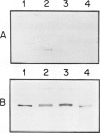
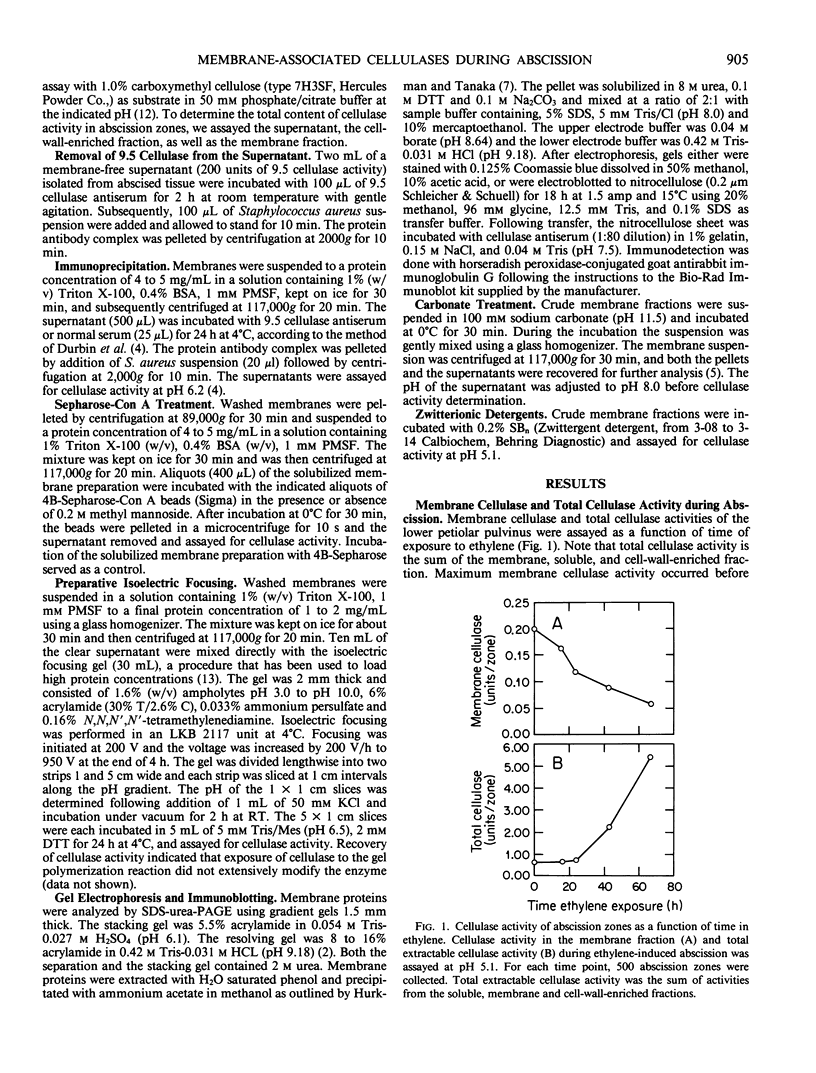
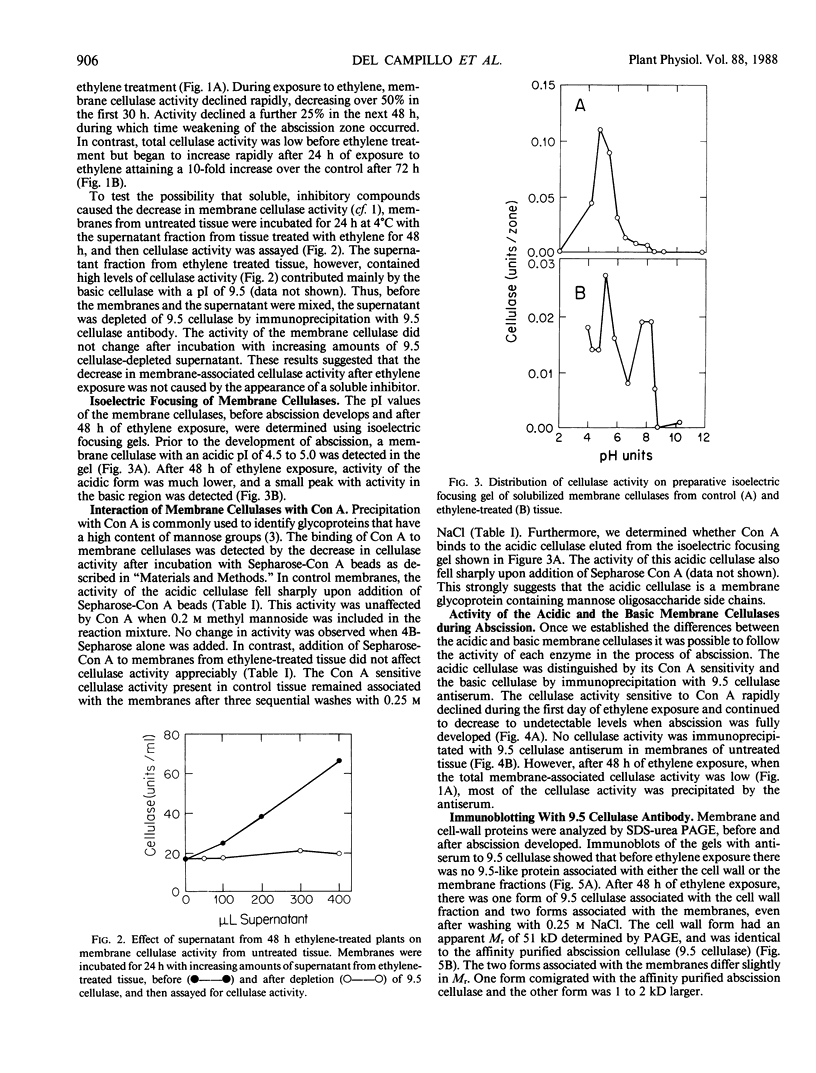
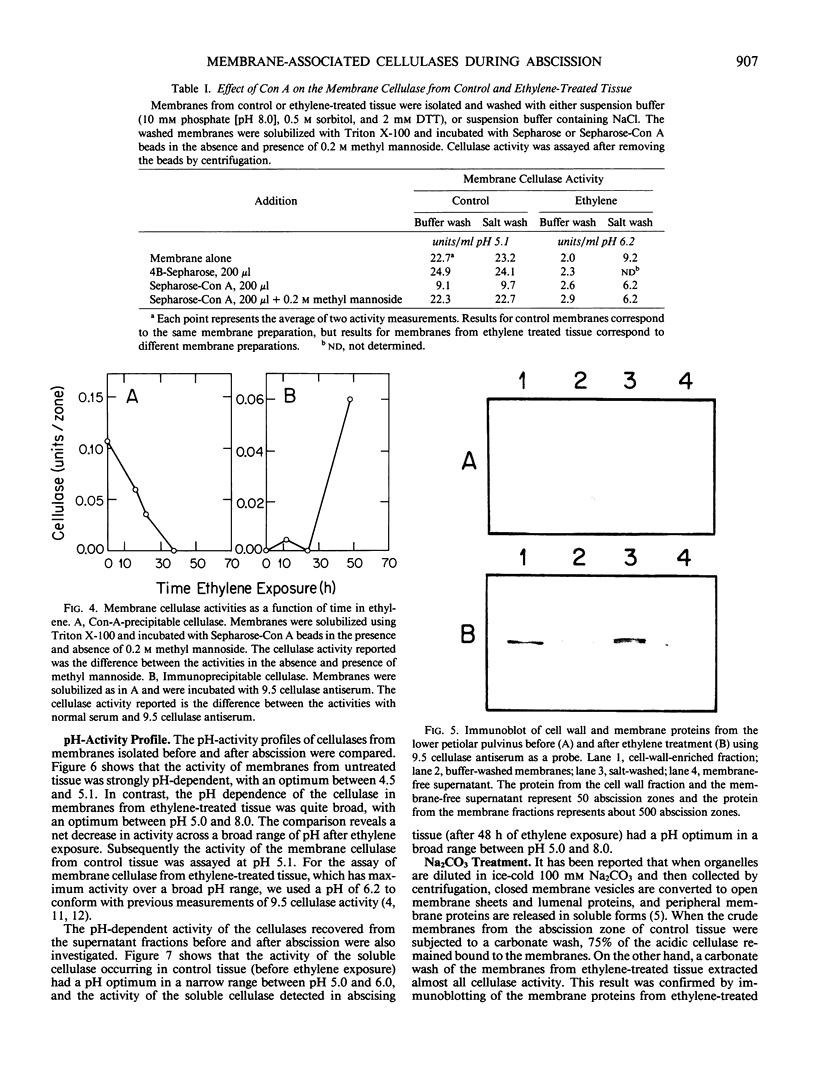
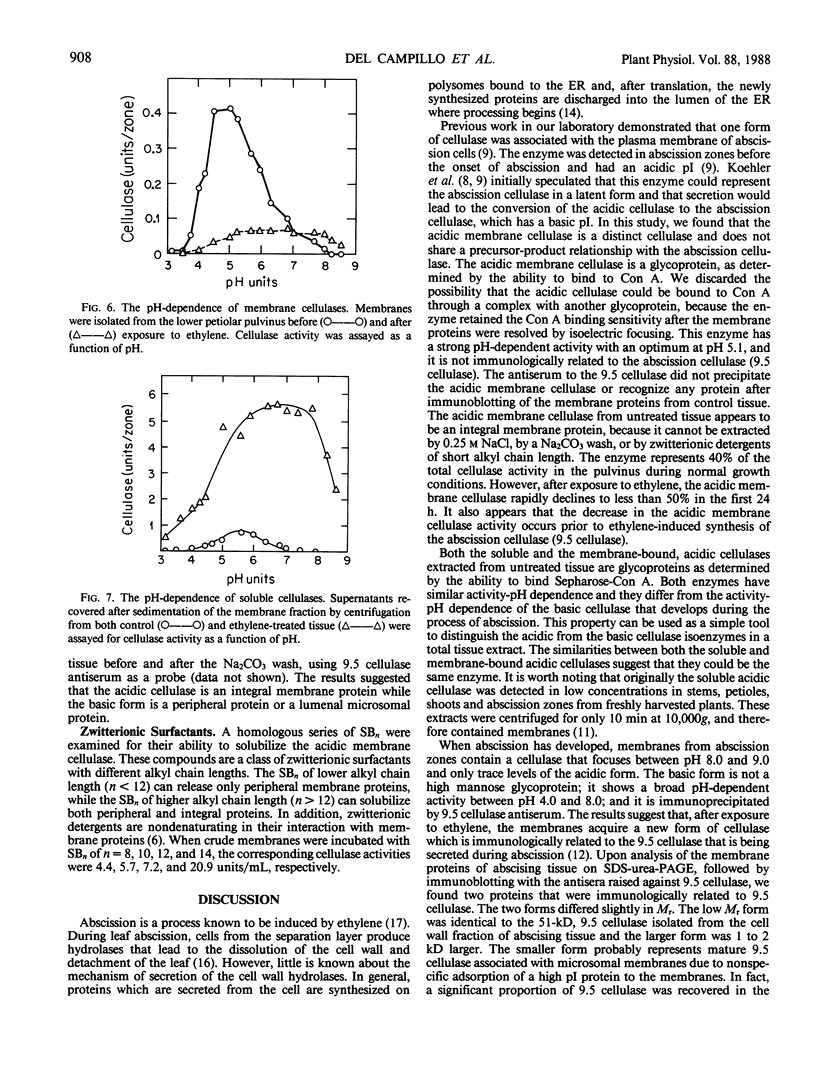
Abstract
Only one form of membrane-associated cellulase was found previously in the lower petiolar pulvinus of Phaseolus vulgaris (cv Red Kidney). The cellulase has an isoelectric point (pI) of 4.5 (DE Koehler, LN Lewis 1979 Plant Physiol 63: 677-679). This enzyme was detected in abscission zones collected before the onset of abscission (control tissue), and was thought to represent a pre-secretory form of another cellulase, the abscission cellulase, which has a basic pI and is secreted during abscission. We now show that this acidic, membrane-associated cellulase is a glycoprotein, tightly bound to the membrane, with maximum activity at pH 5.1, and that it is not immunologically related to the abscission cellulase. Furthermore, when bean explants are induced to abscise with ethylene, the activity of the acidic cellulase