Abstract
The increase in world population growth and its resultant increase in industrial production to meet its need, have continued to raise the volume of wastewater received by treatment plant facilities. This has expectedly, led to an upsurge in the volume of sewage sludge and biosolids generated from wastewater treatment systems. Biosolids are best managed by application on land because of their agronomic benefits. However, this usage has been discovered to negatively affect humans and impact the environment due to the accumulation of minute concentrations of contaminants still present in the biosolid after treatment, hence the need for government regulations. This review article examined the fate and effects of pollutants, especially persistent organic pollutants (PoPs) of concern and emerging contaminants found in biosolids used for land applications, and also discussed government regulations on biosolid reuse from the perspectives of the two major regulations governing biosolid land application-the EU's Sludge Directive and USEPA's Part 503 Rule, in an attempt to draw attention to their outdated contents since enactment, as they do not currently meet the challenges of biosolid land application and thus, require a comprehensive update. Any update efforts should focus on USEPA's Part 503 Rule, which is less stringent on the allowable concentration of biosolid pollutants. Furthermore, an update should include specific regulations on new and emerging contaminants and persistent organic pollutants (PoPs) such as microplastics, pharmaceutical and personal care products (P&PCPs), surfactants, endocrine-disrupting chemicals, flame retardants, pathogens, and organic pollutants; further reduction of heavy metal standard limits, and consideration of soil phosphate-metal interactions to regulate biosolid agronomic loading rate. Future biosolid research should focus on the concentration of TCS, TCC, and emerging pharmaceuticals, as well as Microplastic transport in biosolid-amended soils, soil-plant transfer mechanism, and metabolism of PFAs in the soils; all of which will inform government policies on biosolid application on land.
Keywords: Biosolid, Land application, Agronomic loading rate, Effect of contaminants, Government regulation
1. Introduction
The proliferation of industries, urbanization, and industrialization of suburbs and rural areas over the past decades around the world, has resulted in an increased standard of living [[1], [2], [3]] together with its attendant increase in the volume of wastes generated, especially sewage and industrial wastewater [[4], [5], [6], [7]] (with Asia generating highest volume according to a projection of global wastewater generation, Fig. 1), which has to be treated before discharged into the environment to prevent pollution [8,9]. According to Giacomo and Romano [10], municipal wastewater generated in the world in 2020 was between 360 and 380 cubic kilometers, which is a projection of a 24% and 51% increase by 2030 and 2050 respectively.
Fig. 1.
Projected increase in global wastewater generation since 2015 [11].
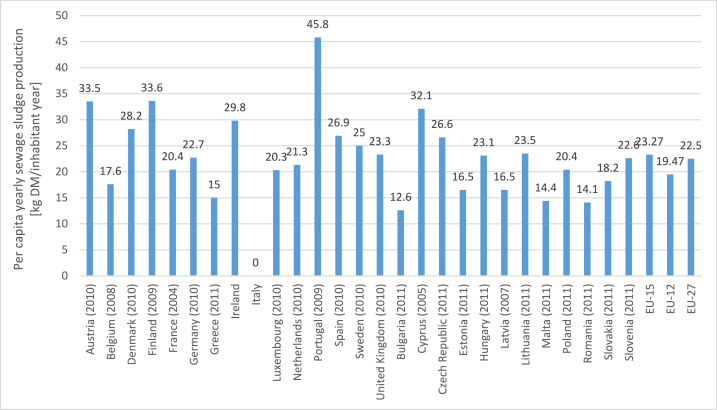
Wastewater treatment generates sewage sludge and effluent as end products [10,12,13]. Due to an increase in wastewater production as a result of population growth in the last couple of years [14], there has been a corresponding increase in sewage sludge production [[15], [16], [17]]. According to Collivignarelli and Abba et al. [18], the total sludge production from urban wastewater plants in the EU-15 countries has increased from 6.5 MT (dry weight) to 9.5 MT (dry weight) over the last two decades and, EU-27 countries saw sludge produced up to 10 MT (dry weight) during the same period; the yearly per capita sludge generation in Europe in kg dry matter per inhabitant per year (kg DM/inhabitant year) is shown in Fig. 2 which revealed that Portugal and Italy both members of EU-15 countries, had the highest and lowest sludge generation per inhabitant respectively. Chow and Pan [19], gave the total sewage sludge produced per day from different treatment plants in Hong Kong as 1200 tons and, this figure is projected to reach 2000 tons by 2030 as a result of the improvement of treatment plant operations [20,21], environmental discharge standard [22] and population increase [10,20,23].
Fig. 2.
Comparison of yearly sewage sludge generation per person in Europe [24].
Raw sewage sludge contains a high level of contaminants whose concentration has to be reduced by subjecting sludge to further treatment or processing before useful applications [[25], [26], [27]]. Land application of raw sludge is restricted because it can result in severe environmental risks [[28], [29], [30]] such as water pollution and soil contamination due to the presence of heavy metals including Chromium (Cr), Copper (Cu), Nickel (Ni), Zinc (Zn),Lead (Pb), Cadmium (Cd) [31,32],Ethers (PBDEs), Di(2-Ethylhexyl) Phthalate (DEHP), Polychlorinated Biphenyls (PCBs), Polycyclic Aromatic Hydrocarbons (PAHs), Polychlorinated Dibenzo-p-dioxins and Dibenzo-p-furans (PCDD/Fs) [33,34], pharmaceuticals, endogenous hormones, synthetic steroids [35] and, organic, antimicrobial pollutants known as Per- and Poly-fluoroakyl substances (PFAs) [36,37]which can all accumulate in soil or washed to surface water during any short or long term sludge use [38,39].
2. Sewage sludge treatment technologies
The large quantities of sewage sludge produced at water treatment plant facilities undergo certain treatment processes to convert it to biosolid (Fig. 3) for more beneficial use. Major reasons for the treatment of sewage sludge are to drastically reduce the amount of disease-causing pathogens and contaminants [40], reduce the volume of sludge for better handling [41], make sludge economically more viable [42], and to reduce the attraction of vectors such as flies, fleas, rodents, mosquitoes, birds; all these are referred to as vectors in the EPA's Biosolid Rule [43]. Some of these treatment technologies, which include anaerobic sludge digestion, incineration, sludge compost, sludge drying, deep-dewatering/thermal hydrolysis, and alkaline stabilization/pH treatment [19], are discussed below.
Fig. 3.
Block flow diagram showing biosolid production from wastewater treatment [49].
2.1. Anaerobic sludge digestion
This sludge stabilization process involves the use of biological microorganisms in the absence of air to convert volatile solids in raw sewage sludge to biogas, carbon dioxide, and water [44]. The biogas produced during this process can be further treated for energy recovery to utilize its methane component as fuel while the stabilized solids (biosolids) can be used for soil conditioning [44]. Anaerobic sludge digestion is the most commonly employed sludge stabilization process and unlike aerobic digestion, it is lower in energy utilization and generates fewer wastes; it also uses fewer chemicals than alkaline stabilization. However, it has a higher initial capital cost and complex operation than the other processes [44].
2.2. Sludge incineration
Incineration of sewage sludge is a thermal treatment process [45] that oxidizes organic sulphur, nitrogen, carbon, and phosphorus in raw sludge to inorganic, mineral solid, and gaseous products [44]. The process may be carried out in a furnace at a high temperature greater than 800 °C so that the organics in sewage sludge may be oxidized [45].
2.3. Sludge composting
This is an aerobic sludge digestion process that uses biological thermophilic or mesophilic microorganisms in the presence of air, to degrade raw sewage sludge combined with other waste materials such as rice hulls, straws, sawdust, and recycled compost (amendments) and, shredded green wastes, tyres, and wood scrapes (bulking agents) to produce a pasteurized solid product [44]. The stages involved in sludge composting include 1). Mixing raw sludge with amendments or bulking agents at the preprocessing stage, 2). Aerating the mixture for decomposition using air provided by a mechanical pumping device, 3). Recovery of undegraded additives (amendments and bulking agents), 4). Stabilizing the product formed by curing and storing and, 5). Screening, grinding, or comminution to remove undegraded materials and produce a uniform product [44].
2.4. Sludge drying
This is an intermediate thermal intervention process for treating wet sewage sludge that involves reducing/evaporating the moisture content of raw sludge thus, significantly lowering its weight and volume for better handling and recovery to meet important applications such as its use as fuel for incineration plants, compost material, and fertilizer [46].
2.5. Deep-dewatering/thermal hydrolysis
Raw sewage sludge can be treated by first subjecting the sludge to a deep-dewatering process before thermal hydrolysis. Deep-dewatering, which involves removing moisture from sludge to reduce its overall volume for better operational handling and subsequent cost of transportation [47], is carried out to thicken sludge to about 16–18% of dry weight [48]. The partially thickened sludge is then exposed to high temperature, between 160 and 180 °C, and a pressure of about 6 bars in the thermal hydrolysis step to sterilize the sludge and kill pathogens [48].
2.6. Alkaline stabilization/pH treatment
This is a chemical treatment method employed to reduce pathogenic colony, putrescence or decay, and odour during the sludge treatment process. It involves the use of slaked or hydrated lime (calcium hydroxide) to raise the pH of raw sludge to create a high alkaline system [44]. The amount of lime used may be reduced by dosing waste solid materials into the system thereby making the system cost-efficient [44].
3. Biosolid management and disposal methods
Biosolid management technologies are employed to dispose of the large quantities of biosolids generated from sewage sludge treatment. Many researchers have described biosolids as treated sewage sludge materials produced to meet Environmental Protection Agency (EPA) standards for agriculture and land application [12,36,43,50,51]. Kinney and Furlong et al. [52] in their study, defined biosolids as carbon-rich, organic beneficial solids, produced during wastewater treatment to meet local and United States Environmental Protection Agency (USEPA) regulations for pathogen, metal, and nutrient content, suitable for land application and, Thomas Burke [13], noted that biosolids are the main organic solids from municipal wastewater treatment that can be valuably recycled to amend agricultural soil.
Biosolid scan be classified as class A or class B solids and this classification is based on sewage sludge treatment methods [53] and the extent of pathogen reduction [13,43,54]. Class A biosolids are produced when sewage sludge is treated so that the presence of pathogens is below the detectable amount [27]. It can be bagged, sold to the public, and used without restrictions based on its pathogen content [43,53]. Class B biosolids, on the other hand, are produced when sewage sludge is treated to only reduce pathogen concentration which may still be detectable [27]. They are regulated for use on land to prevent man and animal exposure to contaminants, or environmental degradation [43,53].
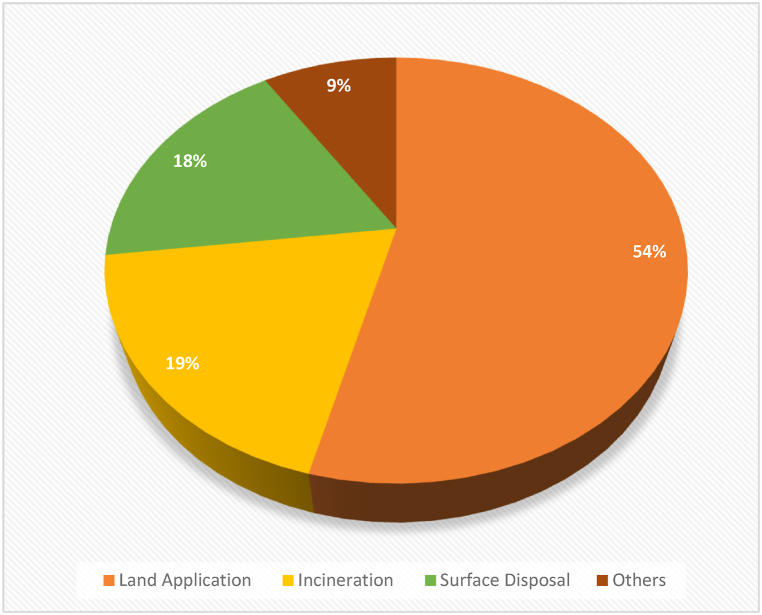
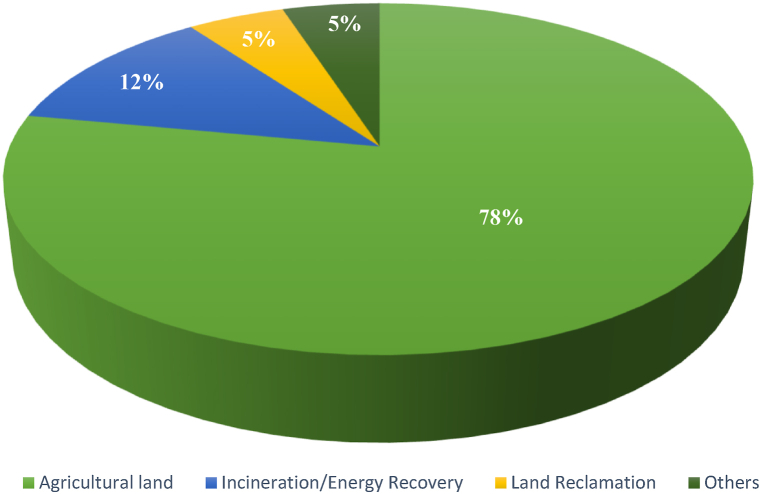
Biosolid management and disposal techniques have evolved over the years with many countries favouring land/agricultural application to incineration, landfilling/surface disposal, or production of building materials, etc. [55]. In the United States for instance, of the 8.2 million tons of biosolid produced in 2010, 54% were land applied compared to 18% and 19% that were landfilled (surface-applied) and incinerated respectively (Fig. 4) [56]. In the same vein, the United Kingdom Water Industry Research (UKWIR) [50], noted that 150,000 ha of agricultural land in the U.K. utilized a total, between 3 and 4 million tons of biosolids produced annually for land application, representing more than 75% of the total production quantity compared to other sludge management practices including incineration/energy recovery which utilized 12% of total sludge produced, land reclamation 5%, and others 5% (Fig. 5) while, in China, approximately 39.04 million tons of biosolids were produced annually (2019 data) from which 29.3% was disposed via land application, 26.7% incinerated, and 20.1% landfilled [57]. Some of the disposal routes of biosolids currently in use include land application, incineration, sanitary landfilling/surface disposal, and production of construction materials [51,57] are discussed in the following section.
