Abstract
The planula larvae of the sea anemone Aiptasia have so far not been reported to complete their life cycle by undergoing metamorphosis into adult forms. This has been a major obstacle in their use as a model for coral–dinoflagellate endosymbiosis. Here, we show that Aiptasia larvae actively feed on crustacean nauplii, displaying a preference for live prey. This feeding behavior relies on functional stinging cells, indicative of complex neuronal control. Regular feeding leads to significant size increase, morphological changes, and efficient settlement around 14 d postfertilization. Surprisingly, the presence of dinoflagellate endosymbionts does not affect larval growth or settlement dynamics but is crucial for sexual reproduction. Our findings finally close Aiptasia’s life cycle and highlight the functional nature of its larvae, as in Haeckel’s Gastrea postulate, yet reveal its active carnivory, thus contributing to our understanding of early metazoan evolution.
Keywords: endosymbiosis, gastrulation, settlement, nutrition, basal metazoan evolution
Coral reefs are biodiversity hotspots threatened by global climate change, including by coral bleaching, a loss of the symbiotic relationship that corals form with algae during their larval/juvenile stage (1). Anthozoan larval growth and settlement as a prerequisite for symbiont uptake has so far been elusive due to the difficulty of establishing settlement under laboratory conditions. While triggers for cnidarian larval settlement are chemical or other environmental cues, we focused on the dual nutrition sources of diet and algal symbionts in the sea anemone Aiptasia (Exaiptasia diaphana), a model for cnidarian endosymbiosis (Fig. 1A) (2, 3). Similar to coral and sea anemone larvae (4), Aiptasia planulae readily ingest animal homogenates or symbiotic algal cells, but these have not resulted in growth or settlement (5). Here, we demonstrate that Aiptasia larvae actively feed on live nauplii of the copepod Tisbe, leading to substantial growth, settlement, and metamorphosis. Endosymbionts are not necessary for these processes, but crucial for gametogenesis. Our findings highlight the predatory nature of these late gastrulae/early planulae, shedding light on the early evolution of (eu)metazoans and providing a breakthrough in the Aiptasia model system.
