Abstract
Background
Cocaine’s increase of dopamine is strongly associated with its reinforcing properties and, thus, agents that reduce dopamine have received much attention as candidate cocaine-dependence treatments. The potential efficacy of reserpine, a dopamine depletor, for treating cocaine dependence is suggested by both pre-clinical research and a small clinical trial.
Method
One hundred and nineteen participants who met DSM-IV criteria for cocaine dependence were enrolled into this 12-week, double blind, placebo controlled outpatient trial. Participants received either reserpine (0.5 mg/day) or matching placebo. All participants received one hour of manualized individual cognitive behavioral therapy on a weekly basis. Outcome measures included cocaine use as determined by self-report confirmed with urine benzoylecgonine results, cocaine craving, addiction severity index scores, and clinical global impression scores. Safety measures included adverse events, EKGs, vital signs, laboratory tests, and the Hamilton Depression Inventory.
Results
Seventy-nine participants (i.e., 66%) completed the 12-week trial. The safety results suggest that reserpine was safe and well tolerated by the participants. The efficacy measures indicated no significant differences between reserpine and placebo.
Conclusion
These results do not support the efficacy of reserpine as a cocaine dependence treatment.
Keywords: cocaine dependence, reserpine, clinical trial, double-blind
1. Introduction
There is strong evidence that the addictive nature of cocaine stems from its ability to increase dopamine levels dramatically in the mesolimbic reward centers of the central nervous system (Di Chiara and Imperato, 1988). A rapid increase of dopamine and other amines in the synaptic cleft is believed to contribute to cocaine-induced subjective responses such as stimulation, euphoria and craving. Consequently, agents acting on the dopamine system have been widely studied as possible treatments for cocaine dependence. One agent of interest is reserpine, a rauwolfia alkaloid used since the 1950’s for the treatment of hypertension. Reserpine destabilizes the membranes of the vesicles which store catecholamines in neurons (Henry et al., 1994). As a result, the storage vesicles rupture causing the neurotransmitters (e.g., dopamine and norepinephrine) to spill out into the cytoplasm and to be metabolized by the enzyme monoamine oxydase (MAO). No new dopamine is available to be discharged into the synaptic junction until new vesicles become available, which requires protein synthesis and takes several days to weeks. During this period, individuals exposed to reserpine hypothetically would not experience the euphoric effects of cocaine, as it would not be possible for cocaine to augment the synaptic concentration of dopamine in the nucleus accumbens. Consistent with this hypothesized effect, pre-clinical research has found that disruption of vesicular stores of dopamine and norepinephrine using reserpine results in an attenuation of the neurochemical and behavioral responses to cocaine (Florin et al., 1995).
Reserpine has been evaluated in a National Institute on Drug Abuse (NIDA)-sponsored Cocaine Rapid Efficacy and Safety Trial (CREST). The outpatient, 60-participant four-arm study compared the safety and efficacy of reserpine (at a 0.50 mg dose), together with two additional drugs, gabapentin and lamotrigine, against a single unmatched placebo (Berger et al., 2005). The target dose of reserpine (0.50 mg) was selected to minimize potential sedative and dysphoric effects. Analysis of self-report and interviewer-administered instruments suggested that all four study groups decreased their cocaine use and craving. However, only the participants in the reserpine group significantly decreased their cocaine use as assessed by urine benzoylecgonine (BE) levels at endpoint when compared to baseline (two-sided p < 0.05). Some early reports suggested an association between reserpine and depressive episodes (Goodwin & Bunney, 1971; Wendy, 1974; Bant, 1978; Widmer, 1985); thus, depressive symptoms were monitored carefully during the course of the CREST study. The analysis of the Beck Depression Inventory and Hamilton Depression Inventory (HAM-D) revealed no statistically significant changes between baseline and endpoint for any of the four patient groups in the case of the HAM-D, and that all four medication groups improved significantly on Beck Depression Inventory ratings during the course of the study. These findings are consistent with more recent reports that have noted that the causal relationship between the use of reserpine and depression has never been adequately established (Baumeister et al., 2003). Analysis of the safety assessments, including laboratory tests and adverse events (AE) reporting revealed that reserpine was well-tolerated by the participants.
The findings of the CREST study together with the fact that reserpine requires only once-daily dosing make reserpine an attractive pharmacologic treatment. The present study, which included a larger sample and employed a double-blind, placebo controlled design, evaluated the safety and efficacy of reserpine treatment for cocaine dependence. It was predicted that reserpine would prove both safe and effective for cocaine-dependent individuals.