Fig. 4.
Biosolid management and disposal practice in the U.S [5].
Fig. 5.
The UK biosolid disposal pathways [50].
The pie diagrams shown above (Fig. 4, Fig. 5), clearly revealed that land application of biosolids is the most popular/common biosolid management practice in the U.S. and UK.
3.1. Land application
Biosolid land application involves spreading the stabilized, treated solids on surface soil or incorporating them into the ground on the basis of government regulations and guidelines [5]. This practice can occur at sites such as forests, mine reclamation sites, agricultural lands, disturbed lands, golf courses, or parks [5]. Land application of biosolid is sustainable, cost-effective, unlikely to cause environmental degradation, and generally improves soil fertility making it a widely accepted technique in many regions [50,56,57].
3.2. Biosolid incineration
Biosolid incineration is an “end-of-useful-life” approach to biosolid management. This is because after subjecting biosolid to high-temperature combustion in the presence of air and converting it to carbon, ash, and char, it is taken to a landfill site for surface disposal [57]. Incinerating biosolids before landfilling kills and inactivates pathogens that could cause environmental pollution [56]. Incineration as a biosolid management technique is discouraged as a result of the release of toxic, greenhouse gases into the environment thereby, causing air pollution [57].
3.3. Sanitary landfilling/surface disposal
The biosolid landfilling/surface disposal management option involves dumping biosolids in excavated spaces and then compacting and leveling the area to reduce waste volume [58]. The disposal can be on a mono-disposal landfill where only solid wastes from wastewater treatment are dumped; this type of landfill is regulated by the 40 CFR Part 503 legislation or, on a mixed landfill (co-disposal landfill) where biosolid wastes are combined with municipal solid wastes (MSWs), which is regulated by the 40 CFR Part 258 legislation for MSWs [5,56]. Management of biosolids by landfilling should be distinguished from land application practice, where biosolids are applied to soil for enhancements [56]. It is important to point out that landfilling biosolids can result in severe public and environmental health complications due to the uncontrolled release of contaminants into the environment [56].
3.4. Production of construction materials
Application of biosolids in the field of construction is one of the sustainable disposal routes of biosolids, which is dependent on the material's geotechnical properties. Modified/stabilized biosolid composites and aggregates applied in construction can be used for embankment filling, land reclamation, brick manufacture, and the development of other construction materials [49]. Biosolid's use in this way is a major contribution to the circular economy ecosystem. The addition of substances like lime, cement, bauxson, glass, and fly ash has been reported to greatly enhance the stability, compaction characteristics, shear, and compressive strengths of biosolids [49]. Usually, before composites are applied in construction, standard tests such as large-scale direct shear tests, vane shear tests, cone penetration tests, triaxial tests, California bearing ratio, consolidation tests, compaction tests, etc. [49].
The present review article adopted the narrative research design to provide a contributory insight into the evidence of environmental hazards associated with biosolid land application disposal routes and discussed government legislations that regulate this biosolid management technique including how application practices can influence government policies. A flowchart of the research concept is shown in Fig. 6A scoping search was initially carried out to identify the knowledge gap in the field and develop a research question. Using keywords and phrases in the research question, which are combined with Boolean operators, an elaborate search for literature in major databases such as Scopus, Google Scholar, and Science-Direct was done. The total number of relevant articles obtained after applying inclusion/exclusion criteria such as year of publication, title, originality of the study, whether the study was peer reviewed or not, methodology, and reported outcome or conclusion, was 150. These articles were each assessed for limitation and bias using the Cochrane Collaboration Tool with a low overall risk of bias.
Fig. 6.
Flow chart of research concept.
4. Biosolid land application and the circular economy
The need for land or agricultural soil amendment especially in an arid or semi-arid region where crop yields are very low, as a result of the combined effects of harsh climatic conditions like land degradation occasioned by erosion, long and persistent drought conditions, poor cultivation practices, etc. [59,60], has necessitated biosolid application practices on land for agricultural purposes [61]. This practice of recycling biosolids as organic fertilizer on land is an aspect of the circular economy [62] that tends to eliminate large volumes of sewage sludge produced annually around the world, especially in the developed world, and thus, reduce its environmental pollution by reprocessing and consuming it, forming a circle of sludge regenerative production and consumption [63].
Being a widely practiced and economically viable biosolid management method, biosolid land application in agriculture as soil amender and conditioner [12] has been reported to enhance the diversity of soil beneficial bacteria (rhizosphere bacteria) community, plant performance, and growth, improve soil properties [64], provide nutrient and stable organic matter to soil [12,50,65]. The organic nature of biosolids makes them better alternatives to mineral (inorganic) fertilizers [61] as they are high in plant nutrients such as nitrogen, potassium, phosphorus, organic matter and, can improve the yield and quality of crops [12]. Also, biosolids’ slow-release of plant nutrients (which may potentially reduce pollution caused by leaching of excess nutrients, a common occurrence with mineral fertilizer)make them readily available to plants and increase their efficiency for plant growth and soil enhancement; these and together with their low cost make them more attractive than mineral fertilizer [12,54,66].
4.1. Biosolid land application best practices
Recycling biosolids to agricultural soils, rangelands, forests, and damaged lands to replenish soil organic matter, supply soil nutrients, and enhance land reclamation is known as biosolid land application [66]. Such land application has been observed to increase plant drought tolerance and improve soil texture and water holding capacity [67], which favour root and shoot growth and, remediate disturbed land area [66]. Application of biosolids on land, however, can result in environmental pollution due to the bioaccumulation of excess nutrients from repeated use as organic fertilizer [68]. It is therefore important to apply biosolids while observing certain management practices (Best Management Practices) which can allow for proper usage and protection of human and environmental health [54].
-
(a)
Following Strict Biosolid Agronomic Loading Rate: This describes the frequency at which solid or liquid biosolid is applied (or injected) to an agricultural area of known dimension [69,70]. High loading rates may result in the accumulation of nutrients like phosphorus on soil surfaces thereby, increasing the likelihood of runoff (caused by erosion) to streams causing water pollution [54]. The application rate of biosolids should be determined by soil nitrogen requirement if the phosphorus index of the soil is low or medium and, by reducing phosphorus content if the index is high [54]. Application rate should also consider soil nutrient content and crop requirement (by considering trace element content of the soil) as well as, nutrient content of the biosolid (considering trace elements content of the biosolid) [54].
-
(b)
Application Site Slope Limitation: Biosolid land application should consider site steepness or slope to avoid excess nutrient runoff to nearby plateaus or streams [70]. Usually, for sites with 0–6 unit slopes, the application rate is not limited but may be varied when erosion is a concern and soil conservation practices are employed on sites with 7–12 unit slopes [71]. Biosolids can be applied on grass vegetation sites with more than 80% of the site covered, for slopes of 12 units and above [71].
-
(c)
Application Site pH Limitation: The availability of soil nutrients for plant growth is affected by soil pH value [72]. Depending on the soil solution pH test (whether salt or water solution test) conducted, biosolid application on sites should be avoided when pH is within the acidic region of less than 6.0 or alkaline region of greater than 7.5 for a salt test or, 6.5 and 8.5 for a water test [71].
-
(d)
Establishing Buffer Zones: Biosolid application is discouraged on neutral land areas around natural water bodies like sink boreholes, ponds, wells, etc., for environmental protection [73]. These buffer zones (neutral areas) can be established about 300 feet from water bodies, 150 feet from residential areas or dwellings, 100 feet from wetlands, and 50 feet from intermittent flowing streams [71].
-
(e)
Restriction of Public Access to Biosolids: General public use of biosolids should depend on the type and government approval for public use. Class A biosolids to be applied on home gardens, root and vegetable crops gardens, and other public sites must comply with strict regulations and must be approved by the government before distribution or use [71,74]. Class B biosolids should not be applied on public sites, turf lawns, or residential farms, unless they are produced by a regulated industry (i.e. incorporated) [71].
-
(f)
Encouraging Biosolid Application Deferment on Grazing Land: Biosolid land application should be discouraged immediately after livestock grazing or crop harvesting on agricultural fields to prevent pollution caused by nutrient washout (run-off) which may occur after these activities as the soil becomes prone to erosion [69,70]. Generally, a 30-day period should be allowed before biosolid application on land after grazing or harvesting [69].
-
(g)
Encouraging Soil Conservation via Biosolid Application: Biosolid application on land can protect topsoil from erosion and maintain soil productivity and fertility [75], as the organic matter present in biosolid help to increase soil water holding capacity, reduce compaction [67,76], and provide a platform for microbial activity [71].
-
(h)
Discouraging Biosolid Application on Saturated or Frozen Soil: Biosolid land application should be discouraged on frozen, snow-covered, or saturated soil unless restriction controls can be employed to prevent pollution from snowmelt or water runoff [71]. If biosolid must be applied on frozen soil, then the land must have a maximum of 2 or 6 units of slope and a buffer area of 100 or 300 feet respectively and must be allowed between a water body and the application site [71,77,78].
-
(i)
Protection of Threatened Biological Species: Biosolid land application should be practiced in such a way that it does not harm endangered soil biota such as bacteria, fungi, earthworms, protozoa, nematodes [71,79], insects [69] or, threaten their habitat [80].
5. Fate, environmental hazard, and effects of contaminants in Biosolid applied on land
Although biosolids are beneficial to crops when used as organic fertilizer and soil conditioner [81], they do contain contaminants at minute concentrations in nanogram/liter (ng/L) or lower (the concentrations of contaminants in biosolids are usually below regulatory standards), which can accumulate in soils [82] due to repeated land application and, eventually cause environmental pollution [83]. These emerging contaminants discovered in biosolids may include pharmaceuticals and personal care products (P&PCPs) such as prescription drugs that may be found in human faeces and urine [84,85] and, chemicals in soaps, detergents, shampoo, etc. [83], endocrine-disrupting chemicals (EDCs), surfactants, flame retardants, per-and poly-fluoroalkyl substances (PFAS) [84], microplastics (or nanoparticles) in toothpaste and laundry wastewater, etc. [[86], [87], [88]], which all enter the wastewater treatment plant and thus, sewage sludge through the sewer system, remaining in biosolid after sludge treatment as treatment does not completely remove these contaminants [27]. When contaminants enter the environment through biosolid application on land, they may be persistent, degraded, undergo additional transformations, or converted into by-products with hazardous effects on the environment [84].
Researchers have studied the effects and potential hazards of biosolid contaminants on the cell structure, reproduction, biochemistry, growth, and mortality of the flora and fauna colony in an environmental ecosystem [89,90]. While some of the literature reviewed agreed that contaminants may have a minor effect at low soil accumulation on soil microbes and animals [89], others presented evidence of a major effect on terrestrial and aquatic animals [[91], [92], [93]]. For instance, Diclofenac, a pharmaceutical drug detected in soil amended with biosolid, was linked to a drastic decrease in the vulture population in Pakistan [91]. Also, Garric et al. [92] in their study, demonstrated the effect of Ivermectin on the development of aquatic invertebrates and Lange et al. [93] demonstrated the endocrine disruptive effect of Ethinylestradiol (a drug used for contraception in humans) in fishes. It is therefore imperative to understand the fate and hazardous effects of emerging contaminants and persistent organic pollutants (PoPs) found in biosolid used for land application in order to prevent environmental pollution and inform government regulations on biosolid use.
5.1. Heavy metals
Biosolid application on land may be limited by its heavy metals content [35]. Heavy metals such as arsenic (As), mercury (Hg), selenium (Se), molybdenum (Mo), titanium (Ti), antimony (Sb) [94], zinc (Zn), cadmium (Cd), copper (Cu), chromium (Cr),lead (Pb), and nickel (Ni) [95] are commonly found in biosolids and, their concentrations depend on the nature of wastewater and region of origination (Table 1) [96], wastewater treatment process [[96], [97], [98]], and the industrial manufacturing process [95]. Domestic or household wastewater usually has a lower heavy metal concentration than industrial wastewater [95]. While some heavy metals in biosolids are appropriate for plant growth at low concentrations (Mn, Zn, Cu), others (Pb, Cd, Hg, As) are not [99]. The accumulation of heavy metals in the soil becomes high over a long-term period of biosolid application and even, short-term use has been observed to raise their concentrations considerably [95].
Table 1.
Variation in concentrations of heavy metals in biosolids due to region and nature of wastewater generated.
| Country | Zn | Cu | Cr | Pb | Ni | Cd | Hg |
|---|---|---|---|---|---|---|---|
| USA (1977) | 1740.0 | 850.0 | 890.0 | 300.0 | 82.0 | 19.0 | 3.0 |
| USA (2002) | 750.0 | 511.0 | 35.0 | 65.0 | 23.0 | 2.3 | 1.5 |
| France (1999) | 761.0 | 286.0 | 4.5 | 107.0 | 35.0 | 4.5 | 2.1 |
| France (2017) | 60–200 | 20–100 | 30–100 | 70–100 | 15–70 | 0.5–1.5 | 0.1–1.0 |
| China (2018) | 674.0 | 204.0 | 236.0 | 26.0 | 334.0 | 0.9 | 0.9 |
| Concentration limit, EPA (2002) | 7500.0 | 4300.0 | ND | 840.0 | 420.0 | 85.0 | 57.0 |
ND = not determined. All values are in mg/kg [98].