Fig. 1.
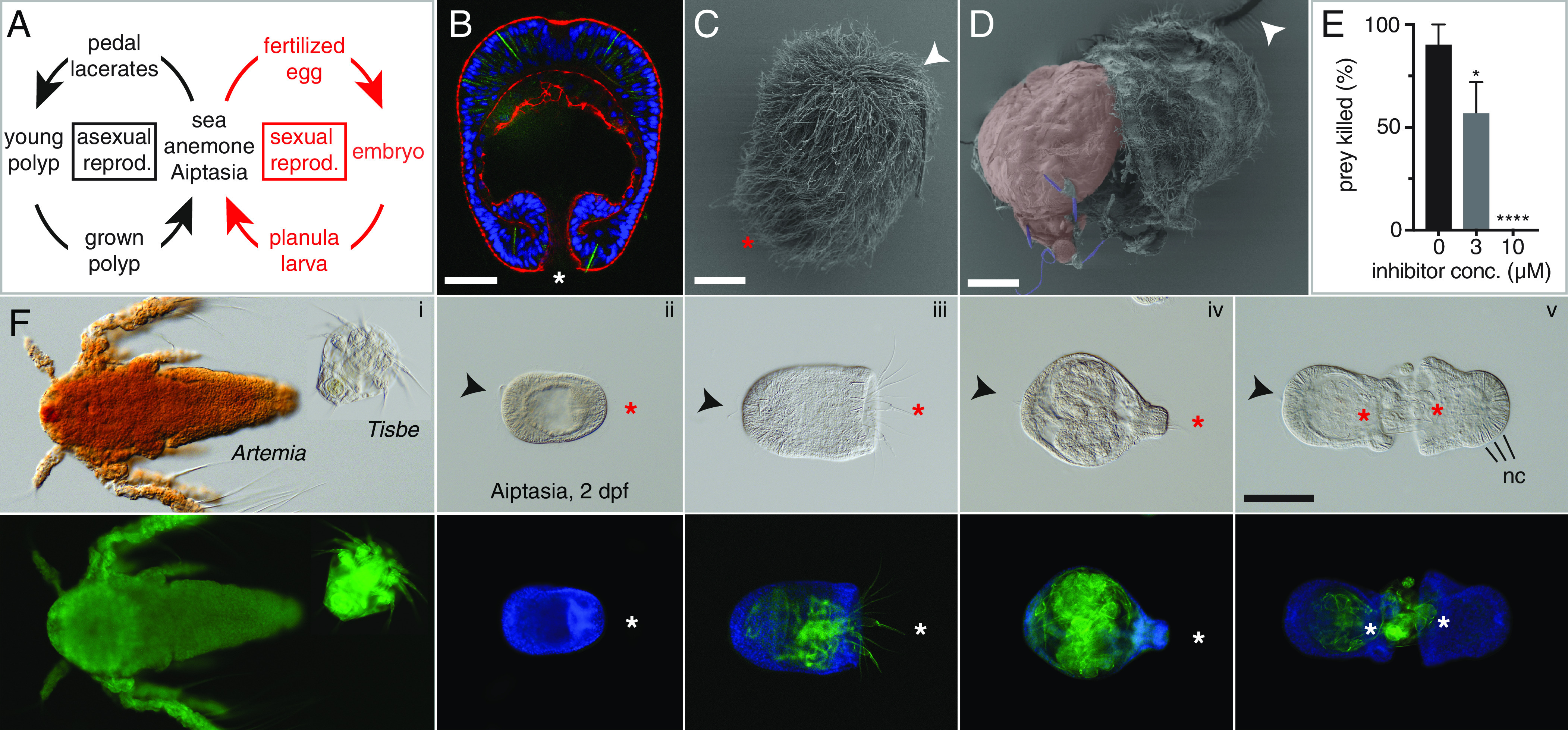
Carnivorous larvae of Aiptasia. (A) Life cycle of Aiptasia. (B) Representative image of a late gastrula/early planula 2 dpf (also in F). Blue, Hoechst; red, phalloidin; green, autofluorescence of nematocysts. The blastopore corresponds to the mouth. (C and D) Scanning electron micrographs of planulae (3 dpf) with cilia (C); ingestion of a Tisbe nauplius (red) penetrated by nematocysts (purple) (D). (E) Impaired nematocyst activity by a small molecule inhibitor (fraction of killed prey is mean ± SD from three independent experiments, Student’s t-test). (F) Aiptasia larvae 2 dpf and prey under DIC (Upper row) and fluorescence (Lower row) microscopy. Blue, Hoechst; green, autofluorescence of chitin. Tisbe but not Artemia nauplii (i) can be ingested by young planulae (ii). Tisbe nauplii are predated by one (iii and iv) or multiple larvae (v) and ingested (iii and iv) [Scale bars, 25 µm (B–D), 100 μm (F)]. B–D and F: Asterisks, mouth; arrows, aboral apical tuft; nc, nematocysts.
Results
We attempted feeding Aiptasia planulae with the copepod Tisbe (6). Surprisingly, we found that even planulae at the gastrula stage, 2 d postfertilization (dpf) (Fig. 1B), were able to hunt Tisbe nauplii (Fig. 1 C, D, and F and Video S1), caught by the planulae’s nematocysts (Fig. 1 B and D–F) and ingested into the gastric cavity (Fig. 1 D and F and Video S2). Larvae fed with Tisbe nauplii grew continuously in size, followed by eventual metamorphosis and settlement (Fig. 2 A–E). In contrast, unfed larvae developed as described (5), i.e., size and endoderm thickness shrank over time (Fig. 2 B–D). Live prey were preferred to heat-killed nauplii (Fig. 2D), and after 8 d of daily feeding, planulae also hunted and ingested Artemia. Inhibition of nematocyst discharge by the [2.2] paracyclophane compound 1 (7) prevented prey capture and led to increased prey survival (Fig. 1E).
Fig. 2.
