Abstract
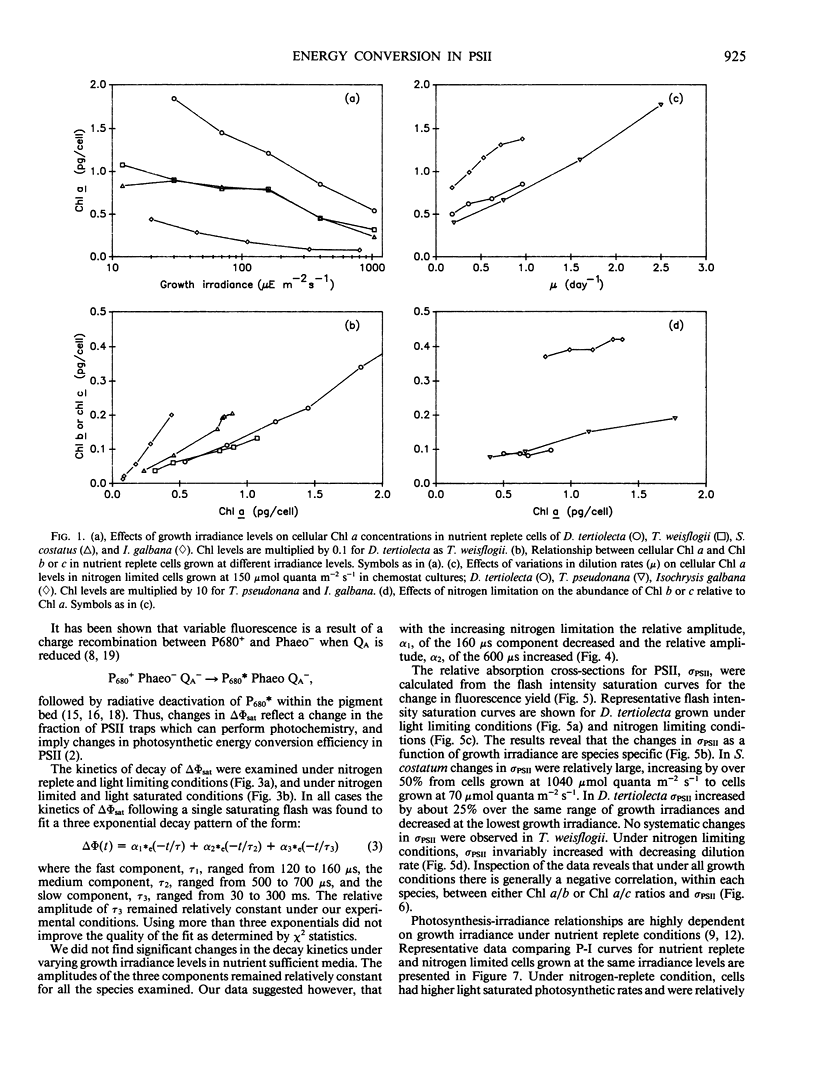
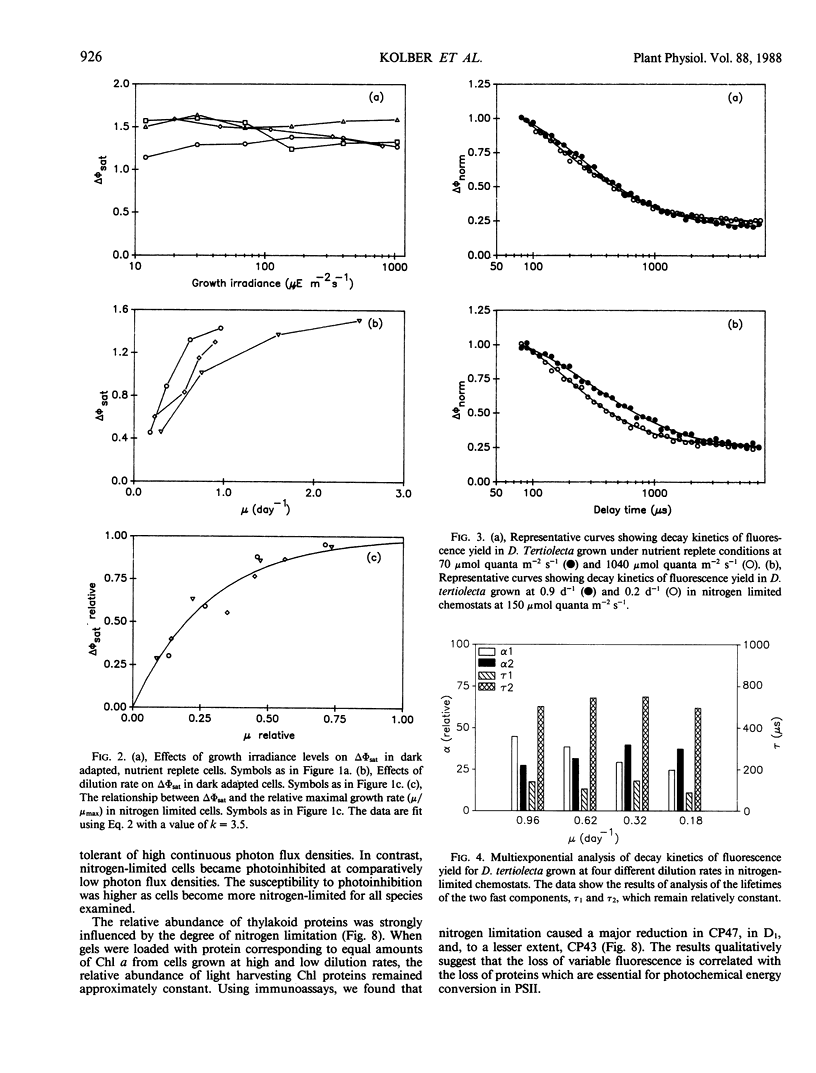
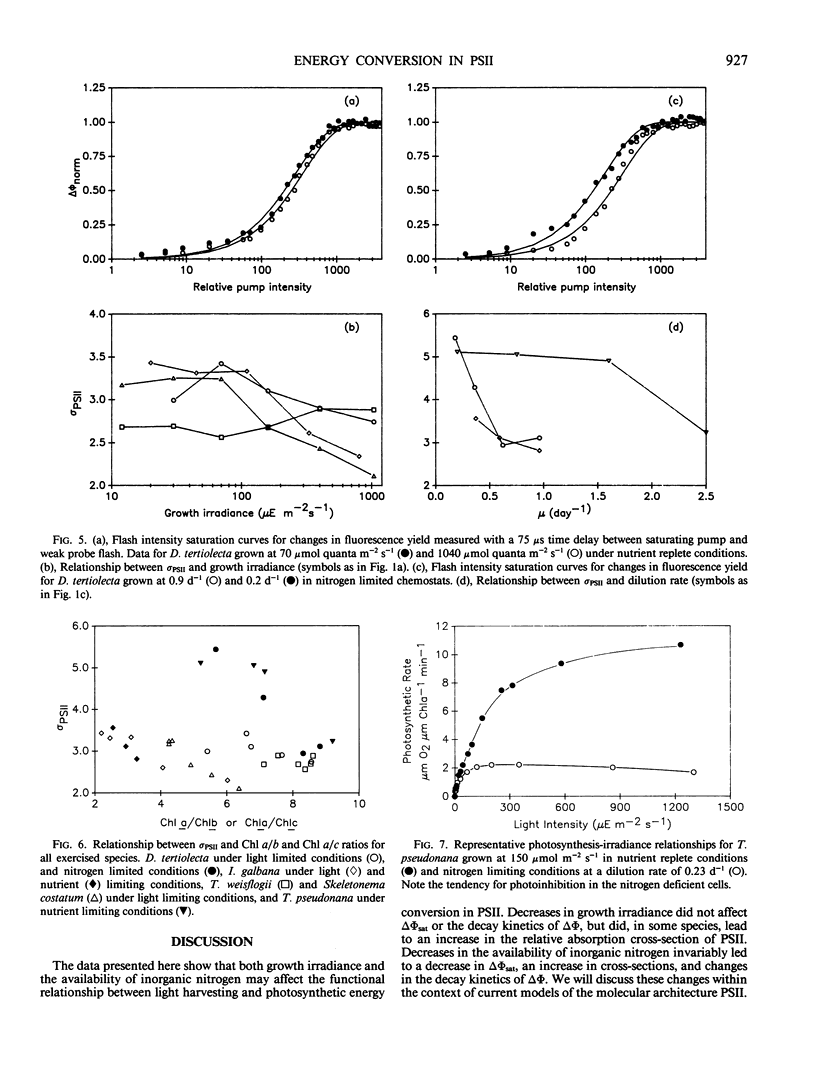
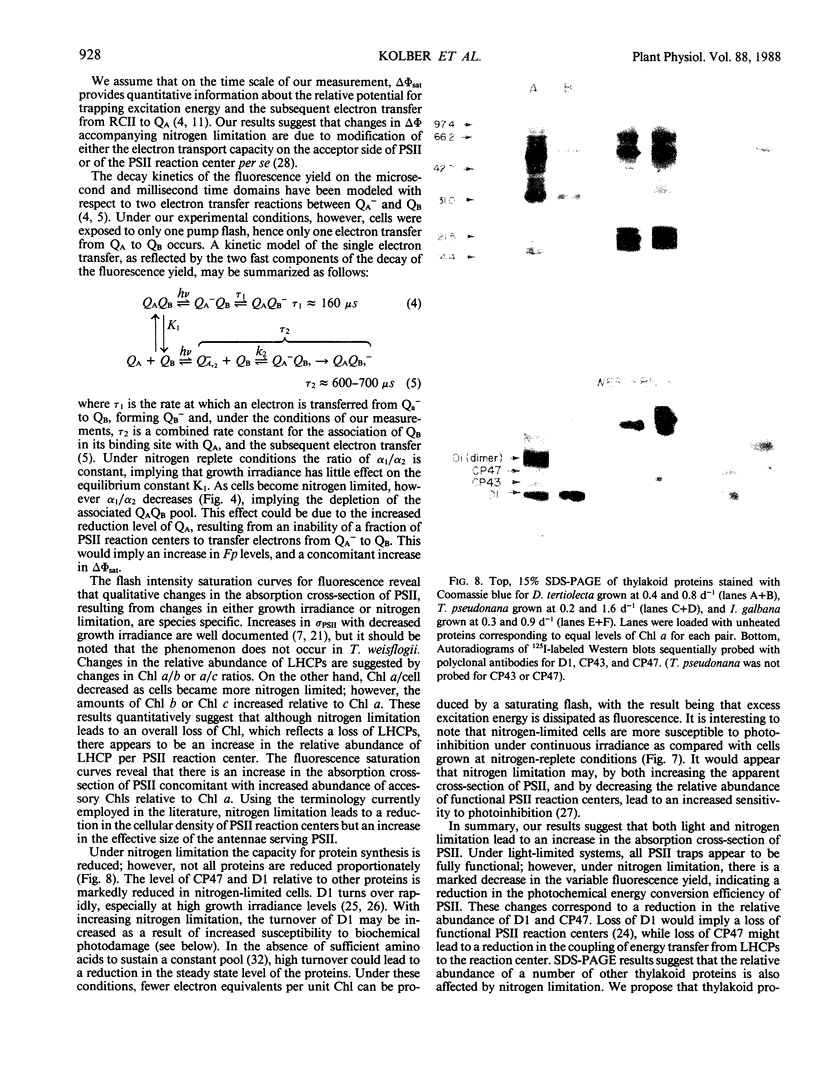
Photosynthetic energy conversion was investigated in five species of marine unicellular algae, (Dunaliella tertiolecta, Thalassiosira pseudonana, T. weisflogii, Skeletorema costatum, Isochrysis galbana) representing three phylogenetic classes, which were grown under steady state conditions with either light or inorganic nitrogen as a limiting factor. Using a pump and probe fluorescence technique we measured the maximum change in variable fluorescence yields, the flash intensity saturation curves for the change in fluorescence yields and the kinetics of the decay in fluorescence yields. Under all growth irradiance levels nutrient replete cells exhibited approximately the same changes in fluorescence yields and similar fluorescence decay kinetics. The apparent relative absorption cross-section of photosystem II, calculated from the slope of the flash intensity saturation curves, generally increased as cells shade adapted. The decay kinetics of the fluorescence yield following a saturating pump flash can be expressed as the sum of three exponential components, with half-times of 160 and 600 microseconds and 30 to 300 milliseconds. The relative contribution of each component did not change significantly with