5.2. Per- and poly-fluoroalkyl substances (PFAs)
These synthetic, organic chemical compounds, used in several applications such as water and heat-resistant fabrics [100,101], cleaning products, dental floss [102], shampoo, and cosmetics [103], paint production, industrial emulsifier and surfactant manufacture, non-sticky cookware, oil-repelling containers, aqueous film-forming foams for firefighting operations [84], etc., are found in sewage sludge and have been detected in soil amended with biosolids [36,104,105].
The number of PFA variants (Table 2) synthesized after the first (Polytetrafluoroethylene or PTFE) was produced in 1938, has been on a steady increase since the early 1950s and presently, there are over 5000 PFA compounds that have been commercially manufactured [106]. Of these PFA variants, long-chain PFAs such as polyfluorooctane sulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) are most detected in polluted soils [107] as they are more persistent and bioaccumulative in the environment [36,106,108] due to their preferential bonding to the hydrophobic or protein binding site of the soil [106]. Because of the persistent fate of PFAs especially PFOA and PFOS in environmental matrices, their concentrations are elevated within a short period of biosolid application on agricultural soil [109,110] with the attendant phytotoxic effect on certain plant species [111]. This characteristic of PFAs has been demonstrated by researchers in various research, for instance, Damien [106], reported the phototoxic effect of PFOA on Thale Cress (used in genetic experiments) at a concentration in excess of 181 ng/L in a study to investigate the fate, behaviour, and ecological impact of biosolid-derived PFAs and, Li et al. [111], carried out a metabolomic study of Lettuce exposed to elevated concentrations of PFOS and PFOA, between 500 and 5000 ng/L and observed between 88 and 95 significantly disturbed metabolites (amino acids, prenol lipids, phenolic compounds, carboxylic acids)for PFOS and PFOA exposure respectively. Public health hazards of human and animal exposure to high levels of PFAs include reproductive defects, neurological development delays, low birth weight, bone variation, accelerated puberty, changes in liver and kidney functions, thyroid disease, testicular cancer, etc. [94,112].
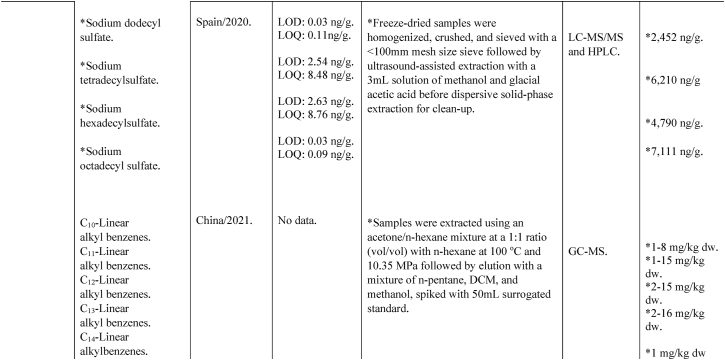
Table 2.
Pollutants and emerging contaminants recently discovered in biosolid. Cl-PFCA: Chlorine substituted PFCA; UPLC-ESI-MS/MS: Ultra Performance liquid Chromatography with Mass Spectrometry and Positive Electrospray Ionization; PFHxS: Perfluorohexanesulfonic acid; ASE: Accelerated solvent extraction; PFHpA: Perfluoroheptanoic acid; PFEA: Perfluoroalkyl ether alcohol; H-PFCA: Hydro substituted PFCA; HPFSA: Hydro substituted PFSA; HPLC-FD: High performance liquid chromatography with fluorescence detection; LC-MS/MS: Liquid chromatography with mass spectrometry; LOD-limit of detection; FTS: Fluorinated telomer sulfonate; LOQ-limit of quantification; OBS: P-perfluorous nonenoxybenzenesulfonate; PFBS: Perfluorobutanesulfonic acid; Cl-PFESA: Chlorine substituted perfluoroalkyl ether sulfonate; GC/ECNI-MS: Gas chromatography/mass spectrometry operated in negative ionization mode; PFBA: Perfluorobutanoic acid; PFOS: Perfluorobutanesulfonic acid; PFOA: Perfluorooctanoic acid; PFPeA: Perfluoropentanoic acid; P&PCPs: Pharmaceutical and personal care products; PFSM: Perfluoroalkyl sulfonamide; SPE: Solid-phase extraction; UPFCA: Unsaturated PFCA; PFHxA: Perfluorohexanoicacid; HPFESA: Hydro substituted perfluoroalkyl ether sulfonate; PFAS: Poly & Per fluoroalkyl substances; UPFA: Unsaturated perfluoroalkyl alcohol [84].
In a study conducted by Oliveira et al. [113], the researchers reported that the concentration of Zn, Ni, Cr, and Cu in a biosolid-amended soil rose sharply over a relatively short period of two years. The speciation, bioavailability, and fate of heavy metals after biosolid application to the soil ecosystem is a function of the soil's (ionic strength, pH, and dissolved organic carbon) and biosolid's chemical characteristics [95]. Most metals are not biodegradable or chemically degradable and are, thus persistent in the environment [114,115] leading to metal leaching through soil profile and contamination of groundwater [99]. Heavy metals in the soil's rhizosphere (where toxicokinetic and toxicodynamic processes take place) in the form of dissolved free cations and negative, positive, or neutral species, are absorbed by plants through the root system [116], causing bioaccumulation of these metals [114]. Environmental effects related to heavy metal accumulation in the soil are severe on plants, animals, and humans alike [117]. Plant exposure to high concentrations of heavy metals may result in stunted growth, reduced yield, altered metabolism, etc. [116]. The effect of heavy metals on humans is the result of the biomagnification of these toxic chemicals in the body which affects different organs [118]. These effects on humans, which may be acute or chronic [118,119] include nervous system disorders, gastrointestinal and kidney dysfunction, immune system dysfunction, skin lesion, birth defect, vascular damage, and cancer [118].
5.3. Excess nutrients in biosolid
The major plant nutrients in biosolids are magnesium (Mg), nitrogen (N), potassium (K), phosphorus (P), sulphur (S), calcium (Ca), and micronutrients [120]. Among these nutrient types, P is commonly found in excess in biosolid-amended soils [121] because most of the phosphates in wastewater are retained in sewage sludge (biosolid)during the tertiary treatment step [122] and, because farmers by following government regulatory guidelines, apply biosolid to soil based on plant requirement of N, [121]; P accumulation in the soil thus increases rapidly as the ratio of N to P varies [123]. Long-term application of biosolid leads to excess concentration of P in top soils [121] which can be washed into surface water resulting in algae invasion and river eutrophication [123]. Increased phosphate concentration in the soil has also been observed to affect soil metal lability [124]. Mossa et al. [124], in a study, reported that with rising levels of metal concentration due to prolonged biosolid application, there is an interaction with increasing phosphate concentration, which reduces the lability of Pb and Cd metals and, increases lability of Zn, Ni, and Cu metals as a result of weak soil binding. These interactions between soil phosphate and heavy metal concentrations should be taken into account when the government sets regulatory standards [124].
5.4. Microplastics (MPs)
These emerging contaminants are plastic particles with diameters less than 5 mm [[86], [87], [88]]. MPs commonly found in biosolids include microbeads (Fig. 7) and microfibers (both primary MPs) manufactured by industries to act as fillers, abrasives [125], viscosity control agents [126], and for aesthetics purposes in fabrics, personal care products, consumer plastics [127,128], etc.; secondary MPs are formed by processes such as biodegradation, photo-oxidation, or mechanical abrasion of large plastic debris in the environment [129].
Fig. 7.
Microbeads used as abrasive in toothpaste [130].
Most MPs are collected in sewage sludge during wastewater treatment and are not completely removed when the sludge is treated to produce biosolids [86]. When MPs enter the environment through the application of biosolids on land, they are hardly removed (they can persist in the environment for many years) but can be fragmented into smaller-sized particles (nanoplastics have a size range less than 1 μm) by environmental forces [[86], [87], [88]]. MPs have been reported to act as vectors (carriers) for pollutants, heavy metals, hazardous additives, and pathogens, which can cause health complications in humans and animals [131]; these pollutants can be spread through contamination pathways such as water and food ingestion [132] as water and food samples have been discovered to contain them (e.g. salt, seafood, tap water, honey, and bottled water) [133].
5.5. Pharmaceuticals and personal care products (P&PCPs)
P&PCPs (structure of common pharmaceuticals in biosolids are shown in Fig. 8) are a wide range of manufactured products used for medical care and cosmetic purposes and, include substances like anti-inflammatory agents, steroidal hormones, antibiotics, detergents, perfumes and active ingredients in soaps [84]. Prescription drugs used by patients for the treatment of certain diseases are not fully absorbed by the body as remnants of these drugs and their metabolites, are passed out of the body during excretion into the sewer system [134,135]. From the sewer, the remnant drugs are transported to the wastewater treatment plant and then degraded by the secondary treatment process [136]. Most of the remnant drugs are trapped in the sewage sludge after wastewater treatment while a small concentration is contained in biosolid after sludge treatment [136].
Fig. 8.
Chemical structure of (a) acetaminophen (b) sulfadiazine (c) sulfapyridine (d) diclofenac (e) ibuprofen (f) atenolol (g) metoprolol (h) bezafibrate (i) clofibric acid (j) ethynylestradiol (k) gestodene (l) testosterone (m) fluoxetine (n) carbamazepine (o) caffeine and (p) cortisone which are commonly found pharmaceuticals in Biosolid [84].
The fate and persistence of pharmaceuticals in the soil following biosolid application depend on the drug's biodegradability [84], its physicochemical properties, and the molecular structure of its constituent chemicals but not on the soil's properties or bioactivity [137]. This persistence can affect plant uptake of, and bioaccumulation of these drugs which can determine their effect on humans as a result of ingestion [136]. For example, the pharmaceutical drug Carbamazepine was found to have a limit of quantitation (LOQ) as high as 52 μg/g and 33 μg/g in radish and ryegrass respectively, in a study to compare plants' uptake of pharmaceuticals in soil; whereas the LOQ for sulfamethazine was far lower at 0.01 μg/g in the same study [136]. The effects of bioaccumulation of Carbamazepine on plants include leaf chlorosis, tissue necrosis, inability to produce fruit, etc. [138]. Phototoxicity of Sulfamethazine at high levels in plants on the other hand causes root and stem elongation, root decay, necrosis, etc. [139]. In humans, the toxic effects of Carbamazepine trigger life-threatening allergic reactions such as Toxic Epidermal Necrolysis (TEN) and Stevens-Johnson syndrome (SJS), decrease the number of blood cells produced in the body, causing unusual bleeding in the gum, nose, heavy menstruation, shortness of breath, mouth sores, etc. [140]. Also, Sulfamethazine causes anaphylaxis, angioedema, and urticaria hypersensitive reactions in the human body and can result in skin rash, drug fever, agranulocytosis, polyarthritis, and hemolytic anemia, etc. [141]. Another effect of applying pharmaceutical-contaminated biosolids on agricultural land is the development of antibiotic-resistant bacteria or ARBs in the soil [136].
Triclocarban (Fig. 9a), Triclosan (Fig. 9b) and Triclocarban (Fig. 9c) are used as antibacterial ingredients in fast-moving consumer (FMCG) or personal care products that enter the wastewater treatment system from household wastewater (kitchen and bath wastewater) because of their use in liquid and bar soaps [142], are not completely eliminated from the sludge during wastewater treatment [143]. These chemicals also called 3, 4, 4′-trichlorocarbanilide and 2, 4, 4′-trichloro-2′-hydroxydiphenyl ether respectively, whose structure are shown in Fig. 9b and (c) respectively, have a low biodegradation potential and high persistence in the soil after biosolid application [[143], [144], [145]]; their degradation may increase with the amount of aeration available in the soil i.e. degradation rate is faster in aerobic than in anaerobic soil [144,145]. Negative effects of TCS and TCC in the environment can be seen in algae growth inhibition [146], fauna endocrine disruption [126], toxic or carcinogenic compound (such as chlorinated anilines, dioxins, and chloroform) formation on environmental biota, and chemical bioaccumulation especially in snails and earthworms [143].
Fig. 9.
Structure of (a) Triclocarban (b) Triclosan, and (c) Triclocarban [143].
5.6. Surfactants
These are organic chemicals often called surface-active agents because they help to reduce the surface tension of liquid media [84]. They are used for a wide range of applications including soap, detergent, dish cleaner and shampoo manufacture, production of lubricants and mining flocculates, and in wastewater treatment, textile industries, and petroleum recovery operations [84]. Commonly found surfactants in biosolid-amended soil are quaternary ammonium compounds, linear alkyl benzene sulfonates, alkyl ethoxylate, alkyl phenol ethoxylates, alkyl sulfates, and alkyl ethoxy sulfates [84]. The biodegradability of certain surfactants (linear alkyl benzene sulfonates) in environmental matrices, which is dependent on their isomerization process is more rapid under aerobic conditions (with removal rate up to 99%) than anaerobic conditions [84]. Thus, linear alkyl benzene sulfonates have short-term persistence in the environment than alkyl phenol ethoxylates, which are further degraded into by-products such as nonyl and octyl phenols, increasing their environmental persistence and bioaccumulation [84,147]. The effect of benzalkonium chloride exposure is evident in soil microbe resistance to antibiotics [84]. Also, alkyl phenol ethoxylate exposure causes the production of vitellogenin in male fishes at low concentrations [148] just as its by-products, nonyl, and octyl phenols have been associated with certain types of cancer and, low sperm count in men [147].