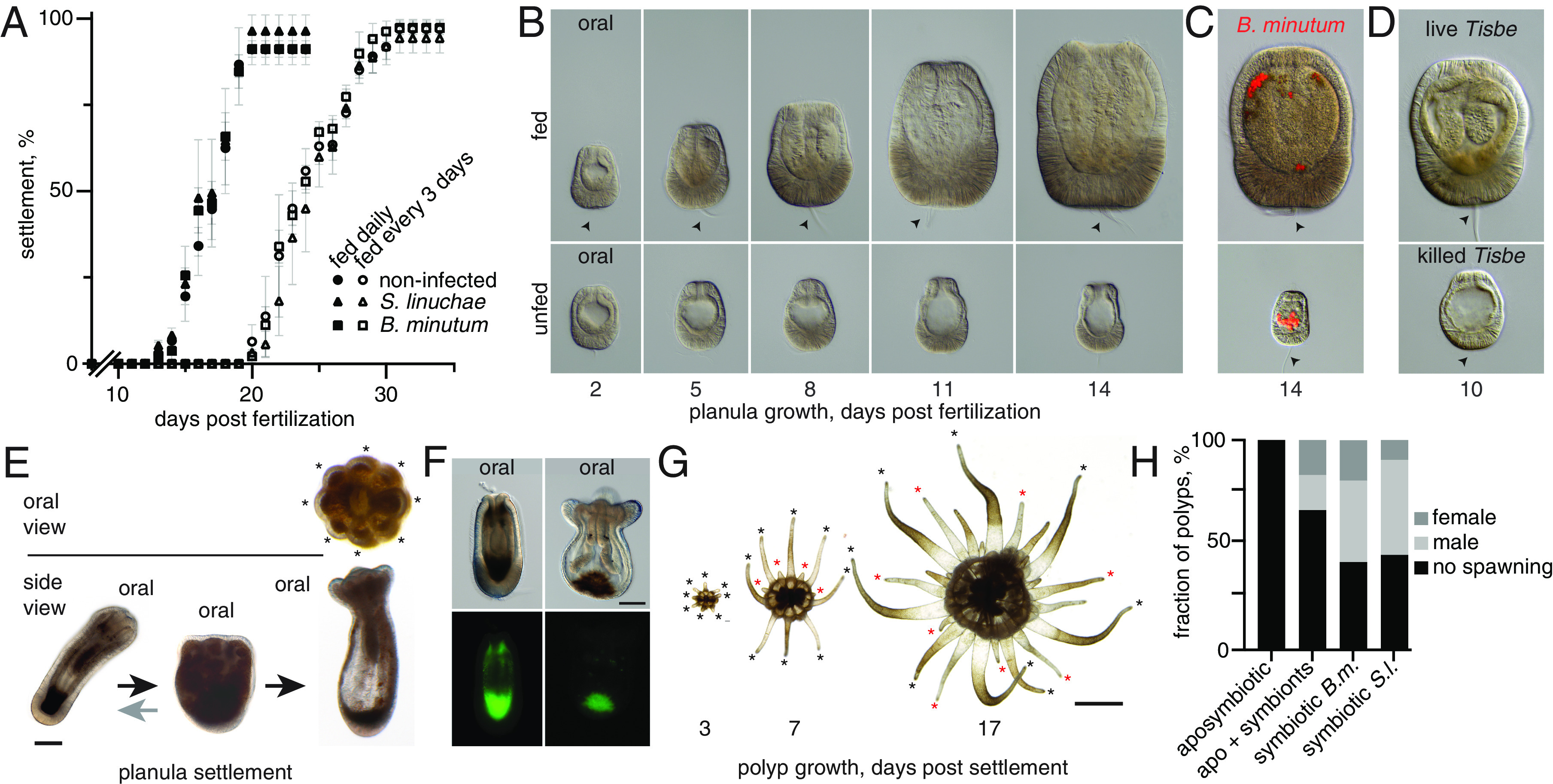
Aiptasia larval and polyp development. (A) Settlement of fed planulae hosting either Symbiodiniaceae symbiont strain (mean ± SD of four replicate experiments with 72 larvae per condition, Student’s t-test). No unfed larvae underwent metamorphosis or settlement in any tested condition. (B–D) Planula growth in different feeding regimes. (B) Growth with (Upper row) or without (Lower row) daily feeding over 14 dpf. (C) Fed with addition of symbiont strain Breviolum minutum. (D) Fed with live (Upper row) or heat-killed (Lower row) Tisbe after 10 dpf. (E) Planula settlement and metamorphosis. Note cycling between elongated and laterally flattened forms before settlement. Arrows, aboral apical tuft. (F) Autofluorescence in live planulae appears 5–8 dpf and gets brightest around settlement. (G) Tentacle formation in growing polyps 3–17 dps. Black asterisks, symmetric first; red asterisks, asymmetric second tentacle wave (H). Sexual reproduction (spawning) in grown primary polyps without symbionts (“aposymbiotic”), aposymbiotic polyps infected with the Symbiodiniaceae strains (“apo + symbionts”), or polyps from planulae with symbionts (“symbiotic” in A) [Scale bars, 50 µm (B), 100 μm (E and F), and 1,000 µm (G)].
