1128/aac.40.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodey G P. Fungal infections complicating acute leukemia. J Chronic Dis. 1966;19:667–687. doi: 10.1016/0021-9681(66)90066-x. [DOI] [PubMed] [Google Scholar]
- 4.Brummer E, Stevens D A. Synergy of human neutrophils with fluconazole in killing Candida species. Mycopathologia. 1996;134:115–120. doi: 10.1007/BF00436717. [DOI] [PubMed] [Google Scholar]
- 5.Garcha U K, Brummer E, Stevens D A. Synergy of fluconazole with human monocytes or monocyte-derived macrophages for killing Candida species. J Infect Dis. 1995;172:1620–1623. doi: 10.1093/infdis/172.6.1620. [DOI] [PubMed] [Google Scholar]
- 6.Gujral S, Brummer E, Stevens D A. Role of extended culture time on synergy of fluconazole and human monocyte-derived macrophages in clearing Candida albicans. J Infect Dis. 1996;174:888–889. doi: 10.1093/infdis/174.4.888. [DOI] [PubMed] [Google Scholar]
- 7.Klein R S, Harris C A, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high risk patients as the initial manifestations of the acquired immune deficiency syndrome. N Engl J Med. 1984;311:354–356. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 8.Natarajan U, Brummer E, Stevens D A. Effect of granulocyte colony-stimulating factor on the candidacidal activity of polymorphonuclear neutrophils and their collaboration with fluconazole. Antimicrob Agents Chemother. 1997;41:1575–1578. doi: 10.1128/aac.41.7.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Natarajan, U., N. Randhawa, E. Brummer, and D. A. Stevens. Effect of granulocyte macrophage-colony stimulating factor on candidacidal activity of neutrophils, monocytes or monocyte-derived macrophages and synergy with fluconazole. J. Med. Microbiol., in press. [DOI] [PubMed]
- 10.Sanguineti A, Carmichael K, Campbell K. Fluconazole resistant Candida albicans after long term suppressive therapy. Arch Intern Med. 1993;153:1122–1124. [PubMed] [Google Scholar]
- 11.Schroter G P J, Hoelscher M, Putman C W, Porter K A, Starzl T E. Fungus infections after liver transplantation. Ann Surg. 1977;186:115–122. doi: 10.1097/00000658-197707000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winston D J, Gale R P, Meyer D V, Young L S. Infectious complications in human marrow transplantation. Medicine. 1979;58:1–31. doi: 10.1097/00005792-197901000-00001. [DOI] [PubMed] [Google Scholar]