5.7. Flame retardants
These are lipophilic compounds used in applications such as firefighting equipment manufacture, transportation, construction, electrical/electronic industries, etc. [84]. They are designed to provide lifesaving opportunities in a disaster. For instance, they can extend personnel escape time in an industrial fire incident thus, reducing the number of casualties [84]. Flame retardants enter the wastewater treatment plants through the drainage system and because of their lipophilic (hydrophobic) nature, they are trapped in sewage sludge and reach the environment through biosolid land application, incineration, plastic, and e-waste recycling, etc. [84]. Common flame retardants such as organo-phosphate and brominated flame retardants (BFRs) [84] have long-term persistence in the soil and will undergo transformation, translocation, sorption, and root-uptake processes once in the environment [149]. They negatively affect public health and are toxic to the environment [84].
5.8. Endocrine-disrupting chemicals
These are natural (e.g. daidzein, coumestrol, and genistein found in human and animal foodstuff) or synthetic (e.g. bisphenol, perchlorate, and polychlorinated biphenyls used in manufacturing plastics, epoxy resins, lubricants, industrial solvents, pesticides, electrical equipment, and will certain pharmaceuticals) organic chemicals found in industrial/domestic wastewater, sewage sludge, and biosolids and, can impede the normal functioning of the endocrine or hormonal system [84,150]. These chemicals, which are examples of a group of PoPs found in biosolids, get into the environment via waste discharge including sewage effluent disposal and biosolid land application [151], and are extremely stable and persistent, resulting in high bioaccumulation [84]. The accumulation of endocrine disruptors (EDs) has made them ubiquitous and human exposure pathways included ingesting contaminated food, and water, breathing contaminated air, and contact with domestic/industrial chemicals [84]. Exposure to bisphenol can lead to infertility and the early onset of puberty in adolescents [84] while high levels of perchlorate impede thyroid gland iodine intake, reducing the production of hormones in the thyroid gland [97].
6. Regulations on biosolid land application
For the benefits of biosolid application in agriculture and land reclamation to be fully realized while mitigating its adverse effects on humans and the environment, government agencies around the world have promulgated guidelines for biosolid application practices [152]. Some of these legislations are examined in the following section.
6.1. The sludge directive and regulations (directive 86/278/EEC)
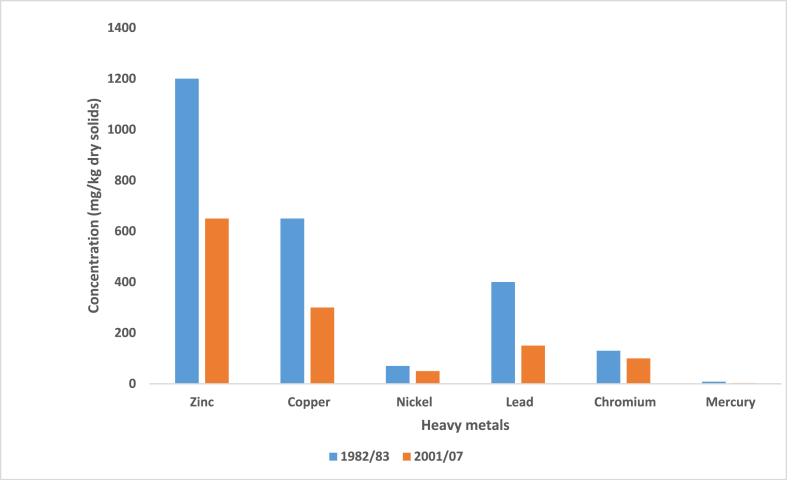
Biosolid land application in the European Union (EU) is controlled by the number of heavy metals it contains which is specified in the EU regulation called The Sludge Directive or simply, The Directive, first enacted in 1986 [153]. This regulation sought to promote sustainable biosolid use in agriculture and control its land application in order to prevent harmful effects on public health and environmental contamination; since treatment and recovery of biosolid on land is one of its important uses [154,155]. Contaminants such as organic compounds and pathogens also present in biosolids, are not considered under The Sludge Directive [18]. Since the enactment of the regulation, the United Kingdom (UK) has made great improvements in the heavy metal content (measured in mg/kg dry solids) of biosolids used for land application as seen in Fig. 10, which showed that between 1982 and ’83, the concentration of Zinc, Copper, Lead, and Chromium allowable in biosolid were 1200, 650, 400, and 130 mg/kg dry solids compared to more recent years between 2001 and 2007, when the allowable concentration of these metals had been scaled down to 650, 300, 150, and 100 mg/kg dry solids respectively, as a way of reducing their environmental impact [50]. The Sludge Directive specified that the soil and biosolid samples are to be analyzed (Table 3) before biosolid usage on land to limit heavy metal concentrations below maximum permissible levels in the soil (Table 4) [156].
Fig. 10.
Heavy metal improvement in biosolid in the UK over a period of two decades [50].
Table 3.
Soil and Biosolid analyses as stipulated by The Sludge Directive [156].
| S/N | Soil Analysis | Sludge Analysis |
|---|---|---|
| Frequency | Determined by soil mass. | Bi-annually or annually if there's no change in parameters. |
| Sampling method | Samples should be taken from 25 cm below the surface and not less than 10 cm minimum depth, a minimum of 25 core samples of soil over an area of 5 ha should be taken. | Wet or dry sludge samples can be used for analysis. |
| Parameters | To be tested should include Zn, Pb, Ni, Cd, Cu, Cr, and pH. | Should include organic matter, pH, dry matter, Zn, Pb, Ni, Cd, Cu, Cr, pH, Hg, N, and P. |
| Test method (s) | Atomic absorption spectrometry | Atomic absorption spectrometry, Spectrophotometry. |
Table 4.
The EU Council standard limit for heavy metals in soil and biosolid for agricultural applications.
| Parameter | Soil (mg/kg dm) | Sludge (mg/kg dm) | Loading Amount (kg/ha/yr) |
|---|---|---|---|
| Hg | 1–1.5 | 16–25 | 0.1 |
| Cd | 1–3 | 20–40 | 0.15 |
| B | 30–75 | 300–400 | 3.0 |
| Cu | 50–140 | 1000–1750 | 12.0 |
| Pb | 50–300 | 750–1200 | 15.0 |
| Zn | 150–300 | 2500–4000 | 30.0 |
The standard permissible limits for heavy metals in soil and biosolid samples used for agricultural applications in 1986, according to The Sludge Directive are given in Table 4; the high concentrations of metals allowable in both soil and sludge samples are noticeable from this table. Although this regulation is almost four decades since it was first enacted, it is still relevant as many other regulations on biosolid reuse have been derived from it (EU member states' individual regulations) [35], its provisions may be reviewed and updated to meet present-day realities [155]. With new and emerging pollutants not covered by the regulation now discovered in biosolids [[86], [87], [88],136,144,145], any potential environmental pollution caused by biosolid samples containing these pollutants will affect far more people in the world today, with the population in 2023 put at 8,045,311,477, at a percentage growth rate of 0.88% than in 1986 (year The Sludge Directive was passed) when the world's population was just 85,804,185 and 2.65% growth rate (Fig. 11) [157].
Fig. 11.
World population data from 1950 and projections up to the year 2100 [157].
[156].
EU member states have scaled down the concentration level of heavy metals in biosolids since the enactment of The Sludge Directive, by promulgating individual national legislations on further reduction in the limits of these metals, keeping values lower than the EU Council's directive, in a bid to further protect humans and the environment (Fig. 12a, b, c, and d showed the comparison of standard limits of selected heavy metals) [35]. In Fig. 12, it can be seen that the majority of EU states including Belgium, Croatia, Denmark, Finland, Germany, Hungary, Latvia, Malta, Netherlands, Slovakia, etc., allowed a national standard limit of heavy metals much below the EU Council's lower limit, which is shown on the figure by the red dotted lines (upper and lower limits). The comparison in Table 5 is important as it gives a glance-view of the national heavy metal standard limits and organic compounds concentration between EU member states.
Fig. 12.
a: Comparison of the limits of (i) Cadmium and (ii) Copper in biosolid for land application set by EU member states and that set by the EU council's Sludge Directive. Red dotted lines represent the EU Sludge Directive's lower and upper limits. b: Comparison of the limits (in mg/kg dw) of (i) Nickel and (ii) Lead in biosolid for land application set by EU member states and that set by the EU council's Sludge Directive. Red dotted lines represent the EU Sludge Directive's lower and upper limits. c: Comparison of the limits (in mg/kg dw) of (i) Mercury and (ii) Chromium in biosolid for land application set by EU member states and that set by the EU council's Sludge Directive. Red dotted lines represent the EU Sludge Directive's lower and upper limits. d: Comparison of the limits (in mg/kg dw) of (i) Zinc and (ii) Arsenic in biosolid for land application set by EU member states and that set by the EU council's Sludge Directive. Red dotted lines represent the EU Sludge Directive's lower and upper limits [35].
Table 5.
Comparison of the standard limits of heavy metals and organic compounds in biosolid among EU states with the EU Council's Sludge Directive.a, b, c, d, etc. represents values from different categories measured. PCDD/F: polychlorinated dibenzo-p-dioxins and furan; AOX: absorbable organic halogens; DEHP: di (2-ethylhexyl) phthalates; NP: nonylphenols; PCB: polychlorinated biphenyls; NPE: nonylphenol ethoxylates; PAH: polycyclic aromatic hydrocarbons; LAS: linear alkylbenzene sulfonates [18].
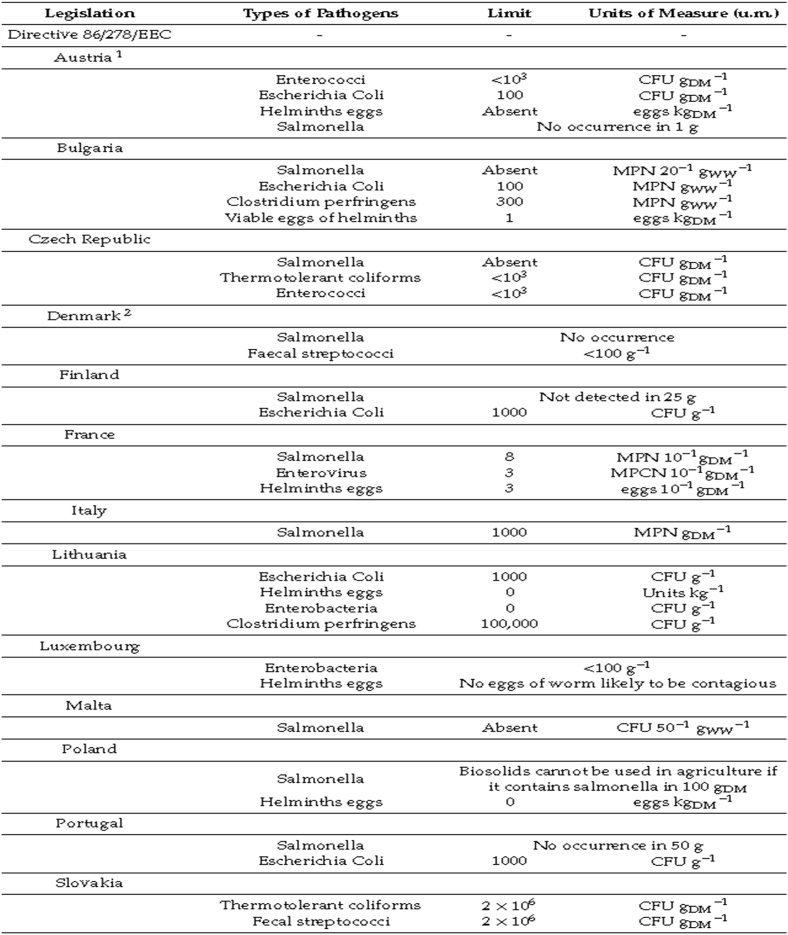
Permissible heavy metal constituent of biosolid shown in Table 4 (EU Council Sludge Directive, data from 1986), Table 5 (national regulation of EU member states, data from 2019) and Fig. 12 revealed a significant reduction over 3 decades, indicative of the need for lower values and thus, better environmental health. Maria et al. [18] provided the standard limit for pathogens in biosolids in selected EU states based on national legislation, which is not captured by The Sludge Directive. This is represented in Table 6. Data in Table 6 showed that most pathogen standard limits allowable in biosolids are less than or equal to 1000 CFU per gram dry matter (CFU/gDM); this has been adopted in many other states in Europe today [18].
Table 6.
Standard limit for pathogens in Biosolids used in agriculture in the EU.1 = three regions were involved in measurement, 2 = advanced sewage sludge treatment was employed; DM: dry matter; CFU: colony-forming unit; MPCN: most probable cytopathic number u.m.: units of measure; WW: wet weight; MPN: most probable number [18].
6.2. The 40 CFR part 503 regulation
The United States Environmental Protection Agency (USEPA) developed a regulation titled Standards for the Use of Sewage Sludge (popularly referred to as the Part 503 Rule, enacted in 1993), as part of the Clean Water Act of the U.S. Congress [158]. The Rule specifies general minimum requirements for applying biosolids on land for agronomic applications in the United States [159,160]. According to the Part 503 Rule, biosolids used for agricultural applications must meet the ceiling concentration (maximum limit) for heavy metal content before usage; this is given in Table 7.
Table 7.