2. Methods
2.1 Participants
Three study sites, located in Boston Massachusetts, Cincinnati Ohio, and Dayton Ohio, recruited participants. Recruitment methods included clinic referrals, flyers, and television, radio, and newspaper advertisements about a treatment study for individuals who frequently use cocaine. All participants were given a thorough explanation of the study and signed an informed consent form that was approved by the Institutional Review Boards and the VA Medical Center Research and Development committees of the participating sites.
Eligible participants were at least 18 years of age and in good physical health as determined by a medical history, physical exam, electrocardiogram, and standard laboratory tests. Participants were required to have at least one positive urine toxicology screen for the cocaine metabolite benzoylecgonine (BE) (i.e., > 300 ng/mL) during the two-week screening period, to meet DSM-IV criteria for cocaine dependence as assessed by the Structured Clinical Interview for DSM-IV (SCID; First,1996), and to be seeking treatment for cocaine dependence. Participants were excluded from the study if they required detoxification from alcohol, met DSM-IV criteria for dependence for any substance other than cocaine, alcohol, nicotine or marijuana, or if they were court-ordered for cocaine-dependence treatment. Other exclusion criteria included any serious psychological disorder requiring ongoing treatment such as psychosis or bipolar disorder, a Hamilton Depression score greater than 15, and a history of suicide attempts or current suicidal ideation. Individuals were also excluded if they were currently taking reserpine, had a medical condition that could be exacerbated by reserpine, were taking a medication that could adversely interact with reserpine, or had a known or suspected hypersensitivity to reserpine. Women were ineligible for the study if they were pregnant or unwilling to use an adequate method of birth control.
One hundred and sixty-six participants who signed consent were not randomized into the trial. The most common reasons for failing to be randomized were failing to attend clinic visits (30%), meeting one of the depression ineligibility criteria (e.g., Hamilton Depression score greater than 15, suicidal ideation, etc; 22%), having a serious medical illness (15%), failing to provide a urine positive for cocaine (8%), reporting no longer being interested in participating (6%), failing to meet criteria for cocaine dependence (5%), and having a serious psychological disorder requiring treatment (5%). The non-randomized participants had significantly fewer years of education compared to the randomized participants (t=3.70, df=280, p<.01) but otherwise did not differ significantly on age, gender, race/ethnicity, marital status, employment status and last 30 days of cocaine use.
2.2 Measures
The primary outcome measure was cocaine non-use days (self-report confirmed or disproved by urine benzoylecgonine (BE) levels) expressed as the weekly proportion of non-use days to the total number of non-missing study days that week; this variable was assumed to be continuously distributed. At each of the thrice weekly visits, a urine sample was collected for BE analysis and the Substance Use Inventory (SUI), a self-report assessment of the participant’s use of drugs of abuse for each day of the study, was completed. The SUI was completed using the Timeline Follow-Back procedure (Sobell and Sobell, 1992; Fals-Stewart, 2000). Proportion of cocaine non-use days was determined using a combination of the self-report and the thrice weekly quantitative measures of urine BE levels. By using the known pharmacokinetics of BE (using a procedure similar to Preston’s rules (Preston et al., 1997)), an attempt was made to confirm whether or not the participant’s self report of cocaine use for each day of the study is correct or not. A summary of the rules used to calculate weekly proportion of non-use days is provided in Table 1. The rules used for this outcome variable are somewhat complicated and are generally incorporated into a computer program (one can be provided by the lead author upon request).
Table 1.
Summary of Scoring Instructions for Calculating Weekly Proportion of Cocaine Non-Use Days
| Measure | Scoring Summary | |
|---|---|---|
| Proportion of Non-Use Days | To calculate the weekly fraction of cocaine non-use days:
|
Secondary outcome measures included quantitative urine BE, cocaine craving, addiction severity, and clinical global impression scores. Cocaine craving was assessed on a weekly basis with the Brief Substance Craving Scale (BSCS) (Mezinskis et al., 1998). The Fifth Edition of the Addiction Severity Index (ASI), a structured clinical interview that yields scores for seven areas of functioning, was administered by a research staff member having a master’s level degree (McLellan et al., 1992) at baseline and study weeks 4, 8, and 12. Both clinician and participant global impression ratings were obtained on a weekly basis using the CGI-Self and the CGI-Observer (Tracy et al., 2000).
