Abstract
The 2A and 3C proteases encoded by human rhinoviruses (HRVs) are attractive targets for antiviral drug development due to their important roles in viral replication. Homophthalimides were originally identified as inhibitors of rhinovirus 3C protease through our screening effort. Previous studies have indicated that the antiviral activity of certain homophthalimides exceeded their in vitro inhibitory activity against the viral 3C protease, suggesting that an additional mechanism might be involved. Reported here is the identification of homophthalimides as potent inhibitors for another rhinovirus protease, designated 2A. Several homophthalimides exhibit time-dependent inhibition of the 2A protease in the low-micromolar range, and enzyme-inhibitor complexes were identified by mass spectrometry. Compound LY343814, one of the most potent inhibitors against HRV14 2A protease, had an antiviral 50% inhibitory concentration of 4.2 μM in the cell-based assay. Our data reveal that homophthalimides are not only 3C but also 2A protease inhibitors in vitro, implying that the antiviral activity associated with these compounds might result from inactivation of both 2A and 3C proteases in vivo. Since the processing of the viral polyprotein is hierarchical, dual inhibition of the two enzymes may result in cooperative inhibition of viral replication. On the basis of the current understanding of their enzyme inhibitory mechanism, homophthalimides, as a group of novel nonpeptidic antirhinovirus agents, merit further structure-action relationship studies.
The major etiologic agents of the common cold in humans are human rhinoviruses (HRVs), which include over 100 different serotypes and belong to the picornavirus family (5). These small plus-strand RNA viruses translate their genomic information into a single large polyprotein with a size of 220 kDa (for reviews, see references 13 and 14). Maturation cleavage of the polyprotein to generate functional viral proteins is required for viral replication and is performed mainly by two virally encoded proteases, designated 2A and 3C (13, 14). The first cleavage of the polyprotein is believed to be catalyzed by the 2A protease as a cotranslational event (13, 14). This cleavage, taking place at the junction of capsid protein VP1 and the N terminus of 2A itself, separates the viral capsid proteins from the nonstructural ones (13, 14). Most of the remaining cleavages are further processed by either 3C or its precursor 3CD enzyme. In addition, these enzymes have been shown to be responsible for cleavage of several other important cellular proteins, which may lead to inhibition of normal host cell functions (2, 3, 7, 10, 11).
Based on amino acid sequence alignments to known proteases, HRV 2A and 3C proteins display strong similarities to trypsin-like serine proteases, although 2A and 3C both contain a cysteine residue as the active-site nucleophile (13, 14). Inhibition studies using a series of class-specific protease inhibitors also reveal that HRV 2A and 3C enzymes are novel cysteine proteases that are not inactivated by traditional cysteine protease inhibitors such as E-64 (12, 17, 18). Due to their essential roles in viral replication and unique protein structures, the viral 2A and 3C proteases appear to be ideal targets for antiviral chemotherapy.
Several attempts have been made to discover or design HRV 3C protease inhibitors (8, 9, 15, 21). Peptide-based inhibitors for the 3C protease of HRV serotype 14 (HRV 14) have been reported previously (9, 15). More recently, we and Webber et al. have described the syntheses and antiviral activities of nonpeptidic inhibitors for the same enzyme (8, 21). In contrast to the 3C protease, no structure-action relationship studies have been reported for the HRV 2A enzyme nor have any specific 2A inhibitors been described. In this paper, we report the inhibition of HRV 2A protease by homophthalimides, a series of compounds exhibiting in vivo antiviral activities in the low-micromolar range and certain levels of 3C protease inhibition in vitro (8). Herein, we discuss the inactivation of the 2A and 3C proteases by these compounds and the potential mechanism related to their antiviral activities.
MATERIALS AND METHODS
Materials.
Purified HRV14 3C protease was prepared as described previously (1). Purification of recombinant HRV14 2A protease is described in detail elsewhere (20). Briefly, a gene encoding the full-length HRV14 2A protein was inserted into an Escherichia coli expression vector, pH10, which has been described previously for HRV14 3C expression (1). Overproduced 2A protein, predominantly partitioned in inclusion bodies of the transformed bacterial cells, was solubilized, refolded, and then purified to homogeneity by a two-step purification protocol. The 2A protease from HRV2 was obtained from Boehringer Ingelheim (11). Chromogenic peptide substrates for both the 2A and 3C proteases, containing p-nitroaniline (pNA) as the only moiety at the prime side of the scissile bond, were designed based on their native processing sites of the HRV polyprotein precursors and were synthesized and purified as described previously (18–20). Human neutrophil elastase was purchased from Calbiochem. Bovine chymotrypsin and trypsin, human plasma thrombin and factor Xa, and papain were purchased from Boehringer Mannheim.