The EPA Part 503 heavy metal requirements in biosolids applied to land.
| Type of Heavy Metal | Concentration limits for all biosolid types applied to land. (mg/kg)a | Concentration limits for exceptional quality (EQ) and pollutant concentration biosolids (mg/kg)a | Cumulative pollutant loading rate (CPLR) limits for biosolid (kg/ha) | Annual pollutant loading rate (APLR) for biosolids (kg/ha.yr) |
|---|---|---|---|---|
| Chromium | 3000 | 1200 | 3000 | 150 |
| Arsenic | 75 | 41 | 41 | 2.0 |
| Cadmium | 85 | 39 | 39 | 1.9 |
| Mercury | 57 | 17 | 17 | 0.85 |
| Lead | 840 | 300 | 300 | 15 |
| Copper | 4300 | 1500 | 1500 | 75 |
| Nickel | 420 | 420 | 420 | 21 |
| Molybdenumb | 75 | – | – | – |
| Zinc | 7500 | 2800 | 2800 | 140 |
| Selenium | 100 | 36 | 100 | 5.0 |
| Applies to: | All biosolids applied on land. | Bulk and bagged biosolids. | Bulk biosolids | Bagged biosolidsc. |
ab,c = Dry mass basis measurement; molybdenum limits were deleted from the Part 503 Rule after February 1994 amendment, awaiting EPA consideration; biosolids delivered in bags or other similar containers [159].
The 40 CFR Part 503 Rule specifies pathogens reduction in two biosolid categories, Class A and Class B biosolids. The Rule specifies that pathogens in Class A biosolids be reduced below detectable concentrations [160]. In Class B biosolids, however, pathogens are reduced to concentrations unlikely to pose threats to the environment and public health [160]. There are, however, new pathogens (hepatitis A virus and adenovirus) with heat-resisting capabilities that have been detected in biosolids since the enactment of the Part 503 Rule in 1993 [161]. The general requirements for land application of biosolids based on the Part 503 Rule specified that the supplier of biosolid must provide the user with a guide so as to comply with the requirements of Table 7 and the user, on the other hand, must apply biosolids to meet these requirements [159,162]. The supplier must notify government authorities in writing, about the location and time of application, and provide contact information while the user, must notify authorities before the initial application of bulk biosolids [159].
The majority of literature reviewed for this study agreed that The Sludge Directive (i.e. Directive 86/278/EEC) regulations and the Part 503 Rule formed the basis for biosolid use on land in most countries around the world including developing countries [153,154,[160], [161], [162]]. Iranpour et al. [163], compared the standards (Table 8) specified by both regulations and, a national standard of the Netherlands in a review study in which values for heavy metals were normalized against the U.S. pollution standard limit. These pictorials clearly showed that the national limit of the Netherlands is much lower than EU or U.S limits., further underscoring efforts by EU member states to reduce national pollutant standards for better protection of the environment. Also, Table 8 showed that concentration limits in the EU are expected to reduce significantly, over a long-term period [163].
Table 8.
Comparison of heavy metal standard limits for the EU, Netherlands, and the U.S.
| Heavy Metal | EU Limits |
Netherland Limits | U.S. EPA Limits |
|||||
|---|---|---|---|---|---|---|---|---|
| Upper Limits | Lower Limits | Short-term Limits | Medium-term Limits | Long-term Limits | Ceiling Concentration | Limit Concentration | ||
| Chromium | – | – | 1000 | 800 | 600 | 75 | – | – |
| Arsenic | – | – | – | – | – | 15 | 75 | 41 |
| Cadmium | 40 | 20 | 10 | 5 | 2 | 1.25 | 83 | 39 |
| Mercury | 25 | 16 | 10 | 5 | 2 | 0.75 | 57 | 17 |
| Lead | 1200 | 750 | 750 | 500 | 200 | 100 | 840 | 17 |
| Copper | 1750 | 1000 | 1000 | 800 | 600 | 75 | 4300 | 1500 |
| Nickel | 400 | 300 | 300 | 200 | 100 | 30 | 420 | 420 |
| Molybdenum | – | – | – | – | – | – | 75 | – |
| Zinc | 4000 | 2500 | 2500 | 2000 | 1500 | 300 | 7500 | 2800 |
| Selenium | – | – | – | – | – | – | 100 | 100 |
[163].
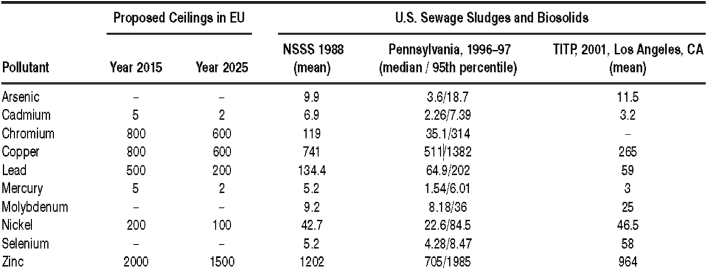
According to Iranpour et al. [163], the EU Council proposed a reduction in heavy metal concentration in biosolids over a 10-year period in line with member states’ leanings. Iranpour and co-researchers compared these proposed (projected) standards with mean values from the U.S. EPA National Sewage Sludge Survey (NSSS), Pennsylvania, and Los Angeles Surveys over a given period (Table 9). The authors observed a general decline in the value of heavy metals except for Pennsylvania (95th percentile) which has the highest values in spite of the average. They concluded that if the U.S. is to adopt EU proposed limits, new technologies (such as adsorption or precipitation from a suitable porous solid) must be developed to further reduce pollutant concentrations in biosolids.
Table 9.
Comparison of EU projected heavy metal limits in biosolid to U.S. current average values. NSSS = National Sewage Sludge Survey; TITP: Terminal Island Treatment Plant [163].
7. Discussion
In terms of agronomic usage and as a fit for the circular economy, biosolid has had beneficial applications, whether in agricultural land application or as a base material for the manufacture of construction materials. It has been used as organic fertilizer (due to their high organic matter and nutrient content, which is slowly released to plants, making biosolids better than chemical fertilizers in preventing the leaching of nutrients to surface water), as soil amender, and as a land repairer. Although biosolids are treated before land application, they still contain low concentrations of harmful emerging and persistent organic pollutants (PoPs) (Table 2) including per- and poly-fluoroalkyl substances (PFAs), heavy metals, P&PCPs, and microplastics, etc., which can have hazardous effects on humans, animals, and the environment at high concentrations without controlled application of biosolids. Some of the effects in the soil include reproductive defects, neurological development delays, low birth weight, bone variation, accelerated puberty, changes in liver and kidney functions, thyroid disease, and testicular cancer caused by PFAs; algal invasion, endocrine disruption, carcinogenic and toxic compound formation such as chlorinated anilines, dioxins, and chloroform, and, chemical bioaccumulation in snails and earthworms caused by the cosmetic antibacterial chemicals TCS and TCC; nervous system disorders, gastrointestinal and kidney dysfunction, immune system dysfunction, skin lesion, birth defect, vascular damage, and cancer caused by heavy metals; water eutrophication and algae invasion caused by excess soil phosphorus content; Toxic Epidermal Necrolysis (TEN) and Stevens-Johnson syndrome (SJS), reduction in red blood cell production, hemolytic anemia, anaphylaxis, etc. These effects on humans and the environment may be mitigated by biosolid land application practices (Section 4.1) which account for the concentration, fate, and persistence of pollutants in the soil and form part of government regulations.
Government regulations are effective in limiting the number of heavy metals, pathogens, and organic pollutants allowable in biosolids applied on land, imposing restrictions on sites, and fostering good agronomic application practices. The EU's Sludge Directive (Directive 86/278/EEC) and USEPA's Part 503 Rule are the two major legislations on biosolid land application from which many countries around the world and even, EU member states have derived national biosolid legislation. A comparison of both legislations revealed that the EU's Sludge Directive specified stricter regulation, with allowable biosolid pollutant concentration values much lower than USEPA's Part 503 Rule (Table 8). Since the enactment of these major legislations (the Sludge Directive was enacted in 1986 and the Part 503 Rule was passed in 1993), there have been new and emerging contaminants detected in biosolids, making it necessary to review and update these regulations to meet current realities and challenges of today's biosolid land applications.
Updating these government regulations (Directive 86/278/EEC and the Part 503 Rule) should focus on their expansion to accommodate specific legislation on new pathogens, organic pollutants, and emerging contaminants such as microplastics and pharmaceuticals. The updated regulations should review downwards, the standard limits of heavy metals in biosolids (with a focus on USEPA's Part 503 Rule with less stringent allowable standard limits) in line with the national regulation of EU member states, using regulations in the Netherlands as a guide (Table 8). The new heavy metal regulations should take into account soil phosphate interaction with metals which causes metal lability in the soil due to high phosphorus content occasioned by the high agronomic loading rate of biosolid on land. Biosolid land application best practices should form an integral part of government regulatory policies on biosolid use.
8. Conclusion and future perspectives
This review study examined the fate of contaminants in biosolids used for land application and the potential effects of this biosolid management practice on humans and the environment while discussing government policies regulating land application. Although there are other methods (including incineration and energy recovery, landfilling, land reclamation, etc.) employed to dispose of the increasing quantities of biosolids generated from large volumes of wastewater treated daily due to an increasing world population, land application on agricultural farms has been adjudged the most economical and sustainable disposal route aside using biosolid as a base material for the manufacture of construction materials. Land application of biosolids is regulated by the government due to the negative effects of its contaminants and the need to protect the environment. Two major biosolid regulations, the EU's Sludge Directive, and USEPA's Part 503 Rule were identified. These regulations require a review and an update, with a focus on USEPA's Part 503 Rule with less stringent biosolid regulations, to meet the current challenges of biosolid land application. The update should include specific regulations on new and emerging contaminants such as microplastics, pharmaceuticals, pathogens, organic pollutants, and other persistent organic pollutants (PoPs) in biosolid, further reduction of heavy metal standard limits (which should be lower than the projected EU regulation in Table 9), and consideration of soil phosphate-metal interactions to regulate biosolid agronomic loading rate. Future biosolid research should focus on the concentration of TCS, TCC, and newly discovered pharmaceuticals, as well as Microplastic transport in biosolid-amended soils, soil-plant transfer mechanism, and metabolism of PFAs in soils, all of which should inform government policies on biosolid application on land.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The first author acknowledges the financial support rendered by Afe Babalola University, Ado-Ekiti, Ekiti State, Nigeria towards the publication of this article.