The CGI-Self, which instructs the participant to use a seven-point scale to rate the global severity of his or her cocaine dependence symptoms, was collected weekly. The participant’s study physician completed the CGI-Observer on a weekly basis immediately after the participant completed the CGI-Self (Tracy et al., 2000). Like the CGI-Self, the CGI-Observer required the physician to rate the global severity of the participant’s cocaine dependence symptoms on a seven- point scale.
Safety measures included vital signs and adverse events (AEs) assessments obtained weekly, laboratory tests, including renal profile, complete blood count with differential, and urinalysis, completed at baseline and study weeks 4, 8, and 12, and electrocardiograms completed at baseline and study weeks 4 and 12. In addition, a 24-item version of the Hamilton Depression Scale, with the first 21 items being from Williams (Williams, 1988) and three items added to assess additional elements of depression, was completed during study weeks 1, 2, 4, 6, 8, 10, and 12.
2.3 Procedures
Study candidates who were determined by telephone or in person interview to be using cocaine, to be seeking treatment, and to be available to come to the clinic for at least 19 weeks were invited to the clinic to receive an explanation of the study purpose and requirements. Candidates signing informed consent entered the screening and baseline phase which entailed the completion of six clinic visits within two consecutive weeks. Stratified randomization, balancing for gender and self-report of cocaine use (< 18 or ≥ 18 days of use in the last 30 days), was used to assign eligible participants to reserpine or placebo within each study site. All participants were scheduled to receive one tablet per day (i.e., one 0.25 mg tablet of reserpine or one placebo tablet) during dose escalation (week 1) and dose taper (week 13). During weeks 2 through 12, all participants were scheduled to receive two tablets per day (i.e., two placebo tablets or two 0.25 mg tablets of reserpine). When dose reduction was warranted, participants were given one tablet (i.e., one placebo tablet or one 0.25 mg tablet of reserpine, depending upon medication condition). Situations considered indicative of dose intolerance included significant hypotension (i.e., a drop of more than 10 mm Hg on either the diastolic or systolic blood pressure with postural changes) or any other AE that, in the opinion of the study physician, warranted a dose reduction.
During the twelve week active trial participants were scheduled to attend three research visits per week. Study participants received $10 in retail scrip or vouchers per research visit; at the end-of-study evaluation (first visit of week twelve), participants received an additional $25 in retail scrip or vouchers because of the larger assessment burden associated with the visit. A master’s level clinician provided each study participant with an hour of individual cognitive behavioral therapy. Therapy was manual-guided (Brief et al., unpublished). Therapists received didactic training on the manual. They were then certified by the therapy training center after a successful review of audiotapes from a training case. All therapy sessions during the study were audio-taped to monitor adherence to the manual-guided therapy. One therapy session, per month, per therapist was reviewed by the training center and scored using an adherence checklist. Feedback was provided to the therapist and additional tapes were requested if there was drift from the manual. In addition, a senior therapist at each site also provided supervision via case note review and audiotape review.
2.4 Specimen Analysis
Urine BE analysis was performed by Northwest Toxicology (Salt Lake City, Utah). Urine samples were assessed for BE with an immunoassay using the Roche Online® reagents and the Hitachi 717 Boehringer Mannheim automated chemistry analyzer.
2.5 Data Analysis
Per study protocol, the primary outcome measure, the weekly mean proportion of cocaine non-use days following medication titration (weeks 2-12), was compared between treatment groups using Generalized Estimating Equations (GEE). All secondary efficacy analyses, as well as the analysis of two safety measures, vital signs and the Hamilton Depression scale, were conducted with a GEE model that included the study data from weeks 1 through 12. For these exploratory analyses, we utilized a model that included baseline as a covariate and the following terms: Medication, Week, and a Medication by Week interaction effect. A significant Medication by Week interaction effect suggests a significant difference in slope over time between the reserpine and placebo groups and, in the absence of a Medication by Week interaction effect, a significant Medication effect suggests an absolute difference between the two groups; thus, both effects are of interest in evaluating the effects of reserpine, compared to placebo, on outcome. Approximately 28% of the data were missing for this twelve week trial, with 17% missing due to participant drop out and 11% missing due to intermittently missed study visits. The GEE analyses used all available data without imputing missing data.
AEs included all untoward events reported by the participants as well as significant changes in laboratory values and vital signs. AEs were tabulated by body system and preferred term, seriousness, and relationship to study medication. The frequencies of AEs by type were compared between treatment groups using Fisher’s exact test.
