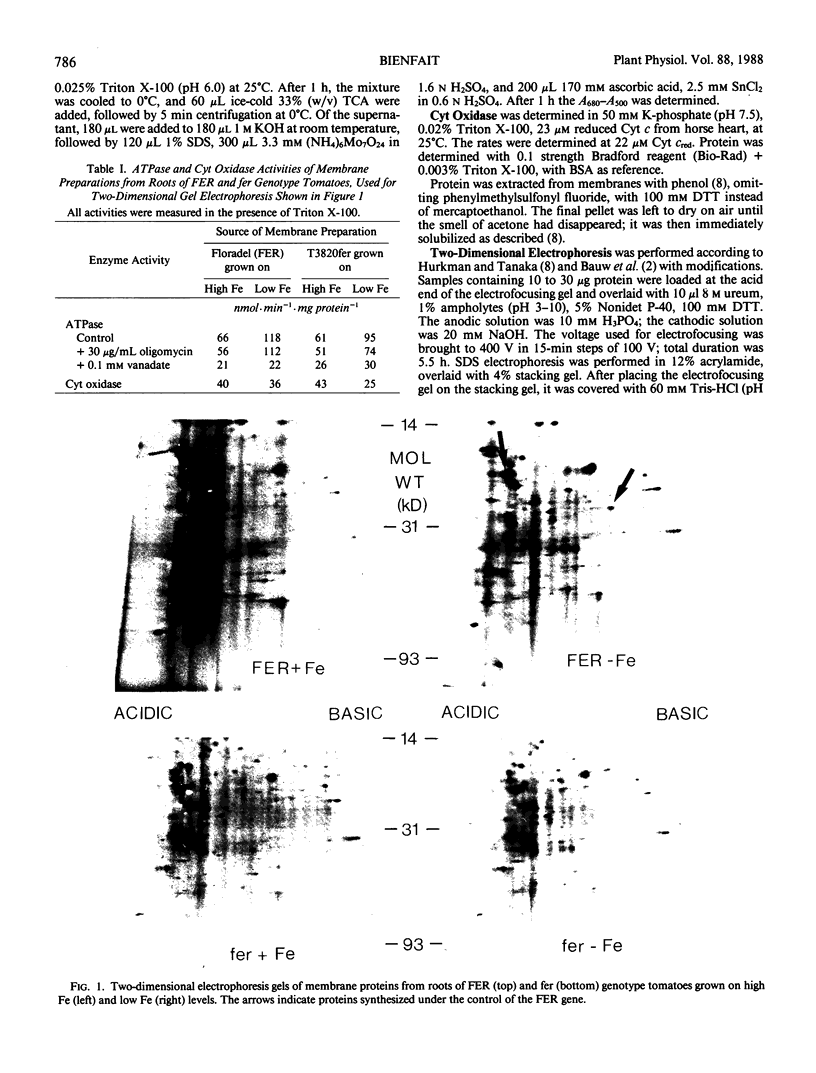
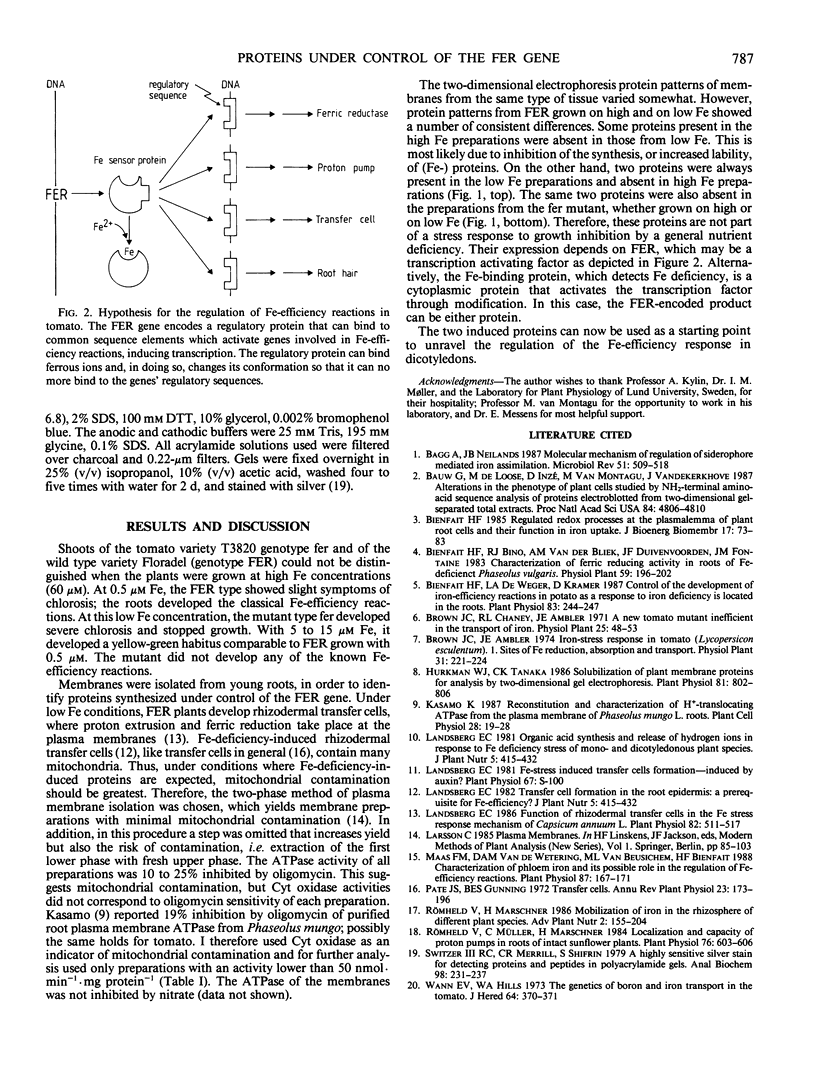
Abstract
Fe-deficient dicotyledons develop Fe-efficiency reactions, such as proton extrusion and ferric chelate reduction activity, which are located in the plasma membranes of the root epidermal cells. The fer mutant of tomato (Lycopersicon esculentum Mill.) cannot develop these reactions. Membranes were isolated from roots of wild-type (FER) and mutant (fer) tomato plants grown on nutrient solution with high and low Fe concentrations. Two proteins were identified which are synthesized under the control of the FER gene.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauw G., De Loose M., Inzé D., Van Montagu M., Vandekerckhove J. Alterations in the phenotype of plant cells studied by NH(2)-terminal amino acid-sequence analysis of proteins electroblotted from two-dimensional gel-separated total extracts. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4806–4810. doi: 10.1073/pnas.84.14.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienfait H. F. Regulated redox processes at the plasmalemma of plant root cells and their function in iron uptake. J Bioenerg Biomembr. 1985 Apr;17(2):73–83. doi: 10.1007/BF00744199. [DOI] [PubMed] [Google Scholar]
- Bienfait H. F., de Weger L. A., Kramer D. Control of the development of iron-efficiency reactions in potato as a response to iron deficiency is located in the roots. Plant Physiol. 1987 Feb;83(2):244–247. doi: 10.1104/pp.83.2.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsberg E. C. Function of Rhizodermal Transfer Cells in the Fe Stress Response Mechanism of Capsicum annuum L. Plant Physiol. 1986 Oct;82(2):511–517. doi: 10.1104/pp.82.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M. Isolation of Functionally Intact Rhodoplasts from Griffithsia monilis (Ceramiaceae, Rhodophyta). Plant Physiol. 1981 Jan;67(1):5–8. doi: 10.1104/pp.67.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas F. M., van de Wetering D. A., van Beusichem M. L., Bienfait H. F. Characterization of Phloem iron and its possible role in the regulation of fe-efficiency reactions. Plant Physiol. 1988 May;87(1):167–171. doi: 10.1104/pp.87.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römheld V., Müller C., Marschner H. Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol. 1984 Nov;76(3):603–606. doi: 10.1104/pp.76.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]