After ~14 d of daily feeding, planulae behavior began to markedly change as a precedent to settlement, exhibiting slower swimming, cycles of lengthening and contraction, and substrate exploration with the apical tuft (Fig. 2E and Video S3); concurrently, autofluorescence appeared at the aboral end (Fig. 2F). Settlement and metamorphosis occurred between 13 and 20 dpf (Fig. 2A), when planulae attached to the substrate at the aboral end, flattened, and displayed eight tentacle primordia (Fig. 2E and Video S3). With feeding every third day, settlement onset was delayed, yet the kinetics and final settlement efficiencies were strikingly similar, suggesting a size tipping point past which settlement was nearly always triggered (Fig. 2A).
We then investigated whether symbionts affected settlement dynamics. Because the parental lines harbor either Symbiodinium linuchae alone (male “CC7”) or in combination with Breviolum minutum (female “F003”), we infected Aiptasia larvae at 2 dpf with either strain under the two feeding regimes (Fig. 2 A and C) (SI Appendix). All larvae displayed indistinguishable settlement dynamics: Only larvae fed with nauplii grew and eventually settled, regardless of symbiotic status (Fig. 2 A–C).
After settlement, Aiptasia primary polyps (F1 generations) developed to reproductive maturity (Fig. 2 E–H) and spawned, giving rise to F2 generations. Most primary polyps displayed radially symmetric tentacle primordia, then bilateral symmetry in the next tentacle wave, before eventual sixfold symmetry (Fig. 2 E and G). Growth patterns were identical in apo- and symbiotic primary polyps when fed at least twice weekly with Artemia nauplii, including asexual reproduction (Fig. 1A) starting 14 d postsettlement (dps). In contrast, sexual reproduction (6 mo postsettlement), relied on symbiotic state: over half of symbiotic polyps hosting either algal strain spawned within two induction cycles (41 of 73), whereas no aposymbiotic polyps spawned (0 of 18). Critically, aposymbiotic polyps infected with symbionts recovered the ability to spawn (4 of 12) (Fig. 2H). Finally, crossing of primary polyps (F2 generation) and back-crossing with parental lines in both combinations produced viable larvae capable of settlement.
Discussion
Our data demonstrate that the predatory gastrula is a crucial step in the metamorphosis and settlement of Aiptasia planula larvae. This finding has significant implications for various fields, including coral–algae symbiosis, embryology, and ecotoxicology. It opens the door to functional genetics and manipulation, allowing the establishment of stable transgenic lines.
Intriguingly, our results reveal that algal symbionts do not appear to affect the growth and settlement of Aiptasia planulae, neither through signaling nor nutritional contribution. The importance of diet outweighs the role of symbiosis during primary polyp growth, development, and early asexual reproduction. However, in adult polyps, the nutritional balance may shift toward the primacy of symbiosis. Our observations indicate a striking reliance on symbiosis for sexual reproduction in Aiptasia, similar to the Hydra/Chlorella symbiosis (2).