3. Results
3.1 Sample Characteristics
The sample characteristics are given in Table 2. The study sample primarily consisted of African American male crack users who were approximately 40 years of age. About half worked full or part time. They were generally high school graduates. Approximately half of the group were or had been married. Chi-Square analyses conducted for the categorical variables revealed that at baseline, there were no significant differences between the study groups. Independent t-tests for the continuous variables revealed that the placebo group had a significantly higher ASI psychiatric score compared to the reserpine group (t=−2.56, df=112, p<.05); all other comparisons were non-significant.
Table 2.
Participant Demographic and Baseline Characteristics
| Variable | Reserpine (N=60) | Placebo (N=59) | X2 or t (df), | p |
|---|---|---|---|---|
| Age (Years) | 41.2 (7.4) | 40.7 (7.9) | 0.40 (117) | .64 |
| Sex (% Male) | 71.6 | 69.5 | 0.07 (1) | .79 |
| Race/Ethnicity (%) | ||||
| African-American | 70.0 | 79.7 | ||
| Caucasian | 25.0 | 11.8 | 3.41 (1)* | .07 |
| Hispanic | 0.0 | 1.7 | ||
| Other | 5.0 | 6.8 | ||
| Marital Status (%) | ||||
| Married/Cohabitating | 20.3 | 22.1 | ||
| Separated/Divorced | 37.3 | 25.4 | 2.01 (2) | .37 |
| Not Married | 42.4 | 52.5 | ||
| Education (Years) | 13.0 (1.9) | 13.0 (2.2) | −0.04 (117) | .97 |
| Employed Full/Part time (%) | 58.3 | 45.7 | 1.88 (1) | .17 |
| Years of Cocaine use | 14.3 (7.7) | 13.2 (7.4) | 0.78 (116) | .43 |
| Days of Cocaine use (past 30) | 16.7 (8.4) | 15.2 (8.5) | 0.96 (116) | .34 |
| Administration Route (%) | ||||
| Smoked | 97 | 100 | 2.00 (1) | .16 |
| Intranasal | 3 | 0 | ||
| ASI Composite | ||||
| Medical | .22 (0.3) | .16 (0.3) | 1.05 (114) | .30 |
| Employment | .57 (0.3) | .65 (0.3) | −1.45 (112) | .15 |
| Alcohol | .20 (0.2) | .20 (0.2) | 0.03 (111) | .98 |
| Drug | .24 (0.1) | .25 (0.1) | −0.37 (113) | .71 |
| Legal | .07 (0.1) | .08 (0.1) | −0.34 (111) | .73 |
| Family/Social | .17 (0.2) | .21 (0.2) | −0.99 (109) | .32 |
| Psychiatric | .05 (0.1) | .11 (0.1) | −2.56 (112) | .01 |
Note. Where not specifically indicated, numbers represent means (standard deviations).
Comparing Caucasian to minority participants.
3.2 Retention
A total of 79 study participants (66%) completed the 12 week active study phase. Study completion rates did not differ significantly between the reserpine (70%) and placebo (63%) treatment groups (X2=0.71, df=1, p>.05). Eighty percent of early terminations resulted from participants failing to attend the clinic visits, with no significant difference between the reserpine (13/60, 22%) and placebo (19/59, 32%) groups (X2=1.68, df=1, p>.05). Fifteen percent of early terminations were participants who withdrew consent, with no significant difference between the reserpine (4/60, 7%) and placebo (2/59, 4%) groups (X2=0.67, df=1, p>.05). Finally, five percent of early terminations resulted from incarceration, with one participant from each group being incarcerated. No participant discontinued the study due to an adverse event.
3.3 Medication Compliance
Medication compliance was assessed via pill count using the procedures outlined by Gorelick and Wilkins (2006). Specifically, medication compliance was calculated by taking the number of tablets dispensed minus the number returned or reported lost divided by the number of tablets prescribed to be taken; this yielded a compliance score between 0 and 1, with 1 representing perfect compliance. In cases where participants failed to return their medication bottles, it was assumed that the participant took zero of the doses. The average compliance score for the reserpine group, .79 (SD=.23), was not significantly different from the placebo group compliance score of .74 (SD=.34), (t=1.13, p=.26).