Syntheses of homophthalimides.
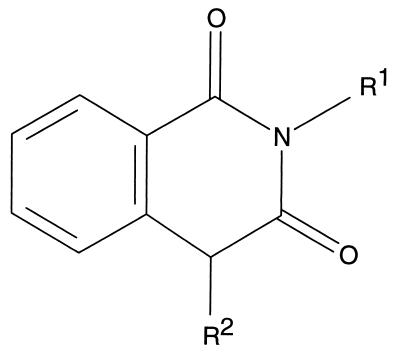
Homophthalimides were synthesized by previously published procedures (8). Briefly, the unsubstituted homophthalimides (Fig. 1) were synthesized through the condensation of homophthalic acid and the corresponding primary amines (8). Further modifications were introduced at the benzylic methylene position by alkylation to produce a set of diversified compounds (8).
FIG. 1.
Chemical structure of homophthalimides. The structure of the homophthalimides is shown with the two potential substitution groups designated R1 and R2.
Colorimetric protease assay.
Two specifically designed chromogenic peptide substrates, R-K-G-D-I-K-S-Y-pNA and E-A-L-F-Q-pNA, were used for the measurement of HRV14 2A and 3C protease activities, respectively (18, 19). Cleavage of these pNA peptides between tyrosine or glutamine at P1 and the pNA moiety at P1′ by the 2A or 3C enzyme releases yellow-colored free pNA, which could be detected at a visible wavelength (18, 19). A typical HRV14 2A protease assay was performed at 25°C for 30 min in a 200-μl reaction mixture containing 25 mM HEPES (pH 8.0), 150 mM NaCl, 1 mM EDTA, 250 μM peptide substrate R-K-G-D-I-K-S-Y-pNA, and 0.2 μM purified 2A protease. The cleavage reaction for the 3C protease (0.2 μM) was carried out at 30°C under the conditions described previously with peptide E-A-L-F-Q-pNA as substrate (8). All reactions were initiated by the addition of the corresponding substrate to the microplate wells containing protease in the absence or presence of inhibitors. Peptide cleavage activity was measured at a wavelength of 405 nm against a blank in which the corresponding enzyme was not included in the reaction mix. HRV2 2A protease activity was measured with a peptide (T-R-P-I-I-T-T-A-pNA) as the substrate as described previously (19). All protease assays were directly performed in microtiter plate wells and were continuously monitored with a temperature-controlled microplate reader (Molecular Devices). Inhibition activities associated with the compounds were expressed as percentages of the control, which contained only the inhibitor solvent. At least six different concentrations of each inhibitor were examined to generate IC50s. The IC50 is the inhibitor concentration at which 50% of the control protease activity is inhibited. All the 2A or 3C protein present in the sample was assumed to be fully active.
Cell-based antiviral assay.
Antiviral assays were conducted in rhinovirus-sensitive H1-HeLa cells purchased from the American Type Culture Collection. A monolayer of HeLa cells was inoculated with HRV14 at a multiplicity of infection of 1 PFU per cell. After an adsorption period of 30 min at room temperature, the infected cells were incubated at 35°C with the medium in the absence or presence of various concentrations of the compounds. At 8 h after virus inoculation, the infected HeLa cells were lysed by freezing and thawing, and the supernatant consisting of culture medium and cell lysate was sonicated for 10 min and centrifuged at 4,000 × g for 15 min at 4°C. The virus in the supernatant was then quantitated by use of the plaque-forming assay in HeLa cells as described previously (6, 8). To determine the effective concentration resulting in 50% inhibition of HRV replication (antiviral IC50), the mean plaque number was calculated from a duplicate series of counts and converted to a percentage of untreated controls. The antiviral IC50 was then calculated from the plot of percentage inhibition of the virus yield versus the logarithm of the inhibitor concentration.
Other methods.