growth irradiance. As cells became more nitrogen limited, however, the maximum change in fluorescence yield decreased, and was accompanied by a decrease in the proportion of a 160 microsecond fluorescence decay component, which corresponds to the transfer of electrons from Qa− to Qb. Changes in fluorescence yields were also accompanied by changes in the levels of D1, a protein which is integral in reaction center II, and CP47, a chlorophyll protein forming part of the core of photosystem II. These results are consistent with a loss of functional photosystem II reaction centers. Moreover, in spite of losses of total cellular chlorophyll, which invariably accompanied nitrogen limitation, the apparent absorption cross-sections of photosystem II increased. Our results suggest that nitrogen limitation leads to substantial decreases in photosynthetic energy conversion efficiency.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Butler W. L., Kitajima M. Fluorescence quenching in photosystem II of chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):116–125. doi: 10.1016/0005-2728(75)90210-8. [DOI] [PubMed] [Google Scholar]
- Demmig B., Winter K., Krüger A., Czygan F. C. Photoinhibition and zeaxanthin formation in intact leaves : a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987 Jun;84(2):218–224. doi: 10.1104/pp.84.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski P. G., Owens T. G., Ley A. C., Mauzerall D. C. Effects of growth irradiance levels on the ratio of reaction centers in two species of marine phytoplankton. Plant Physiol. 1981 Oct;68(4):969–973. doi: 10.1104/pp.68.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUILLARD R. R., RYTHER J. H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Can J Microbiol. 1962 Apr;8:229–239. doi: 10.1139/m62-029. [DOI] [PubMed] [Google Scholar]
- Karukstis K. K., Sauer K. The effects of cation-induced and pH-induced membrane stacking on chlorophyll fluorescence decay kinetics. Biochim Biophys Acta. 1985 Mar 13;806(3):374–388. doi: 10.1016/0005-2728(85)90245-2. [DOI] [PubMed] [Google Scholar]
- King J., Khanna V. A Nitrate Reductase-less Variant Isolated from Suspension Cultures of Datura innoxia (Mill.). Plant Physiol. 1980 Oct;66(4):632–636. doi: 10.1104/pp.66.4.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Light-induced fluorescence changes in Chlorella, and the primary photoreactions for the production of oxygen. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1358–1362. doi: 10.1073/pnas.69.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I., Kyle D. J., Hirschberg J. Light-dependent degradation of the Q(B)-protein in isolated pea thylakoids. EMBO J. 1985 Jul;4(7):1655–1659. doi: 10.1002/j.1460-2075.1985.tb03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