References
- 1.Boyle Michael J., Jasperson Hans D. How Does Industrialization Lead to Urbanization? The Investopedia Team; 2021. https://www.investopedia.com Available at: (Accessed 14 February 2023. [Google Scholar]
- 2.Satterthwaite David, Gordon McGranahan, Tacoli Cecilia. vol. 365. Philosophical Transaction of the Royal Society; 2010. pp. 2809–2820. (Urbanization and its Implication for Food Farming). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The World Bank Understanding poverty: urban development. The International Bank for Reconstruction and Development (IBRD) and, International Development Association (IDA) 2022 https://www.worldbank.org Available at: [Google Scholar]
- 4.Navarro Ferronado, Torretta Vincenzo. Waste management in developing countries: a review of global issues. Int. J. Environ. Res. Publ. Health. 2019;16(6):1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.USEPA Guide for industrial waste management. United States Environmental Protection Agency. 2015 https://www.epa.gov/epaoswer/non-hw/industd/index.htm Available at: [Google Scholar]
- 6.Kanu I., Achi Ome. Industrial effluents and their impact on water quality of recurring rivers in Nigeria. Journal of Applied Technology in Environmental Sanitation. 2011;1(1):75–86. [Google Scholar]
- 7.Sperling Marcos V. vol. 1. Iwa Publishing; London SW1HOQS, UK: 2007. (Wastewater Characteristics, Treatment, and Disposal). Alliance House, 12 Caxton Street. [Google Scholar]
- 8.White Robert Edwin, Torri Silvana I., Stuart Correa Rodrigo. Biosolids soil application: agronomic and environmental implications. Applied and Environmental Soil Science. 2011 10.1155/2011/928973- biosolid-soil-application. [Google Scholar]
- 9.Kabirinejad Shahrzad, Hoodaji Mehran. The effect of biosolids application on soil chemical properties and zea mays nutrition. Kabirinejad and Hoodaji International Journal of Recycling of Organic Wastes in Agriculture. 2012 https://www.ijrowa.com/content/1/1/4 1:4. [Google Scholar]
- 10.Gabriel Di Giacomo, Romano Pietro. UK Govt. Publication; 2020. Evolution and Prospects in Managing Sewage Sludge. Environment Agency Strategy for Safe and Sustainable Sludge Use (Policy Paper) [Google Scholar]
- 11.Quadir Manzoor, Drechsel Pay, Jimenez Cisneros Blanca, Kim Youngy, Amit Pramanik, Mehta Praem, Olaniyan Oluwabusola. Global and regional potential of wastewater as a water, nutrient and energy source. Nat. Resour. Forum. 2020;44(1):40–51. doi: 10.1111/1477-8947.12187. [DOI] [Google Scholar]
- 12.Hu Yecui, Pang Shuang, Yang Junjie, Zhao Xiaoguang, Cao Jirong. Changes in soil microbial community structure following amendment of biosolids for seven years. Environmental Pollutions and Bioavailability. 2019;31(1):24–31. doi: 10.1080/26395940.2019.1569478. 2019. [DOI] [Google Scholar]
- 13.Burke Thomas A. National Academies of Sciences, Engineering, and Medicine, National Academies Press; Washington D.C: 2002. Biosolids Applied to Land: Advancing Standards and Practices.https://nap.nationalacademies.org/10426 [Google Scholar]
- 14.Blanchini Augusto, Pellegrini Marco, Sarcani Cesare. Int’l Journal of Environment and Waste Management; 2016. Sewage Sludge Management in Europe: A Critical Analysis of Data Quality. 2016. [DOI] [Google Scholar]
- 15.Akpan Victor E., Omole David O., Bassey Daniel E. Assessing the public perceptions of treated wastewater reuse: opportunities and implications for urban communities in developing countries. Heliyon. 2020;6(10) doi: 10.1016/j.heliyon.2020.e05246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Englande A.J., Jr., Krenkel Peter, Shamas J. Encyclopedia of Physical Science and Technology; 2015. Waste Treatment and Water Reclamation; pp. 639–670. [Google Scholar]
- 17.Khalid Sana, Shahid Muhammad, et al. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with focus on low and high income countries. International Journal of Environmental Resource and Public Health. 2018;15(5):895. doi: 10.3390/ijerph15050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collivignarelli Maria M., Abba Allessandro, Frattarola Andrea, Mino Marco C., Padovani Sergio, Katsoyannis Joannis, Torretta Vincenzo. Legislation for the reuse of biosolids on agricultural land in Europe: overview. Sustainability. 2019;219(11):6015. [Google Scholar]
- 19.Hoi Yan Chow and Min Pan Fertilization value of biosolids on nutrient accumulation and environmental risks to agricultural plants. Journal of Water, Air, and Soil Pollution. 2020;231:578. doi: 10.1007/s//270.020.049468. 2020. [DOI] [Google Scholar]
- 20.Mateo-Sagasta Javier, Liqa Rasehid Sally, Anne L., Thebo Global wastewater and sludge production, treatment, and use. Researchgate. 2015 doi: 10.1007/978-94-017-9545-6_2. [DOI] [Google Scholar]
- 21.Karagiannidis Avram, Samaras Petro, Kasampalis Themistoklis. Evaluation of sewage sludge production and utilization of Greece in the frame of integrated energy recovery. Desalination and Water Treatment, Researchgate. 2011 doi: 10.5004/dwt.2011.2613. [DOI] [Google Scholar]
- 22.Liang Yuting, Ye Ling, Yue Bo, Meng Bang-bang, Yuan Xusheng. Bibliometric analysis of related literatures on sludge disposal technologies. IOP Conf. Ser. Earth Environ. Sci. 2022;1035 doi: 10.1088/1755-1315/1035/1/012015. 2022. [DOI] [Google Scholar]
- 23.Corcoran E., Nellemann E., Baker R., Bos D., Osborn H.S. Sick water? The central role of wastewater management in sustainable development – a rapid response assessment. United nations environment programme, UN-habitat, grid, arendal. 2010. https://www.grida.no
- 24.Eurostat Population Connected to Wastewater Treatment Plant (online) 2014 https://www.eea.europa.eu/data-and-maps/data/external/population-connected-to-wastewater-collection Available at: September 2015. [Google Scholar]
- 25.Buta Martyna, Hubeny Jaku, Inski Wiktorzie, Harnisz Monika, Korzeniewka Ewa. Sewage sludge in agriculture: the effects of selected chemical pollutants and emerging genetic resistance determinants on the quality of soil and crops- A review. Ecotoxicol. Environ. Saf. 2021;214 doi: 10.1016/j.ecoenv.2021.112070. [DOI] [PubMed] [Google Scholar]
- 26.Hamood A., Khatib J.M. Sustainability of Construction Materials, Science Direct; 2016. Sustainability of Sewage Sludge in Construction; pp. 308–415. (Chapter 24) [Google Scholar]
- 27.Fijalkowski Krzysztof, Rorat Agnieszka, Grobelak Anna, et al. The presence of contaminations in sewage sludge – current situation. J. Environ. Manag. 2017;203 doi: 10.1016/j.jenvman.2017.05.068. 1206-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yakamercan Elif, Ari Akif, Ahmed Aygun. Land application of municipal sewage sludge: human health risk assessment of heavy metals. J. Clean. Prod. 2021;319 [Google Scholar]
- 29.Rajeev Pratap Singh. Agrawal Madhoolika. Potential benefits and risks of land application of sewage sludge. Waste Manag. 2008;28(2):347–358. doi: 10.1016/j.wasman.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 30.Maureen Reilly B.A. The case against land application of sewage sludge pathogens. Can. J. Infect Dis. Med. Microbiol. 2001;12(4):205–207. doi: 10.1155/2001/183583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Briffa Jessica, Sinagra Emmanuel, Blundell Reynald. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon. 2020;6(9) doi: 10.1016/j.heliyon.2020.e04691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alysson Ronberto Baizie Silva. Camilotti Fabio. Risks of heavy metal contamination of soil-plant system by land application of sewage sludge: a review with data from Brazil. IntechOpen. 2014 doi: 10.5772/58384. [DOI] [Google Scholar]
- 33.Koyuncu Serdar. Occurrence of organic micropollutants and heavy metals in the soil after the application of stabilized sewage sludge. Journal of Environmental Health Science and Engineering. 2022;20(1):385–394. doi: 10.1007/s40201-022-00785-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aparicio J., Santos J.L., Alonso E. Limitation of the concentration of organic pollutants in sewage sludge for agricultural purpose: a case study of south Spain. Waste Manag. 2009;29(5):1747–1753. doi: 10.1016/j.wasman.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Gianico Andrea, Camilla M., Braguglia, et al. Land application of biosolids in Europe: possibilities, constraints. and Future Perspective Water. 2021;13:103. doi: 10.3390/w/3/0103. 2021. [DOI] [Google Scholar]
- 36.Dickman Rebecca A., Aga Dana S. Efficient workflow for suspect screening analysis to characterize novel and legacy per-and polyfluoroakyl substances (PFAs) in biosolids. Anal. Bioanal. Chem. 2022;414:4497–4507. doi: 10.1007/s00216-022-04088-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lupton Sara J., Casey Francis, Smith David J., Hakk Heldurr. Perfluorooctanoic acid transport in soil and its absorption and distribution in alfalfa (medicago sativa) J. Food Protect. 2022;85(1):164–172. doi: 10.4315/jfp-21-276. [DOI] [PubMed] [Google Scholar]
- 38.Hashimi Muhammed Z., Wang Shuhong, Ahmed Zulkfil. Elsevier; 2022. Environmental Micro-pollutants. [DOI] [Google Scholar]
- 39.Elliot Sarah M., Erickson Melinda, Krall Aliesha. ResearchGate; 2018. Micropollutants in Groundwater and Soil Downgradient from On-Site Wastewater Discharges.https://www.researchgate.net/publication/321371850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rorat Agnieszka, Coiurtois Pauline, Frank Vandenbulcke, et al. 2019. Sanitary and Environmental Aspects of Sewage Sludge Management. Industrial and Municipal Sludge. [DOI] [Google Scholar]
- 41.Roychoudhury Aryadeep, Das Nilanjana. Sustainable Management and Utilization of Sewage Sludge. Springer; 2022. Sewage sludge treatment and involvement of microbes; pp. 165–181. [DOI] [Google Scholar]
- 42.Kacprzak Malgorzata, Neczaj Ewa, Fijalkowski Krzysztof, et al. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017;156:39–46. doi: 10.1016/j.envres.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 43.Lovingood Tina, Toynosky Jill, et al. U.S. Environmental Protection Agency; 2018. EPA Unable to Assess the Impact of Hundreds of Unregulated Pollutants in Land-Applied Biosolids on Human Health and Environment [Report] [Google Scholar]
- 44.Processing Sludge. Sludge Treatment (online). Benenv. 2020 https://www.sludgeprocessing.com/ Available at: 15-03-2023. [Google Scholar]
- 45.Veolia ) Incineration (online. 2021 https://www.veoliawatertechnologies.co.uk/technologies/incineration Available at: [Google Scholar]
- 46.SUEZ Sludge Drying (online) 2022 www.suez.com/en/water/sludge-and-by-products-management/sludge-drying/ Available at: [Google Scholar]
- 47.Mangone Franco, Gutierrez Soledad, et al. Computer-aided Chemical Engineering; 2018. Sludge Dewatering. 28th European Symposium on Computer-Aided Process Engineering. 2018. [Google Scholar]
- 48.CAMBI How Does Thermal Hydrolysis Work (online)? 2020 www.cambi.com/what-we-do/thermal-hydrolysis/how-does-thermal-hydrolysis-work/ Available at: [Google Scholar]
- 49.Ram Wanare, Kannan Iyer, Dave Trudeep. Application of biosolids in civil engineering: state of the art. Mater. Today: Proceedings, Elsevier. 2022 doi: 10.1016/j.matpr.2022.04.166. [DOI] [Google Scholar]
- 50.UKWIR . 2014. Biosolids: Agricultural Good Practice. United Kingdom Water Industry Research Guidance Leaflet. Project 14/SL/11/7. [Google Scholar]
- 51.Epstein Eliot. Lewis Publishers, CRC Press LLC, 2000NW, Corporate BLVD; Boca Raton, Florida 33431: 2003. Land Application of Sewage Sludge and Biosolids. [Google Scholar]
- 52.Kinney Chad A., Furlong Edward T., Zangg Steven D., et al. Survey of organic wastewater contaminants in biosolids destined for land application. Environmental Science Technology. 2006;140:7207–7215. doi: 10.1021/es0603406. [DOI] [PubMed] [Google Scholar]
- 53.Patel Savakumar, Kundu Sazal, et al. A critical literature review on biosolids to biochar: an alternative biosolid management option. Revised Environmental Science and Biotechnology. 2020;202(19):807–841. [Google Scholar]
- 54.Lu Qin, He Zhenli L., Stoffellla Peter J. Land Application of Biosolids in the U.S.A.: A Review. Applied and Environmental Soil Science. 2012;2014(62) doi: 10.1155/2012/201462. [DOI] [Google Scholar]
- 55.Gefa Biosolid Assessment and Prepared Study (online). Georgia Environmental Finance Authority, Black and Veatch. 2021. https://www.bv.com/ Available at:
- 56.USEPA . U.S. Environmental Protection, Office of Research and Development, National Risk Management Research Laboratory, Centre for Environmental Research Information. EPA/625/R-92-013; Cincinnati, OH: 1999. Control of Pathogen and Vector Attraction in Sewage Sludge. [Google Scholar]
- 57.Wei Liangliang, Zhu Fengyi, Li Qiaoyang, et al. vol. 144. Environmental International, Elsevier; 2020. (Development, Current State, and Future Trends in Sludge Management in China Based on Exploratory Data and CO2-equivalent Emission Analysis). 2020. [DOI] [PubMed] [Google Scholar]
- 58.Ogunwumi Olawale T., Salami Lukumon. In: Solid Waste Management - Recent Advances. Suhaiza Zailan Dr., editor. IntechOpen; Princes Gate Court, London, SW7 2QJ, UK London, UK: 2023. Perspective Chapter: Industrial Waste Landfill; pp. 1–32. [DOI] [Google Scholar]