Electrospray ionization mass spectrometry was performed with a PESciex API III triple-stage quadrupole mass spectrometer. Samples were dissolved in 50:49:1 (vol/vol/vol) acetonitrile:water:acetic acid and were continuously infused into the interface at a rate of 10 to 20 μl/min. The instrument was operated in the positive ion detection mode with an ionspray voltage of 3,500 V and an inlet orifice potential of 55 V (+25 V relative to the rod offset voltage). Mass spectra were collected over a range of 300 to 2,000 μm at 0.1-μm intervals with a dwell time of 1 ms per interval. The final spectrum was generated by averaging a total of 5 to 10 scans.
RESULTS AND DISCUSSION
Rhinovirus 2A and 3C proteases are essential enzymes involved in the maturation processing of viral polyprotein (13, 14), and therefore it would be expected that inactivation of viral protease activity should result in inhibition of viral replication. An effort to discover inhibitors of rhinovirus 3C protease via a high throughput screen was initiated, and several nonpeptidic compounds with inhibitory activities were identified (8). Among these inhibitors, a series of homophthalimides appeared to be worthy of further investigation. These compounds contain several potential modification sites including the aromatic ring, benzylic methylene, and nitrogen atom as seen in Fig. 1. Our initial effort resulted in the discovery of compound LY353349, which displayed improved 3C protease inhibition as well as antiviral activity in cell culture assay (see Table 2). For this molecule in particular, both mass spectrometry and molecular modeling studies have suggested that LY353349 forms a tight binding complex with the 3C protein (8).
TABLE 2.
Anti-HRV14 activities of homophthalimides
| Compound | IC50a (μM) | TC50b (μM) | TIc |
|---|---|---|---|
| LY343813 | 25.1 | >360 | >14.1 |
| LY343814 | 4.2 | 125 | 29.8 |
| LY344453 | 19.6 | >400 | >20.4 |
| LY353348 | 27.7 | 155 | 5.9 |
| LY353349 | 22.1 | 183 | 8.3d |
| LY353352 | 15.8 | >265 | >16.7d |
| LY353354 | 27.3 | NAe | NA |
The antiviral IC50 for each compound was determined as described in Materials and Methods.
TC50, inhibitor cytotoxicity determined as described previously (6).
TI, therapeutic index. TI is defined as TC50/IC50. By convention, true antiviral activity requires a TI ≥10.
Data from reference 8.
NA, not available.
It is interesting to note that the antiviral activity of certain homophthalimides did not always correlate with the level of 3C protease inhibition (8). One possible explanation is that these compounds might have different cell membrane permeabilities and thus display different accumulation levels in the infected host cells. We also observed that several homophthalimides exhibited cellular antiviral activities but did not show significant inhibitory activities against 3C protease in vitro, e.g., LY343813 and LY343814 (8). These data suggest that the antiviral activities associated with homophthalimides might be the result of an additional mechanism of action beyond that of the 3C enzyme inhibition. Based on our preliminary data, we thought that homophthalimides might be inhibiting another viral enzyme, designated 2A, which is an HRV-encoded cysteine protease similar to 3C. Several observations support this hypothesis. First, both 2A and 3C contain an active-site cysteine nucleophile but are structurally more closely related to the trypsin-type serine protease family (for reviews, see references 13 and 14). Second, they demonstrate similar responses in vitro to a series of class-specific protease inhibitors (12, 17, 18). Furthermore, previous studies have shown that both proteases cleave small peptide substrates in vitro with a preference for glycine and proline at the P1′ and P2′ positions, respectively (17). Although HRV14 3C protease prefers a P1 glutamine (4), the 2A protease from HRV2 seems to recognize several amino acids, including alanine, methionine, and tyrosine, at this position (17).
To examine if the 2A protease was targeted by homophthalimides, we cloned and purified the HRV14 2A protease to homogeneity as shown by a silver-stained gel (data not shown). To develop a simple and accurate assay for this enzyme, a chromogenic octapeptide, R-K-G-D-I-K-S-Y-pNA, with its amino acid sequence derived from the authentic HRV14 2A cleavage site of the viral polyprotein, was synthesized with an N-acylated pNA as the only moiety on the prime side. Hydrolysis of this peptide between the tyrosine at P1 and pNA at P1′ releases free pNA, which can be detected at a visible wavelength (Fig. 2). Using this peptide as a substrate, we evaluated the 2A protease activity in the absence or presence of homophthalimides. As seen in Table 1, several compounds demonstrated inhibitory activities against the HRV14 2A protease with IC50s in the low-micromolar range. Compounds such as LY343813 and LY343814 were found to be potent inhibitors for HRV14 2A but not for 3C under the conditions employed (Table 1). In addition, these compounds displayed antiviral activities in cell culture at low-micromolar concentrations (Table 2). It should be noted that all the compounds described herein were designed to take advantage of the 3C protease’s preference for a P1 glutamine (Fig. 1, R1 substituent) and as of yet no attempt to optimize 2A protease inhibition has been made.