3.4 Efficacy Analyses
3.4.1 Weekly proportion of cocaine non-use days
The placebo group had a higher average of non-use days (6%) than the reserpine group at baseline. The placebo group again had a higher average of non-use days (7%) than the reserpine group across the treatment period. Differences in the linear slope over the treatment period (weeks 2–12) of weekly mean proportion of non-use days were compared between treatment groups by GEE. Fitting allowed for differences in mean proportions at baseline (intercept). The two fitted GEE lines for two groups were almost parallel over the treatment period. No difference was detected between slopes of the reserpine group and the placebo group (GEE, p= 0.45). Figure 1 provides a plot of the mean proportion of cocaine non-use days as a function of treatment group and study week.
Figure 1.
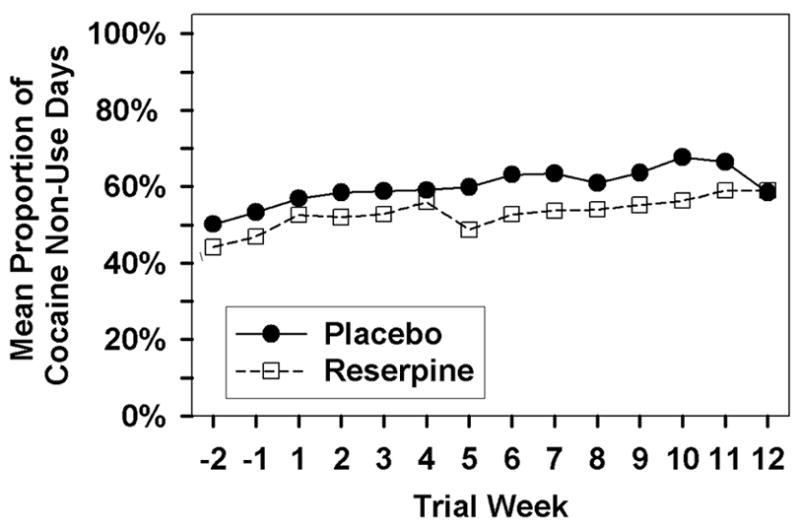
Mean proportion of cocaine non-use days as a function of medication group and study week
3.4.2 Urine BE
For the log10 urine BE results, the GEE analysis did not reveal significance for either effect of interest, the Medication by Week interaction (Z=0.62, p>.05) or the Medication effect (Z=0.89, p>.05), nor did it find significance for the Week effect (Z=−1.94, p>.05). The mean log10 urine BE as a function of treatment group and study week is displayed in Figure 2. These results suggest that neither the reserpine nor the placebo groups had a significant change in urine BE levels during the study.
Figure 2.
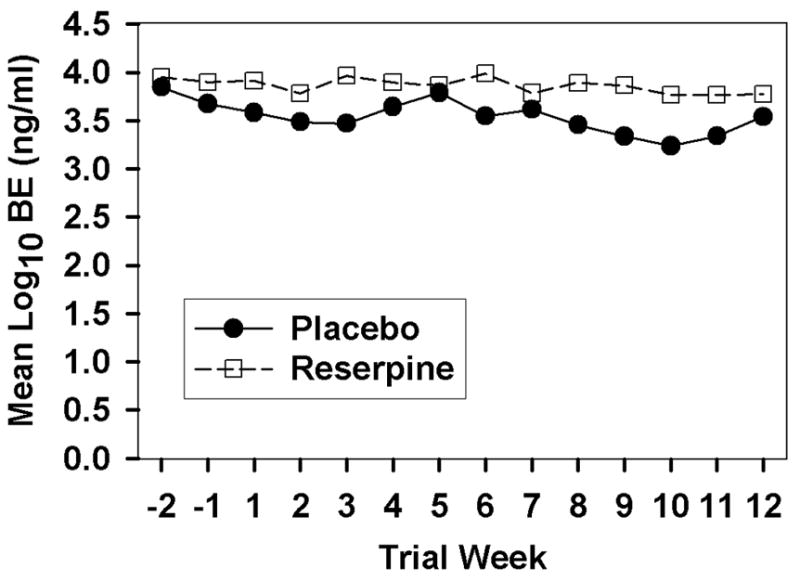
Mean Urine BE level as a function of medication group and study week.
3.4.3 Brief Substance Craving Scale
Average BSCS score as a function of medication group and study week is provided in Table 3. A review of Table 3 suggests that both groups reported less craving over the course of the study, which is consistent with the significant Week effect (Z=−3.76, p<.01) yielded from the GEE analysis. The analysis revealed that there was no significant difference between the groups as indicated by the non-significant Medication by Week (Z=−1.15, p>.05) and Medication (Z=−0.32, p>.05) effects.