The carnivorous nature of Aiptasia larvae is remarkable, as they possess the ability to hunt live food as early as 2 dpf, during the late gastrula/early planula stage. This is in contrast to most scleractinian corals, which develop into planulae after 3 or 4 dpf and settle spontaneously or under the influence of environmental chemical cues (4). The quick development and voracious appetite of Aiptasia are consistent with its lack of lipid-rich yolk in comparison to other cnidarians. This predatory lifestyle likely provides an advantage in heavily human-impacted eutrophic environments where Aiptasia is commonly found.
While symbiosis eventually becomes critical in Aiptasia’s ontogeny, the observed autofluorescence in planulae is consistent with the “beacon” hypothesis of symbiont attraction in juvenile corals (8). However, it is possible that the predatory lifestyle was also an ancestral feature of cnidarian gastrulae. Ancestral anthozoans primarily consisted of taxa with solitary and nonsymbiotic polyps (9), and the yolk-rich embryos of derived cnidarians like hydrozoans might be an adaptation to a benthic lifestyle (10). Thus, although sea anemones are not strictly basal cnidarians, Aiptasia’s predatory, yolk-poor planulae that result from gastrulation by invagination (the ancestral mode) may represent a maintenance of (or return to) deeply conserved traits shared by Anthozoa.
The predatory gastrula of Aiptasia, which is carnivorous rather than the hypothetical filter-feeding gastrula of Haeckel (11), carries significant implications for the evolution of early emerging metazoans. The presence of specialized stinging cells used for prey capture in cnidarians, which is reflected by extrusive organelles in protist eukaryotes (12), ctenophores (13, 14), and planarians (15), suggests that predation played a crucial role in the early origin of metazoans from unicellular eukaryotes, although further analyses into cell types with more taxa are needed. The predatory lifestyle may have been a major driving force for early metazoan evolution and the development of organized nervous systems. Recent evidence points to ctenophores as the sister clade to all metazoans (16). A secondary loss of the predatory life style and extrusive organelles in sponges would support this hypothesis and suggest a shared origin of both toxin-producing cells with extrusive functions and nervous systems between ctenophores, cnidarians, and bilaterians.
Materials and Methods
Aiptasia and axenic Symbiodiniaceae (B. minutum strain SSB01, S. linuchae strain SSA01) were cultured as described (5). Aiptasia planulae were fed with Tisbe nauplii (6) and after sufficient growth also with Artemia. Metamorphosed F1 polyps were induced to spawn after 6 mo. Nematocyst discharge in starved 4 dpf planulae was inhibited for 30 min with “compound 1” [2.2] paracyclophane in DMSO (7). Imaging with epifluorescence, confocal, and scanning electron microscopy followed standard methods. For details, see SI Appendix.
Supplementary Material
Appendix 01 (PDF)
Hunting and feeding of Aiptasia I. Gastrula-like Aiptasia larvae, 2 dpf, hunting on Tisbe nauplii (Interference contrast, real time).
Hunting and feeding of Aiptasia II. Feeding and uptake of a Tisbe nauplius by an Aiptasia larva, 2 dpf (Interference contrast recording of ~60 minutes, time lapse factor 20X).
Settlement of Aiptasia planulae. Fed larvae of Aiptasia, 15 dpf, slow down swimming and elongate before they finally contract and adhere on the substrate to metamorphose into a primary polyp (interference contrast, total recording time ~6 minutes, time lapse factor 2X and real time).
Acknowledgments
This study was supported by German Research Foundation grants to T.W.H. (SFB1324, D.A.C.H) and S.Ö. (SFB1324, Oe416/8-1), an European Research Council Consolidator Grant (724715) to A.G., and by the Centre for Microbiology and Environmental Systems Science, University of Vienna to E.A.H. We thank G. Genikhovich for helpful discussions, the Heidelberg Electron Microscopy Core Facility (C. Funaya, L. Eis) and Nikon Imaging Center (U. Engel, N. Dross) for support, and S. Braese (Karlsruhe Institute of Technology) for nematocyst inhibitor compounds.
Author contributions
I.M. and T.W.H. designed research; I.M. performed experiments; S.R. performed confocal microscopy experiments; I.M., S.Ö., A.G., E.A.H., and T.W.H. analyzed data; and I.M., S.Ö., A.G., E.A.H., and T.W.H. wrote the paper.