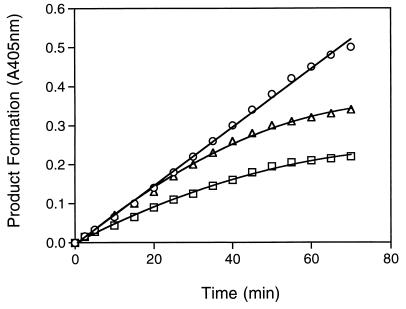
FIG. 2.
Inhibition progress curve of rhinovirus 2A proteases by homophthalimides. HRV14 2A protease activity was measured with the pNA peptide at 250 μM as described in Materials and Methods in the absence (○, no inhibitor) or the presence of compound LY343814 (▵, 6 μM; □, 30 μM). Absorbance at 405 nm was recorded at the indicated time points against a blank containing no enzyme. Reactions were run in duplicate, and data are averages with variations of less than 3%.
TABLE 1.
Inhibition of HRV 2A and 3C proteases by homophthalimidesa
| Compound | Structure
|
IC50 (μM)
|
|||
|---|---|---|---|---|---|
| R1 | R2 | HRV2 2A | HRV14 2A | HRV14 3Cb | |
| LY046601 | -CH3 | -CH2COPh(p-F) | 21.8 | 56.2 | 41.1 |
| LY343813 | -(CH2)3CO2Et | -H | >200 | 18.2 | >200 |
| LY343814 | -n-Bu | -COMe | >200 | 19.7 | >200 |
| LY344453 | -(CH2)3SMe | -H | >200 | 98.3 | >200 |
| LY353348 | -(CH2)2SMe | -CH2COPh | 8.4 | >100 | >200 |
| LY353349 | -(CH2)2SO2Me | -CH2COPh | 3.1 | 44.6 | 22.1 |
| LY353350 | -(CH2)3CO2Et | -COMe | >200 | 26.5 | >200 |
| LY353352 | -(CH2)2CO2Et | -CH2COPh | 3.9 | 63.3 | 55.4 |
| LY353353 | -(CH2)3SMe | -CH2COPh | 23.1 | 56.0 | 130.6 |
| LY353354 | -(CH2)3SO2Me | -CH2COPh | 31.3 | NDc | 25.0 |
| LY355451 | -(CH2)3CO2Et | -CH2CO-proline | >100 | >100 | 56.9 |
Inhibition assays were carried out as described in Materials and Methods. Control sample contained only the inhibitor solvent (dimethyl sulfoxide) and counted as no inhibition. Experiments were run in duplicate, and data are averages with variations of less than 5%. Me, methyl; Et, ethyl; Ph, benzylic.
Data from reference 8.
ND, not done.
Homophthalimides also inhibit the 2A protease from other serotypes. As revealed in Table 1, HRV2 2A protease, sharing 41% identity and 57% similarity with HRV14 2A at the amino acid level (data not shown), was found to be inactivated by some of the compounds. The inhibition profile of HRV2 2A was different from that of either HRV14 2A or 3C (Table 1), suggesting that the amino acids involved in the enzyme-inhibitor interaction might be different between these proteases. Compounds LY353348, 353349, and LY353352 were much better HRV2 2A inhibitors, with over 10-fold more activity against HRV2 2A than the related enzyme derived from HRV14. In contrast, LY343813 and LY343814 inhibited HRV14 2A more efficiently than the 2A from serotype 2. It seems that the acetophenone-substituted compounds (e.g., R2 = CH2COPh [Ph, benzylic]) are excellent inhibitors for HRV2 2A and HRV14 3C but not for HRV14 2A. The latter enzyme appears to prefer a smaller group at that position (R2 = H or COMe [Me, methyl]). Interestingly, LY353349 was found to be over 10-fold more active against HRV2 2A than LY353354, although the only difference between these compounds is that LY353354 has an extra methylene at the R1 position.