declines rapidly to 50% of control levels in the first day. When abscission is fully developed, the membranes contain a basic form of cellulase with a pI of 8.0 to 9.0 and only trace levels of the acidic cellulase. The basic form is not a high mannose glycoprotein; it has maximum activity in a broad pH range (4.0-8.0) and is antigenically related to the abscission cellulase, which is induced during abscission and transported to the cell wall. Antibody raised against the abscission cellulase recognized two proteins in a crude membrane fraction from abscising tissue. One of those proteins comigrated with the abscission cellulase, and the other was 1 to 2 kilodaltons larger. Thus, during abscission, the acidic membrane-associated cellulase rapidly declines before the appearance of the abscission cellulase. We conclude that there is no conversion from the acidic cellulase to the basic cellulase and suggest that the acidic and basic cellulase isoenzymes are proteins derived from two different genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Goldlust A., Icekson I. Control by ethylene of arginine decarboxylase activity in pea seedlings and its implication for hormonal regulation of plant growth. Plant Physiol. 1985 Nov;79(3):635–640. doi: 10.1104/pp.79.3.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg J. C. Glycoprotein detection in nitrocellulose transfers of electrophoretically separated protein mixtures using concanavalin A and peroxidase: application to arenavirus and flavivirus proteins. Anal Biochem. 1982 Dec;127(2):389–394. doi: 10.1016/0003-2697(82)90192-0. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Hurkman W. J., Tanaka C. K. Solubilization of plant membrane proteins for analysis by two-dimensional gel electrophoresis. Plant Physiol. 1986 Jul;81(3):802–806. doi: 10.1104/pp.81.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Leonard R. T., Vanderwoude W. J., Linkins A. E., Lewis L. N. Association of latent cellulase activity with plasma membranes from kidney bean abscission zones. Plant Physiol. 1976 Sep;58(3):324–330. doi: 10.1104/pp.58.3.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler D. E., Lewis L. N. Effect of ethylene on plasma membrane density in kidney bean abscission zones. Plant Physiol. 1979 Apr;63(4):677–679. doi: 10.1104/pp.63.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis L. N., Varner J. E. Synthesis of Cellulase during Abscission of Phaseolus vulgaris Leaf Explants. Plant Physiol. 1970 Aug;46(2):194–199. doi: 10.1104/pp.46.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGillivray A. J., Rickwood D. The heterogeneity of mouse-chromatin nonhistone proteins as evidenced by two-dimensional polyacrylamide-gel electrophoresis and ion-exchange chromatography. Eur J Biochem. 1974 Jan 3;41(1):181–190. doi: 10.1111/j.1432-1033.1974.tb03258.x. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Reid P. D., Strong H. G. Cellulase and Abscission in the Red Kidney Bean (Phaseolus vulgaris). Plant Physiol. 1974 May;53(5):732–737. doi: 10.1104/pp.53.5.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]