- 59.Naorem Anandkhumar, Jayaraman Somasundaram, Yash P., Dang, et al. Soil Constraints in an Arid Environment- Challenges, Prospects, and Implications. Agronomy, MDPI. 2023;13(1):220. doi: 10.3390/agronomy13010220. [DOI] [Google Scholar]
- 60.Boudjabi Sonia, Chenchouni Haroun. On the Sustainability of Land Applications of Sewage Sludge: How to Apply the Sewage Biosolid in Order to Improve Soil Fertility and Increase Crop Yield? Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131122. [DOI] [PubMed] [Google Scholar]
- 61.Sharma Bhavisha, Sarkar Abhijit, Singh Pooja, Rajeev Pratap Singh Agricultural Utilization of Biosolids. A Review on the Potential Effects on Soil and Plants Growth. Journal of Waste Management, ScienceDirect. 2017;64:117–132. doi: 10.1016/j.wasman.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 62.Siqueira Jr Sergio, Mariana Antonio Desouza Perreira, Morishigue Marjuli, et al. Circular Economy in the Biosolid Management by Nexus Approach: A View to Enhancing Safe Nutrient Recycling-Pathogens, Metals, and Emerging Organic Pollutants of Concern. Sustainability. 2022;14 doi: 10.3390/sul142214693. [DOI] [Google Scholar]
- 63.Apec S.O.M. APEC SOM Committee on Economic and Technical Co-operation; 2022. Understanding the Bio-Circular Green (BCG) Economic Model. August 2022. [Google Scholar]
- 64.Stavridou Evagelica, Giannakis Ioannis, Karamichiali Ioannis, Kamou Natalie N., George Lagiolis, et al. Biosolid-amended Soil Enhances Defense Responses in Tomato Based on Metagenomic Profile and Expression of Pathogenesis-related Genes. Plants. 2021;2789(10) doi: 10.3390/plants10122789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banks M.K., Schwab A.P., Cofield N., Alleman J.E., Switzenbaum M., Shalabi J., Williams P. Biosolid-amended Soils: Part I: Effects of Biosolids Application on Soil Quality and Ecotoxicity. Water Environ. Res. 2006;78(11):2217. doi: 10.2175/106143005x86637. [DOI] [PubMed] [Google Scholar]
- 66.USEPA . U.S. Environmental Protection Agency, Office of Water Management; Washington D.C: 2000. Biosolid Technology Factsheet: Land Application of Biosolids. EPA 832-F-0064. [Google Scholar]
- 67.Tsadilas Christos D., Mitsios I.K., Golia Evangelia E. Influence of Biosolid Application on Some Soils' Physical Properties. Communication in Soil Science and Plant Analysis. 2005;36(4–6):709–716. doi: 10.1081/CSS-200043350. [DOI] [Google Scholar]
- 68.Li Simin, Zhu Li, Jin Li, Xin Ke, et al. Influence of Long-term Biosolid Applications on Communities of Soil Fauna and their Metal Accumulation: A Field Study. Environ. Pollut. 2020;260 doi: 10.1016/j.envpol.2020.114017. [DOI] [PubMed] [Google Scholar]
- 69.Oun Amira, Kumar Arun, Harrington Timothy, Angelakis Andreas, Xagoraraki Irene. Effects of Biosolids and Manure Application on Microbial Water Quality in Rural Areas in the. U.S. Water. 2014;6(12):3701–3723. doi: 10.3390/w6123701. [DOI] [Google Scholar]
- 70.Wang Lawrence, Shammar Nazih, Evanylo Gregory. Biosolid Engineering and Management, Handbook of Environmental Engineering and Management. Springer; 2009. Engineering and Management of Agricultural Land Application of Biosolid. [Google Scholar]
- 71.Brune David, Arnold Ken, Dunn John, Carpenter Jerry. University of Missouri; USA: 2022. Best Management Practices for Biosolids Land Application: A Review. [Google Scholar]
- 72.Gentili Rodolfo, Ambrosini Roberto, Montagnani Chiara, Carroni Sarah, Cittero Sandra. Effect of Soil pH on the Growth, Reproductive Investment, and Pollen Allergenicity of Ambrosia Artemisifolia L. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.01335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Torri Silvana, Rodrigo Studart, Renella Giancarlo, Vadecantos Alejandro, Perelomov Leonid. Applied and Environmental Soil Science; Hindawi: 2015. Biosolid Soil Application: Agronomic and Environmental Implication 2014. [DOI] [Google Scholar]
- 74.Newton Jim. United States Environmental Protection Agency, Office of Research and Development; Washington D.C.: 2020. Land Application of Biosolid and Domestic Septage. Process Design Manual. [Google Scholar]
- 75.Chambers B.J., Nicholson Fiona, Aitken M., et al. Benefits of Biosolids to Soil Quality and Fertility. Water Environ. J. 2007;17(3):162–167. https://10.1111/j.1747-6593.2003.tb004555 [Google Scholar]
- 76.Virginia Jin L., Kenneth Potter N., Mari-Vaughn Johnson V., Daren Harmel R., Jeffrey Arnold G. Applied and Environmental Soil Science; 2015. Surface-appllied Biosolids Enhance Soil Organic Carbon and Nitrogen Stocks but Have Contrasting Effect on Soil Physical Quality. Biosolid Soil Application: Agronomic and Environmental Implication 2014 Special Issue. 2015. [DOI] [Google Scholar]
- 77.Mpca . Minnesota Pollution Control Agency; 2019. Animal Feedlots Minnesota Rules.https://www.pca.state.mn.us (Chapter 7020) (online) Available at: [Google Scholar]
- 78.NRCS Nutrient Management, Code 590 (online) Conservation Practice Standard. Naional Resources Conservation Service. 2022 https://www.nrcs.usda.gov/sites/default/files/2022-09/NRCS_590_standard/ Available at: [Google Scholar]
- 79.Nikolaidis N.P., Demetropoulou L., Saru M.L. ResearchGate; 2012. Land Application of Biosolids: Benefits, Risks, and Costs. International Water Association, Water Purification and Reuse, WWPR12. [Google Scholar]
- 80.Coors Anja, Edwards Mark, Lorenz Pascale, et al. Biosolids Applied to Agricultural Land: Influence on Structural and Functional Endpoints of Soil Fauna on a Short-and Long-term Scale. J. Sci. Teach. Educ. 2016;562:312–326. doi: 10.1016/j.scitotenv.2016.03.226. [DOI] [PubMed] [Google Scholar]
- 81.Margui E., Iglesias Monica, Camps Francesc, et al. Long-term Use of Biosolids and Organic Fertilizers in Agricultural Soils: Potential Toxic Element Occurrence and Mobility. Environ. Sci. Pollut. Control Ser. 2016;23(5) doi: 10.1007/s11356-015-5618-9. [DOI] [PubMed] [Google Scholar]
- 82.Nunes Nuno, Ragonezi Carla, Gouveia Carla, Pinheiro de Cavalho Miguel. Review of Sewage Sludge as a Soil Amendment in Relation to Current International Guidelines: A Heavy Metal Perspective. Sustainability. 2021;13(4):2317. doi: 10.3390/su13042317. [DOI] [Google Scholar]
- 83.Mohapatra D.P., Cledon M., Brar S.K., Surampalli R.Y. Application of Wastewater and Biosolids in Soils: Occurrence and Fate of Emerging Contaminants. Water Air Soil Pollut. 2016;227:77. doi: 10.1007/s11270-016-2768-4. [DOI] [Google Scholar]
- 84.Kumar Ravinder, Vuppaladadiyam Arun K., Antunes Elsa, et al. Emerging Contaminants in Biosolids: Presence, Fate, and Analytical Techniques. Emerging Contam. 2022;8:162e194. 2022. keaipublishing.com/cn/journals/emerging-contaminants. [Google Scholar]
- 85.Gworek Barbara, Kijenska Marta, Wrzosek Justyna, Graniewska Magdalena. Pharmaceuticals in the Soil and Plant Environment: A Review. Water Air Soil Pollut. 2021;232(145) [Google Scholar]
- 86.Tang Kuok H.D., Hadibarata Tony. Microplastics Removal through Water Treatment Plants: Its Feasibility, Efficiency, Future Prospects and Enhancements by Proper Waste Management – A Review. Environmental Challenges. 2021;5(2021) [Google Scholar]
- 87.Amir Hossein, Minyangabe Hamidianab. A Review on the Characteristics of Microplastics in Wastewater Treatment Plants: A Source for Toxic Chemicals. Journal of (Leaner Production 2021. 2021;(295) [Google Scholar]
- 88.Zhiqiang, et al. Are Rural and Small Community Aerated Wastewater Stabilization Ponds a Neglected Source of Microplastic Pollution? Water 2021. 2021;13:2833. [Google Scholar]
- 89.Pomati F., Orlandi C., Clerici M., Luciani F., Zucialo E. Effects and Interaction in an Environmentally Relevant Mixture of Pharmaceuticals. Toxicol. Sci. 2008;102:129–137. doi: 10.1093/toxsci/kfm291. [DOI] [PubMed] [Google Scholar]
- 90.Farre M., Perez S., Gonclaves C., Alpendurada M., Barcelo D. Green Analytical Chemistry in the Determination of Organic Pollutants in the Aquatic Environment. Trends Anal. Chem. 2010;29:1347–1362. [Google Scholar]
- 91.Oaks J.L., Gilbert M., Virani M.Z., Watson R.T., Meteyer C.U., Rideout B.A., et al. Diclofenac Residues as the Cause of Vulture Population Decline in Pakistan. Nature. 2004;427:630–633. doi: 10.1038/nature02317. [DOI] [PubMed] [Google Scholar]
- 92.Garri J., Vollat B., Dunis K., Pery A., Junker T., Ramu M., Fink G., Ternes T.A. Effects of the Parasiticide Ivermectin on Cladoceran Daphnia Magna and the Green Algea Pseudo-Kirchneriella Subcapitata. Chemisphere. 2007;69:903–910. doi: 10.1016/j.chemosphere.2007.05.070. [DOI] [PubMed] [Google Scholar]
- 93.Lange R., Hutchinson T.H., Croudace C.P., Siegmund F., Schweinfurth H., Hampe P., et al. Effects of the Synthetic Estrogen 17-α-ethinylestradiol on the Life Cycle Fathead Minow (Pinephales promelas) Environ. Toxicol. Chem. 2001;20:1216–1227. doi: 10.1897/1551-5028(2001)020<1216:eotsee>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 94.Wuana Raymond A., Okieimen Felix E. vol. 402647. International Scholarly Research Network; 2011. pp. 1–20. (Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks, and Best Available Strategies for Remediation). [DOI] [Google Scholar]
- 95.Azevedo Maria L., Ferracciu Luis R., Guimaraes Luiz R. Biosolids and Heavy Metals in Soils: A Review. Sci. Agric. 2003;60(4) doi: 10.1590/s0103-90162003000400029. [DOI] [Google Scholar]
- 96.Agoro Mojeed, Adeniji Abiodun, Martins Adefisoye, Okoh Omobola. Heavy Metals in Wastewater and Sewage Sludge from Selected Municipal Treatment Plants in Eastern Cape Province, South Africa. Water. MDPI. 2020;12(10):2746. doi: 10.3390/w12102746. [DOI] [Google Scholar]
- 97.Shasheen S.M., Shams M.S., Elbehiry F.A., Ibrahim S.M. Applied and Environmental Soil Science; Hindawi: 2012. Influence of Stabilized Biosolid Application on Availability of Phosphorus, Copper, and Zinc. 2012. [DOI] [Google Scholar]
- 98.Ammar Emna, Morin Loic, Sghir Abdelghani. Environmental, Economic, and Ethical Assessment of the Treated Wastewater and Sewage Sludge Valorization in Agriculture. Handb. Environ. Chem. 2020 doi: 10.1007/698_2020_606. [DOI] [Google Scholar]
- 99.Xu Decong, Shen Zhangjun, Dou Chaugming, Dou Zhiyong, Yang Li, Gao Yi, Sun Qingye. Effects of Soil Properties on Heavy Metals Bioavailability and Accumulation in Crops Grains under Different Farmland Use Patterns. Sci. Rep. 2022;12:9271. doi: 10.1038/s41598-022-13140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.USEPA . The U.S. Environmental Protection Agency, Office of Research and Development, National Risk Management Research Laboratory, Centre for Environmental Research Information. EPA/625/R-92-013; Cincinnati, OH: 2022. Our Current Understanding of the Human Health and Environmental Risks of PFAs. [Google Scholar]
- 101.Dhore Raveena, Murthy Ganti. Per-and Poly-fluoroalkyl Substances Production, Applications, and Environmental Impacts. Bio-resources Technology, ScienceDirect. 2021;341 doi: 10.1016/j.biortech.2021.125808. [DOI] [PubMed] [Google Scholar]
- 102.Gluge Juliane, Scheringer Martin, Cousins Ian, et al. An Overview of the Uses of Per-and Poly-fluoroalkyl Substances. Environmental Science Processes Impact. 2020;22:2345–2373. doi: 10.1039/DOEMOO29IG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fda Per- and Poly-fluoroalkyl Substances (PFAs) in Cosmetics. United States Food and Drug Administration. 10903, New Hampshire Avenue, Silver Spring, MD. 2022 20993-0002. [Google Scholar]
- 104.Kim Lazcano, Choi T.Y., Mashtare M.L., Lee L.S. Characterizing and Comparing Per-and Poly-fluoroalkyl Substances in Commercially Availability Biosolids and Organic Non-biosolid-based Products. Environ. Sci. Technol. 2020;39(11):3946–3956. doi: 10.1021/acs.est.9b07281. [DOI] [PubMed] [Google Scholar]
- 105.Blaine A.C., Rich C.D., Hundal L.S., Lau C., Mills M.A., Harris K.M., et al. Uptake of Perfluoroalkyl Acids into Edible Crops via Land Applied Biosolids: Field and Greenhouse Studies. Environ. Sci. Technol. 2013;47(24):14062–14069. doi: 10.1021/es403094q. [DOI] [PubMed] [Google Scholar]
- 106.Damien Terrence Moodie . Environmental Science Department, RMIT University; 2021. Fate, Behaviour, and Ecological Impacts of Biosolid-Derived Per-And Poly-Fluoroalkyl Substances [thesis] [Google Scholar]
- 107.Langebach Blake, Wilson Mark. Per-and Poly-fluoroalkyl Substances (PFAs): Significance and Considerations within Regulatory Framework of the United States. Int. J. Environ. Res. Publ. Health. 2021;18(21) doi: 10.3390/ijerph182111142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sibel Barisci, Suri Rominder. Electro-oxidation of Short- and Long-chain perfluoroalkyl Substances (PFASs) Under Different Process Conditions. Journal of Environmental Chemical Engineering, ScienceDirect. 2021;9(4) doi: 10.1016/j.jece.2021.105323. [DOI] [Google Scholar]
- 109.Sharifan Hamidreza, Bagheri Majid, Wang Dan, et al. Fate and Transport of Per-and Poly-fluoroalkyl Substances (PFAs) in the Vadoze Zone. Sci. Total Environ. 2021;771(10) doi: 10.1016/j.scitotenv.2021.145427. [DOI] [PubMed] [Google Scholar]