Since homophthalimides were able to inactivate both viral 2A and 3C proteases, we examined their inhibitory activities against other readily available serine and cysteine proteases including chymotrypsin, elastase, factor Xa, papain, thrombin, and trypsin. Of the two compounds analyzed, LY343813 at 65 μM was found to be inactive towards all the cellular proteases tested, while LY353352 exhibited ∼45 and 12% inhibition at 90 μM against papain and thrombin, respectively (data not shown). Thus, it seems that these two homophthalimides, which possess true antiviral activities, appear somewhat selective for HRV 2A and 3C enzymes relative to several other cellular proteases. The dual inhibition of 2A and 3C by homophthalimides is possibly due to their similar P1 preferences as discussed below.
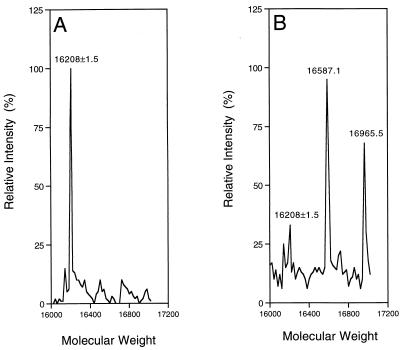
To have a better understanding of the interactions between the homophthalimides and the enzyme, we performed mass spectrometric studies of the 2A sample treated with the potent compounds. Free HRV2 2A was identified as a protein with a mass of 16,208 daltons as expected (Fig. 3A). Modified 2A proteins, with masses corresponding to the enzyme plus one or two inhibitors, were found to be present in the sample treated with the inhibitors (Fig. 3B). These data implied that homophthalimides formed covalent complexes with the 2A protease. Similar results were obtained with the HRV14 2A protease (data not shown). The observation of two inhibitor molecules bound to the enzyme suggests nonspecific interactions can take place with these nonoptimized lead compounds. Detailed characterization studies are in progress to identify the modification sites of the 2A protein treated with inhibitors.
FIG. 3.
Mass spectrometry study of the protease-inhibitor complex. HRV2 2A protease (10 μM) (Mr, 16,208) was incubated with dimethyl sulfoxide or 100 μM LY353352 (Mr, 379.4) at room temperature for 60 min. The samples were then analyzed on a mass spectrometer as described in Materials and Methods. The masses of major peaks are labeled. (A) HRV2 2A protease plus dimethyl sulfoxide as control; (B) HRV2 2A plus LY353352.
Since the 3C protease cleaves the native peptide substrates at the bond between glutamine and glycine (4), several compounds were specifically designed and synthesized with a glutamine- or methionine-like substitution at the R1 site to mimic its conserved P1 glutamine residue (9, 15). Since the 3C protease also has a preference for lypophilic amino acids at the P2 position, e.g., valine or phenylalanine (4), it is likely that the aromatic ring at the R2 substituents of the homophthalimides might interact with the S2 subsite of the 3C protease. Several of these compounds, previously shown to be 3C inhibitors (8), also inhibit 2A proteases. The 2A proteases, as seen in Table 1, were not sensitive to N substitution with sulfone, sulfide, or carbonyl at R1. Thus, compounds with methyl, glutamine-like, or methionine-like substitution exhibited similar inhibitory activities toward the 2A enzymes. This is not surprising as previous studies of HRV2 2A substrate specificity have suggested that this enzyme has no specific requirement for the amino acid residue at P1 (17). Efficient cleavage can be seen with the peptides containing alanine, tyrosine, methionine, and even phenylalanine residues (17). Compared to HRV2 2A, little is known about substrate recognition and cleavage by HRV14 2A. Our enzyme inhibition data suggest that there are differences between these 2A enzymes concerning their substrate specificities, especially for the preference of nonprime-side amino acids. However, a better understanding of the structural requirements of HRV14 2A must await comprehensive specificity studies using small synthetic peptides. As the 2A crystal structure (from any serotype) is not available, elucidation of its substrate specificity features must be obtained in order to effectively design and develop potent viral protease inhibitors with broad-spectrum antipicornaviral activity.
