Abstract
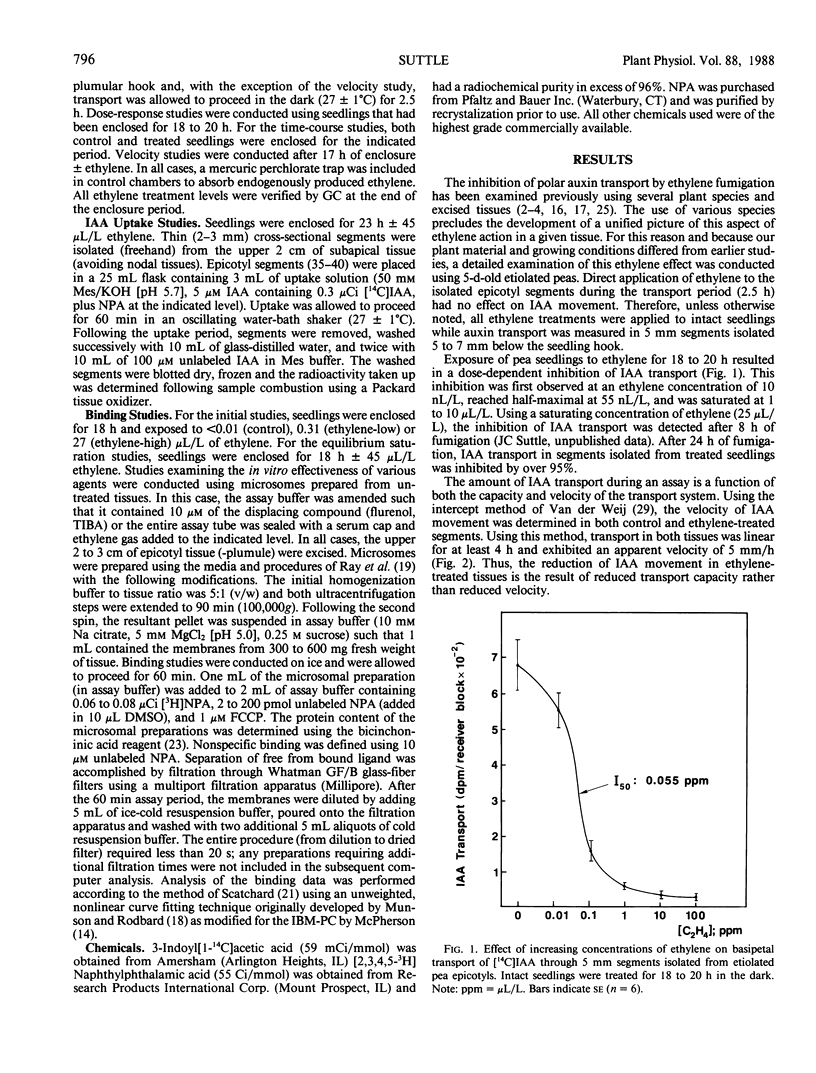
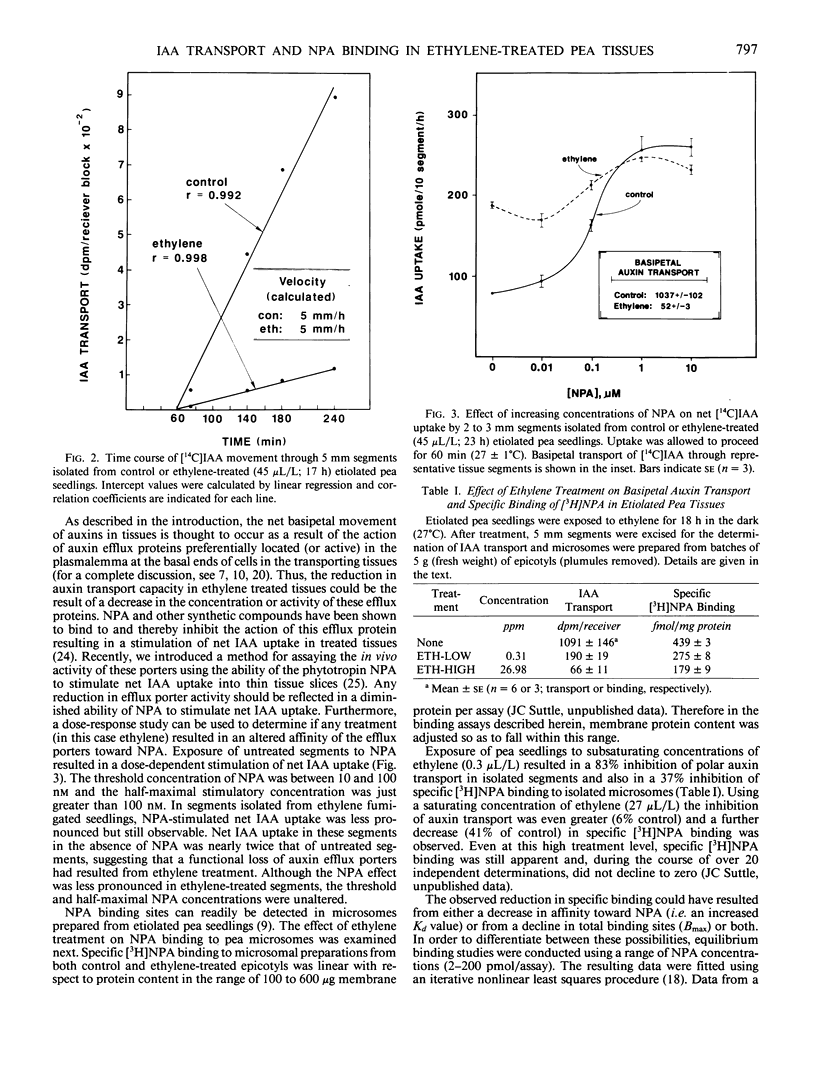
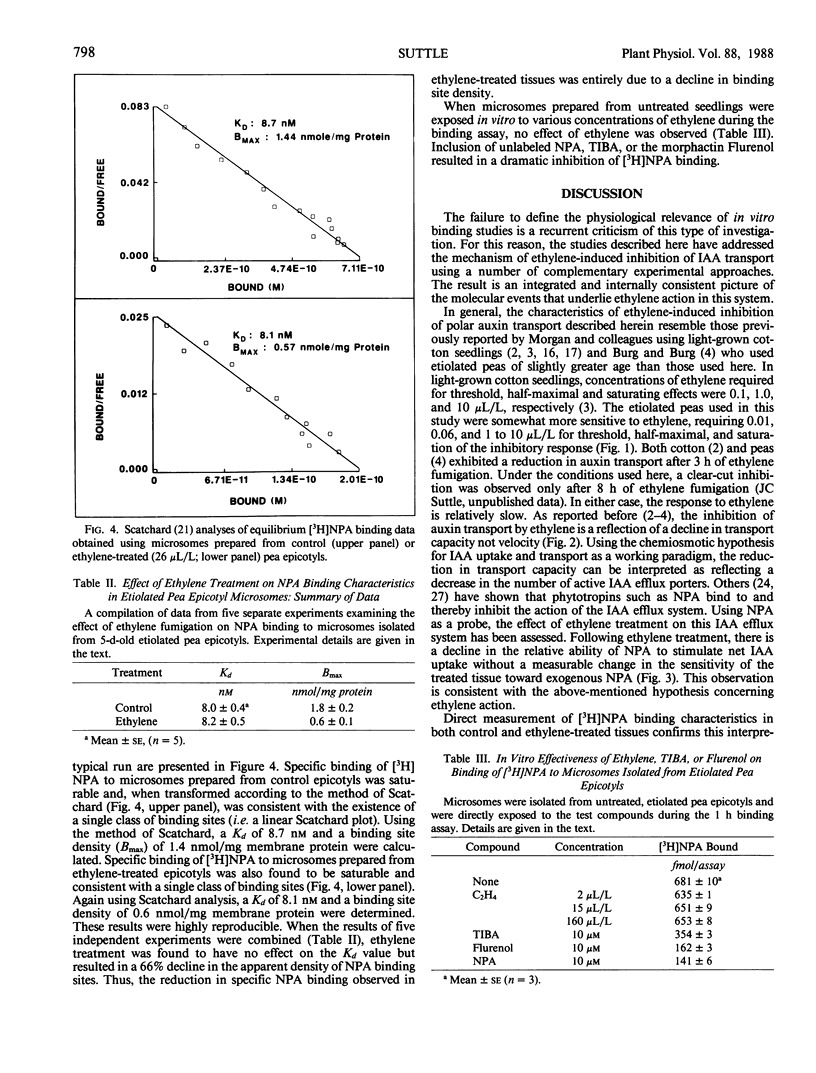
The effect of ethylene treatment on polar indole-3-acetic acid (IAA) transport, net IAA uptake in the presence and absence of N-1-naphthylphthalamic acid (NPA) and [3H]NPA binding characteristics was investigated in tissue segments or microsomes isolated from etiolated pea (Pisum sativum L. cv Alaska) epicotyls. Basipetal IAA transport in 5 millimeter segments isolated from ethylene-treated seedlings was inhibited by ethylene in a dose-dependent manner. Threshold, half-maximal and saturating concentrations of ethylene were 0.01, 0.55, 10.0 microliters per liter, respectively. This inhibition became apparent after 6 to 8 hours of ethylene treatment. Transport velocity in both control and ethylene-treated tissues was estimated to be 5 millimeters per hour. Net IAA uptake was stimulated in ethylene-treated tissues and the relative ability of the phytotropin NPA to enhance net IAA uptake was reduced in treated tissues. Specific binding of [3H]NPA to microsomes prepared from both control and ethylene-treated tissues was saturable and consistent with the existence of a single class of binding sites with an apparent affinity (Kd) toward NPA of 8 to 9 nanomolar. The density of these binding sites (per milligram protein) was lower (36% of control) in ethylene-treated tissues. Direct application of ethylene to microsomal preparations isolated from untreated seedlings had no effect on the level of specific [3H]NPA binding.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Inhibition of polar auxin transport by ethylene. Plant Physiol. 1967 Sep;42(9):1224–1228. doi: 10.1104/pp.42.9.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport T. L., Morgan P. W., Jordan W. R. Auxin Transport as Related to Leaf Abscission during Water Stress in Cotton. Plant Physiol. 1977 Apr;59(4):554–557. doi: 10.1104/pp.59.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Jacobs M., Gilbert S. F. Basal localization of the presumptive auxin transport carrier in pea stem cells. Science. 1983 Jun 17;220(4603):1297–1300. doi: 10.1126/science.220.4603.1297. [DOI] [PubMed] [Google Scholar]
- Katekar G. F., Geissler A. E. Auxin Transport Inhibitors: IV. EVIDENCE OF A COMMON MODE OF ACTION FOR A PROPOSED CLASS OF AUXIN TRANSPORT INHIBITORS: THE PHYTOTROPINS. Plant Physiol. 1980 Dec;66(6):1190–1195. doi: 10.1104/pp.66.6.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson G. A. Analysis of radioligand binding experiments. A collection of computer programs for the IBM PC. J Pharmacol Methods. 1985 Nov;14(3):213–228. doi: 10.1016/0160-5402(85)90034-8. [DOI] [PubMed] [Google Scholar]
- Morgan P. W., Gausman H. W. Effects of ethylene on auxin transport. Plant Physiol. 1966 Jan;41(1):45–52. doi: 10.1104/pp.41.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan P. W., Jordan W. R., Davenport T. L., Durham J. I. Abscission responses to moisture stress, auxin transport inhibitors, and ethephon. Plant Physiol. 1977 Apr;59(4):710–712. doi: 10.1104/pp.59.4.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley D. R., Lefkowitz R. J. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985 Sep 12;317(6033):124–129. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Suttle J. C. Disruption of the Polar Auxin Transport System in Cotton Seedlings following Treatment with the Defoliant Thidiazuron. Plant Physiol. 1988 Jan;86(1):241–245. doi: 10.1104/pp.86.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veen H., Jacobs W. P. Transport and metabolism of indole-3-acetic Acid in coleus petiole segments of increasing age. Plant Physiol. 1969 Aug;44(8):1157–1162. doi: 10.1104/pp.44.8.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]