Competing interests
The authors declare no competing interest.
Contributor Information
Elizabeth A. Hambleton, Email: elizabeth.hambleton@univie.ac.at.
Thomas W. Holstein, Email: thomas.holstein@cos.uni-heidelberg.de.
Data, Materials, and Software Availability
All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.Cleves P. A., Krediet C. J., Lehnert E. M., Onishi M., Pringle J. R., Insights into coral bleaching under heat stress from analysis of gene expression in a sea anemone model system. Proc. Natl. Acad. Sci. U.S.A. 117, 28906–28917 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davy S. K., Allemand D., Weis V. M., Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 76, 229–261 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baumgarten S., et al. , The genome of Aiptasia, a sea anemone model for coral symbiosis. Proc. Natl. Acad. Sci. U.S.A. 112, 11893–11898 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarz J. A., Krupp D. A., Weis V. M., Late larval development and onset of symbiosis in the scleractinian coral Fungia scutaria. Biol. Bull 196, 70–79 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Bucher M., Wolfowicz I., Voss P. A., Hambleton E. A., Guse A., Development and symbiosis establishment in the cnidarian endosymbiosis model Aiptasia sp. Sci. Rep. 6, 19867 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner B., Bau und lebensgeschichte des polypen von Tripedalia cystophora (Cubozoa, class nov., Carybdeidae) und seine beduetung fur die evolution der cnidaria. Helgolander Wiss. 27, 461–504 (1975). [Google Scholar]
- 7.Hofmann D., et al. , A small molecule screen identifies novel inhibitors of mechanosensory nematocyst discharge in Hydra. Sci. Rep. 11, 20627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aihara Y., et al. , Green fluorescence from cnidarian hosts attracts symbiotic algae. Proc. Natl. Acad. Sci. U.S.A. 116, 2118–2123 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McFadden C. S., et al. , Phylogenomics, origin, and diversification of Anthozoans (Phylum Cnidaria). Syst. Biol. 70, 635–647 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Byrum C. A., Martindale M. Q., “Gastrulation in the Cnidaria and Ctenophora” in Gastrulation: From Cells to Embryo, Stern C. D., Ed. (Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 2004), pp. 33–50. [Google Scholar]
- 11.Haeckel E., Generelle Morphologie der Organismen (G. Reimer, Berlin, Germany, 1866). [Google Scholar]
- 12.Hausmann K., “Extrusive organelles in protists” in International Review of Cytology, Bourne G. H., Danielli J. F., Jeon K. W., Eds. (Academic Press, 1978), vol. 52, pp. 197–276. [DOI] [PubMed] [Google Scholar]
- 13.Townsend J. P., Sweeney A. M., Catecholic compounds in ctenophore colloblast and nerve net proteins suggest a structural role for DOPA-like molecules in an early-diverging animal lineage. Biol. Bull. 236, 55–65 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Sebé-Pedrós A., et al. , Early metazoan cell type diversity and the evolution of multicellular gene regulation. Nat. Ecol. Evol. 2, 1176–1188 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith J. P. S. III, Tyler S., Thomas M. B., Reiger R. M., The morphology of turbellarian rhabdites: Phylogenetic implications. BioScience 33, 55–56 (1983). [Google Scholar]
- 16.Schultz D. T., et al. , Ancient gene linkages support ctenophores as sister to other animals. Nature 618, 110–117 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Hunting and feeding of Aiptasia I. Gastrula-like Aiptasia larvae, 2 dpf, hunting on Tisbe nauplii (Interference contrast, real time).
Hunting and feeding of Aiptasia II. Feeding and uptake of a Tisbe nauplius by an Aiptasia larva, 2 dpf (Interference contrast recording of ~60 minutes, time lapse factor 20X).
Settlement of Aiptasia planulae. Fed larvae of Aiptasia, 15 dpf, slow down swimming and elongate before they finally contract and adhere on the substrate to metamorphose into a primary polyp (interference contrast, total recording time ~6 minutes, time lapse factor 2X and real time).
Data Availability Statement
All study data are included in the article and/or supporting information.