As mentioned above, several compounds such as LY343813 and LY343814 did not inhibit 3C activity significantly in vitro, although they exhibited antiviral activities in cell-based assays as shown in Table 2. It seems reasonable to suggest that these compounds might demonstrate antiviral activities by inactivating the 2A protease. In some cases, dual inhibition of 2A and 3C had also been observed with a few potent compounds. For instance, LY353352, previously shown to be a potent 3C inhibitor with true antiviral activity (8), exhibited 2A inhibitory activity as well (Tables 1 and 2). Based on these data, we conclude that the observed antiviral activities of homophthalimides might be a result of their combined inhibition of both 2A and 3C in vivo. Since the maturation cleavages of the viral polyprotein precursor by 2A and 3C are hierarchical (13, 14), simultaneous inactivation of these enzymes might produce cooperative inhibition of viral replication. We did observe that one compound, LY344453, displayed relatively poor IC50s against both proteases but nevertheless acted as a true antiviral compound in the tissue culture assay. This apparent discrepancy might be rationalized on the basis of its structural features. LY344453 lacks both the acetophenone moiety (R2 = CH2COPh) and a hydrogen bond acceptor at the R1 position, thus explaining the apparent low affinity for the isolated enzymes (8). On the other hand, this compound is smaller in size and possesses a lipophilic R1 substituent, and thus, it may penetrate infected cells more readily than the other homophthalimides tested. It should be noted that the concentration of the viral proteases in the in vitro inhibition assay (0.2 μM) is probably much higher than that found in infected cells. At this time, we cannot absolutely exclude alternative mechanisms of its action; however, the low cytotoxicity exhibited by LY344453 (Table 2) suggests there is not significant interaction with host cell functions.
In summary, we report here that homophthalimides, originally designed as 3C protease inhibitors, are also able to inhibit viral cysteine protease 2A. To the best of our knowledge, this is the first study describing potential inhibitors of HRV 2A protease. We have shown that knowledge of the enzyme active site and substrate specificity allow the optimization of these compounds as HRV 3C protease inhibitors (8). Additional information regarding the substrate recognition of the 2A enzyme should inspire the design of more potent and selective 2A inhibitors with improved antiviral activities. However, it still remains to be seen if a more potent enzyme inhibitor will result in a compound which displays sufficient potency and activity against enough picornaviral serotypes to warrant clinical development. Nevertheless, based on the results described in this report, homophthalimides are promising, nonpeptidic anti-HRV agents that merit further investigation.
ACKNOWLEDGMENTS
We thank Gregory Cox, John Richardson, and Mark Walkuchik for technical assistance. We are also grateful to W. Sommergruber of Boehringer Ingelheim for providing us with the purified HRV2 2A protease.
REFERENCES
- 1.Birch G M, Black T, Malcolm S K, Lai M T, Zimmerman R E, Jaskunas S R. Purification of recombinant human rhinovirus 14 3C protease expressed in E. coli. Protein Expr Purif. 1995;6:609–618. doi: 10.1006/prep.1995.1080. [DOI] [PubMed] [Google Scholar]
- 2.Clark M E, Liberian P M, Berg A J, Dasgupta A. Direct cleavage of human TATA-binding protein by poliovirus protease 3C in vivo and in vitro. Mol Cell Biol. 1993;13:1232–1237. doi: 10.1128/mcb.13.2.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark M E, Hämmerle T, Wimmer E, Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991;10:2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cordingley M G, Callahan P L, Sardana V V, Garsky V M, Colonno R J. Substrate requirements of human rhinovirus 3C protease for peptide cleavage in vitro. J Biol Chem. 1990;265:9062–9065. [PubMed] [Google Scholar]
- 5.Couch R B. Rhinoviruses. In: Fields B N, Knipe D M, editors. Virology. New York, N.Y: Raven Press; 1990. pp. 607–629. [Google Scholar]