- 110.Chen Hong, Zhang Chan, Han Jianbo, Yu Yixuan, Zhang Peng. PFOS and PFOA in Influents, Effluents, and Biosolids of Chinese Wastewater Treatment Plants and Effluent-receiving Marine Environments. Environmental Pollution, ScienceDirect. 2012;170:26–31. doi: 10.1016/j.envpol.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 111.Li P., Oyang X., Xie X., Li Z., Yang H., Xi J., Guo Y., Tian X., Liu B., Li J., Xiao Z. Phototoxicity Induced by Perfluorooctanoic Acid and Perfluorooctane Sulfonate via Metabolics. J. Hazard Mater. 2020;389 doi: 10.1016/j.jhazmat.2019.121852. [DOI] [PubMed] [Google Scholar]
- 112.CDC . Centre for Disease Control and Prevention. The U.S. Department of Health and Human Services; 2017. Perfluorooactanoic Acid. National Biomonitoring Program. [Google Scholar]
- 113.Oliveira F.C., Mattiazzo M.E. Heavy Metals in an Oxisol Treated with Sewage Sludge and in Sugarcane Plants. Scientia Agricola, ResearchGate. 2001;58(3):581–593. [Google Scholar]
- 114.AnYan, Wang Yamn, Tan Swee Ngin, et al. Phytoremediation: A Promising Approach for Revegetation of Heavy Metal Polluted Land. Front. Plant Sci. 2020;11:369. doi: 10.3399/fpls.2020.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanyahu Bieby V., Abdullah Siti R.S. Hassan Basri, Mushrifah Idris, Nurina Anuar, and Muhammad Mukhlisin. A Review on Heavy Metals (As, Pb, and Hg) Uptake by Plants through Phytoremediation. International Journal of Chemical Engineering. 2011 [Google Scholar]
- 116.Uchimiya Minori, Bannon Desmond, Nakanishi Hiromi, Murray McBride, Williams Marc, Yoshihana Toshihiro. Chemical Speciation, Plant Uptake, and Toxicity of Heavy Metals in Agricultural Soils. J. Agric. Food Chem. 2020;68(46):12856–12869. doi: 10.1021/acs.jafc.0c00183. [DOI] [PubMed] [Google Scholar]
- 117.Mitra Saikat, Chakraborty Arka J., Tareq Abu M., et al. Impact of Heavy Metals on Environmental and Human Health: Novel Therapeutic Insights to Counter the Toxicity. Journal of King Saud University-Science, ScienceDirect. 2022;34(3) [Google Scholar]
- 118.Balali-mood Naseri, Tahergorabi, et al. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Frontier Pharmacology. 2021;12 doi: 10.3389/fphar.2021.643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jaishanker Monisha, Tseten Tenzin, Anbalagan Naresh, et al. Toxicity Mechanism and Health Effects of Some Heavy Metals. Interdiscipl. Toxicol. 2015;7(2):60–72. doi: 10.2478/intox-2014-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Hailin. Division of Agricultural Sciences and Natural Resources, Oklahoma State University Extension, Agricultural Hall, Oklahoma State University; Stillwater, OK 74078: 2017. Using Biosolids as a Plant Nutrient Source. [Google Scholar]
- 121.Jorge Antonio Silva-Leal, Perez-Vidal Andrea, Torres-Lozada Patricia. Effects of Biosolids on the Nitrogen and Phosphorus Content of the Soil Used for Sugar Plantation. National Library of Medicine, Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Markunas Yulia, Bostan Vadim, Larsen Andrew, Payne Michael, McCarthy Lynda. Applied and Environmental Soil Sciences; Hindawi: 2016. Vertical Phosphorus Migration in a Biosolid-Amended Sandy-loam Soil in a Laboratory Setting: Concentration in Soils and Leachates. [DOI] [Google Scholar]
- 123.Murray McBride. Long-term Biosolid Application on Land: Beneficial Recycling of Nutrients or Eutrophication of Agroecosystem? Soil System. 2022;2022(6):9. doi: 10.3390/soilsystems6010009. [DOI] [Google Scholar]
- 124.Adul-Wahab Mossa, Elizabeth H., Bailey, Usman Abida, Young Scott D., Crout Neil M.J. School of Biosciences, University of Nottingham; Sutton, Bonington Campus, Leicester, LE12 SRD, UK: 2020. The Impact of Long-Term Biosolid Application (>100 Years) on Soil Metal Dynamics. [Google Scholar]
- 125.Venghaus Daniel, Berjenbruch Mathias. Technical Transaction I, Environmental Engineering; 2017. Microplastics in Urban Water Management. 2017. [DOI] [Google Scholar]
- 126.Halden Rolf U., Lindeman Avery E., Aiello Allison E., et al. The Florence Statement on Triclosan and Triclocarban. Environmental Health Perspectives. National Library of Medicine. 2017;125(6) doi: 10.1289/EHP1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bashir Saidu M., Kimiko Sam, Mak Chu-Wa, James Kar-Hei Fang, Goncalves David. Personal Care and Cosmetic Products as a Potential Source of Environmental Contamination by Microplastics in a Densely Populated Asian City. Front. Mar. Sci. 2021;8 doi: 10.3389/fmars.2021.683482. [DOI] [Google Scholar]
- 128.Praveena Sarva M., Shaiffudin Siti N.M., Akizuki Shinichi. Exploration of Microplastics from Personal Care and Cosmetic Products and its Estimated Emissions to Marine Environment: An Evidence from Malaysia. Mar. Pollut. Bull. 2018;136:135–140. doi: 10.1016/j.marpolbul.2018.09.012. [DOI] [PubMed] [Google Scholar]
- 129.Jak Lara D., Exposito Nora, Rovira Joaquin, Macher Karim, Valero Fernando, Sierra Jordi. Screening of MIS in Water and Sludge Lines of a Drinking Water Treatment Plant in Catalonia, Spain. Water Res. 2022;2022(225) doi: 10.1016/j.watres.2022.119185. [DOI] [PubMed] [Google Scholar]
- 130.Cleanpng Microbeads Denitifricio Microplastic Esfoliazione per la Cura Personale. 2018. https://www.it.cleanpng.com/png.p810bz Available at:
- 131.Campanale Claudia, Marssarelli Carmine, Savion Illaria, et al. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. International Journal of Environmental Research and Public Health, National Library of Medicine. 2020;17(4):1212. doi: 10.3390/ijerph17041212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pironti Concentta, Ricciadi Maria, Motta Oriana, et al. Microplastic in the Environment: Intake through Food Web, Human Exposure and Toxicological Effects. Toxics, MDPI. 2021;9(9):224. doi: 10.3390/toxics9090.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mintenig S.M., Loder M.G.J., Pimpke S., Gerdts G. Low Members of the Microplastics Detected in Drinking Water from Groundwater Sources. J. Sci. Teach. Educ. 2019;648:631–635. doi: 10.1016/j.scitotenv.2018.08.178. [DOI] [PubMed] [Google Scholar]
- 134.Garza Aaron Z., Park Sharon B., Kocz Remek. Drug Elimination: StatPearls (online). National Library of Medicine. 2022 https://www.ncbi.nlm.nih.gov/books/NBK547662 Available at: [Google Scholar]
- 135.Blanco Victoria, Hernandora Carolina, Scibona Paula, Belloso Waldo, et al. Acute Kidney Injury and Pharmacokinetic Changes and, its Impact on Drug Prescription. Healthcare, MDPI. 2019;7(1):10. doi: 10.3390/healthcare7010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Carter Laura J., Harris Eleanor, Williams Mike, Ryan Jim J., Kookana Rai S., Alistair B.A., Boxall Fate and Uptake of Pharmaceuticals in Soil-Plant Systems. J. Agric. Food Chem. 2014;64:816–825. doi: 10.1021/jf404282y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Monteiro Sara C., Boxall Alistair B.A. Factors Affecting the Degradation of Pharmaceuticals in Agricultural Soils. Environ. Toxicol. Chem. 2009;28(2):2546–2554. doi: 10.1897/08-657.1. [DOI] [PubMed] [Google Scholar]
- 138.Knight Emma R., Carter L.J., McLaughlin M.J. Bioaccumulation, Uptake, and Toxicity of Carbamazepine in Soil-Plant Systems. Environ. Toxicol. Chem. 2018;37(4):1122–1130. doi: 10.1002/etc.4053. [DOI] [PubMed] [Google Scholar]
- 139.Piotrowicz-Cieslak A., Adomas B., Nalecz-Jawecki Michalczyk Phototoxicity of Sulfamethazine Soil Pollution to Six Legume Plant Species. J. Toxicol. Environ. Health, Part A. 2010;73(18) doi: 10.1080/152873794.2010.492006. [DOI] [PubMed] [Google Scholar]
- 140.MedlinePlus Carbamazepine (online). National Library of Medicine. 2022 https://www.medlineplus.gov Available at: [Google Scholar]
- 141.Mercer Mellisa A. Merck Manual, Maryland College of Veterinary Medicine; Virginia: 2022. Sulfonamides and Sulfonamide Combination Use in Animals. [Google Scholar]
- 142.Lozano Nuria, Rice Clifford P., Ramirez Mark, Alba Torrents The Fate of Triclocarban, Triclosan, and Methyltriclosan during Wastewater and Biosolid Treatment Processes. Water Resources, National Library of Medicine. 2013;47(13):4519–4527. doi: 10.1016/j.waters.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 143.Cha Jongmun, Cupples Alison M. Detection of the Antimicrobials Triclocarban and Triclosan in Agricultural Soils Following Land Application of Municipal Biosolid. Water Res. 2009;43:2522–2530. doi: 10.1016/j.watres.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 144.Yu Ban, Zheng Guodi, Wang Xuedong, Wang Min, Chen Tongbin. Biodegradation of Triclosan and Triclocarban in Sewage Sludge during Composting Under Three Ventilation Strategies. Front. Environ. Sci. Eng. 2019;13(41) doi: 10.1007/s11783-019-1125-4. [DOI] [Google Scholar]
- 145.Ying Guang-Guo, Xiang-Yang Yu, Rai Kookana. Biological Degradation of Triclocarban and Triclosan in a Soil under Aerobic and Anaerobic Conditions and Comparison with Environmental Fate Modeling. Environ. Pollut. 2008;150(3):300–305. doi: 10.1016/j.envpol.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 146.Singh Dhillon Gurpreet, Kaur Surinder, Pulicharia Rama, et al. Triclosan: Current Status, Occurrence, Environmental Risks, and Bioaccumulation Potential. International Journal of Environmental Resources and Public Health, National Library of Medicine. 2015;12(5):5657–5684. doi: 10.3390/ijerph120505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Scott Matthew J., Jones Malcolm N. The Biodegradation of Surfactants in the Environment. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2000;1508(1–2):235–251. doi: 10.1016/50304-4157(00)00013-7. [DOI] [PubMed] [Google Scholar]
- 148.Ying Guang-Guo. Fate, Behaviour, and Effects of Surfactants and their Degradation Products in the Environment. Environ. Int. 2006;32(2006):417–431. doi: 10.1016/j.envint.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Qing, Mei Weiping, Jiang Longfei, Zheng Qian, Luo Chunling, Zhang Gan. Environmental Fate and Effects of Organo-phosphate Flame Retardants in the Soil-Plant System. Soil Ecology Letters. 2021;3(3):178–188. doi: 10.1007/s42832-021-0084-4. 2021. [DOI] [Google Scholar]
- 150.NIH . National Institute of Environmental Health Sciences; 2023. Endocrine Disruptors (Online)https://www.niehs.nih.gov/health/topics/agents/endocrine/index.cfm Available at: [Google Scholar]
- 151.Kumar Manoj, Sarma Devojit Kumar, Shubham Swasti, Kumawat Manoj, Verma Vinod, Prakash Anil, Tiwari Rajnarayan. Environmental Endocrine-Disrupting Chemical Exposure: Role in Non-Communicable Diseases. Frontier Public Health, Sectional Environmental Health, and Exposome. 2020;8 doi: 10.3389/fpubh.2020.553850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.FAO Status of the World’s Soil Resources. Food and Agricultural Organization of the United Nations Main Report. 2015 https://www.fao.org Available at: [Google Scholar]
- 153.Hudcova Hana, Jan Vymazal, Rozkosny Milos. Present Restrictions of Sewage Sludge Application in Agriculture within the European Union. Soil Water Res. 2019;14(2):104–120. [Google Scholar]
- 154.Tsiarta Christina, Watson Steven, Hudson Joe. Final Implementation Report for Directive 86/278/EEC on Sewage. Report for Director General on the Environment, European Commission. The European Commission. 2018 ENV.C.2/FRA/2013/0023. [Google Scholar]
- 155.EUREAU . European Federation of National Associations of Water Resources; 2013. Sewage Sludge Directive. 86/278/EEC- Stakeholder Questionnaire: EUREAU Responses. 2013. [Google Scholar]
- 156.The European Commission Legislation of Sewage Sludge: Directive 86/278/EEC on Sewage Sludge. European Commission Director General on the Environment. 2006 https://www.europe.eu.int/comm/environment/waste/index.htm Available at: [Google Scholar]
- 157.MACROTRENDS . 2023. World Population between 1950 and 2100. Macro-Trends LLC.https://www.macrotrends.net/countries/WLD/world/population Available at: [Google Scholar]
- 158.USEPA Biosolid Laws and Regulations. United States Environmental Protection Agency. 2002 https://www.epa.gov/biosolids/biosolids-laws-and-regulations Available at: [Google Scholar]
- 159.USEPA . Office of Water Management, United States Environmental Protection Agency; 1994. A Plain English Guide to the EPA Part 503 Biosolid Rule. EPA/832/R-93/003. [Google Scholar]
- 160.Evanylo G.K. Virginia Co-operation Extension Publication. Department of Crop and Soil Environmental Sciences; Virginia Tech: 2009. Agricultural Land Application of Biosolids in Virginia: the Regulations; pp. 302–452. [Google Scholar]
- 161.Gerba C.P., Pepper I.L., Whitehead L.F. A Risk Assessment of Emerging Pathogens of Concern in the Land Application of Biosolids. Water Sci. Technol. 2002;46(1):225–230. [PubMed] [Google Scholar]
- 162.Brobst Robert. EPA Region VIII, 18 Str.; Denver Colorado 80202: 2001. Biosolid Regulations. Biosolid Management Handbook. [Google Scholar]
- 163.Iranpour R., Cox H.H.J., Kearney R.J., Clerk J.H., Prince A.B., Daigger G.T. Regulations for Biosolid Land Application in U.S. and European Union. Journal of Residual Science and Technology. 2014;1(4) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.