- 6.Heinz B A, Tang J, Labus J M, Chadwell F W, Kaldor S W, Hammond M. Simple in vitro translation assay to analyze inhibitors of rhinovirus proteases. Antimicrob Agents Chemother. 1996;40:267–270. doi: 10.1128/aac.40.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hellen C U T, Fäcke M, Kräusslich H-G, Lee C-K, Wimmer E. Characterization of poliovirus 2A proteinase by mutational analysis: residues required for autocatalytic activity are essential for induction of cleavage of eukaryotic initiation factor 4F polypeptide p220. J Virol. 1991;65:4226–4231. doi: 10.1128/jvi.65.8.4226-4231.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jungheim L N, Cohen J D, Johnson R B, Villarreal E C, Wakulchik M, Loncharich R J, Wang Q M. Inhibition of human rhinovirus 3C protease by homophthalimides. Bioorg Med Chem Lett. 1997;7:1589–1594. [Google Scholar]
- 9.Kaldor S W, Hammond M, Dressman B A, Labus J M, Chadwell F W, Kline A D, Heinz B A. Glutamine-derived aldehydes for the inhibition of human rhinovirus 3C protease. Bioorg Med Chem Lett. 1995;5:2021–2026. [Google Scholar]
- 10.Lamphear B J, Yan R, Yang F, Waters D, Liebig H-D, Klump H, Kuechler E, Skern T, Rhoads R E. Mapping the cleavage site in protein synthesis initiation factor eIF-4γ of the 2A proteases from human coxsackievirus and rhinovirus. J Biol Chem. 1993;268:19200–19203. [PubMed] [Google Scholar]
- 11.Liebig H-D, Ziegler E, Yan R, Hartmuth K, Klump H, Kowalski H, Blaas D, Sommergruber W, Frasel L, Lamphear B J, Rhoads R, Kuechler E, Skern T. Purification of two picornaviral 2A proteinases: interaction with eIF-4γ and influence on in vitro translation. Biochemistry. 1993;32:7581–7588. doi: 10.1021/bi00080a033. [DOI] [PubMed] [Google Scholar]
- 12.Molla A, Hellen C U T, Wimmer E. Inhibition of proteolytic activity of poliovirus and rhinovirus 2A proteinases by elastase-specific inhibitors. J Virol. 1993;67:4688–4695. doi: 10.1128/jvi.67.8.4688-4695.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmenberg A C. Proteolytic procession of picornaviral polyprotein. Annu Rev Microbiol. 1990;44:603–623. doi: 10.1146/annurev.mi.44.100190.003131. [DOI] [PubMed] [Google Scholar]
- 14.Porter A G. Picornavirus nonstructural proteins: emerging roles in virus replication and inhibition of host cell functions. J Virol. 1993;67:6917–6921. doi: 10.1128/jvi.67.12.6917-6921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shepherd T A, Cox G A, McKinney E, Tang J, Wakulchik M, Zimmerman R E, Villarreal E C. Small peptidic aldehyde inhibitors of human rhinovirus 3C protease. Bioorg Med Chem Lett. 1996;6:2893–2895. [Google Scholar]
- 16.Skern T, Sommergruber W, Auer H, Volkmann P, Zorn M, Liebig H-D, Fessl F, Blaas D, Kuechler E. Substrate requirements of a human rhinoviral 2A proteinase. Virology. 1991;181:46–54. doi: 10.1016/0042-6822(91)90468-q. [DOI] [PubMed] [Google Scholar]
- 17.Sommergruber W, Ahorn H, Zöphel A, Maurer-Fogy I, Fessl F, Schnorrenberg G, Liebig H-D, Blaas D, Kuechler E, Skern T. Cleavage specificity on synthetic peptide substrates of human rhinovirus 2 proteinase 2A. J Biol Chem. 1992;267:22639–22644. [PubMed] [Google Scholar]
- 18.Wang Q M, Johnson R B, Cox G A, Villarreal E C, Loncharich R J. A continuous colorimetric assay for rhinovirus-14 3C protease using p-nitroaniline peptide as substrate. Anal Biochem. 1997;252:238–245. doi: 10.1006/abio.1997.2315. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q M, Sommergruber W, Johnson R B. Cleavage specificity of human rhinovirus-2 2A protease for peptide substrates. Biochem Biophys Res Commun. 1997;235:562–566. doi: 10.1006/bbrc.1997.6830. [DOI] [PubMed] [Google Scholar]
- 20.Wang Q M, Johnson R B, Cox G A, Villarreal E C, Churgay L M, Hale J E. Enzymatic characterization of refolded human rhinovirus type 14 2A protease expressed in Escherichia coli. J Virol. 1998;72:1683–1687. doi: 10.1128/jvi.72.2.1683-1687.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Webber S E, Tikhe J, Worland S T, Fuhrman S A, Hendrickson T F, Matthews D A, Love R A, Patick A K, Meador J W, Ferre R A, Brown E L, DeLisle D M, Ford C E, Binford S L. Design, synthesis, and evaluation of nonpeptidic inhibitors of human rhinovirus 3C protease. J Med Chem. 1996;39:5072–5082. doi: 10.1021/jm960603e. [DOI] [PubMed] [Google Scholar]