Table 3.
Results from the BSCS, CGI-S, CGI-O, ASI – Drug Composite Score, and HAM-D Total, as a function of medication group and study week, means shown with (standard deviations)
| Week | BSCS | CGI-S | CGI-O | ASI-Drug | HAM-D | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reserpine | Placebo | Reserpine | Placebo | Reserpine | Placebo | Reserpine | Placebo | Reserpine | Placebo | |||||||||||
| n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | n | X (SD) | |
| Baseline 1 | 57 | 6.31 (2.66) |
58 | 5.93 (2.75) |
56 | 4.74 (1.66) |
57 | 4.58 (1.45) |
57 | 4.90 (1.16) |
57 | 4.91 (0.99) |
||||||||
| Baseline 2 | 58 | 5.86 (2.85) |
55 | 5.62 (2.86) |
58 | 4.24 (1.51) |
56 | 4.25 (1.44) |
58 | 4.71 (1.21) |
56 | 4.59 (1.02) |
58 | 0.24 (0.09) |
57 | 0.25 (0.09) |
60 | 4.43 (4.07) |
59 | 4.31 (3.81) |
| 1 | 60 | 5.09 (2.91) |
58 | 5.29 (2.89) |
60 | 4.21 (1.43) |
59 | 4.19 (1.38) |
60 | 4.32 (1.08) |
59 | 4.53 (0.92) |
56 | 3.00 (3.18) |
53 | 3.59 (3.39) |
||||
| 2 | 53 | 4.34 (2.88) |
52 | 4.48 (2.89) |
52 | 4.12 (1.45) |
52 | 3.57 (1.59) |
51 | 4.35 (0.96) |
50 | 3.83 (1.20) |
43 | 2.16 (2.25) |
48 | 2.81 (2.65) |
||||
| 3 | 51 | 4.43 (2.5) |
47 | 4.84 (2.65) |
52 | 3.96 (1.53) |
44 | 3.52 (1.34) |
52 | 4.17 (1.11) |
44 | 3.77 (1.22) |
||||||||
| 4 | 51 | 4.45 (2.86) |
45 | 4.31 (2.86) |
51 | 3.77 (1.32) |
46 | 3.46 (1.49) |
48 | 4.02 (1.04) |
46 | 3.81 (1.21) |
50 | 0.20 (0.08) |
46 | 0.19 (0.10) |
44 | 2.84 (3.13) |
44 | 3.18 (3.52) |
| 5 | 46 | 3.91 (2.41) |
44 | 4.21 (2.79) |
45 | 3.53 (1.14) |
43 | 3.31 (1.60) |
46 | 3.87 (1.05) |
42 | 3.64 (1.25) |
||||||||
| 6 | 47 | 4.33 (2.68) |
38 | 4.23 (3.29) |
48 | 3.61 (1.21) |
36 | 3.30 (1.51) |
47 | 3.84 (1.23) |
36 | 3.62 (1.40) |
39 | 1.97 (2.15) |
30 | 3.10 (3.00) |
||||
| 7 | 40 | 3.95 (2.60) |
36 | 3.72 (3.13) |
40 | 3.48 (1.24) |
35 | 3.43 (1.56) |
39 | 3.90 (1.19) |
35 | 3.66 (1.28) |
||||||||
| 8 | 41 | 3.76 (2.84) |
37 | 3.76 (3.11) |
39 | 3.45 (1.20) |
37 | 3.37 (1.67) |
39 | 3.72 (1.17) |
36 | 3.56 (1.23) |
40 | 0.16 (0.09) |
33 | 0.15 (0.11) |
36 | 2.14 (2.45) |
31 | 3.03 (3.84) |
| 9 | 38 | 2.97 (2.40) |
34 | 3.79 (3.26) |
37 | 3.88 (1.01) |
31 | 3.13 (1.50) |
39 | 3.74 (1.34) |
29 | 3.52 (1.15) |
||||||||
| 10 | 40 | 3.38 (2.56) |
36 | 3.73 (3.06) |
37 | 3.22 (1.27) |
35 | 3.14 (1.42) |
37 | 3.95 (1.43) |
34 | 3.50 (1.05) |
26 | 2.04 (2.89) |
32 | 4.19 (4.32) |
||||
| 11 | 38 | 2.58 (2.17) |
33 | 3.76 (2.77) |
37 | 2.95 (1.45) |
31 | 3.13 (1.31) |
37 | 3.48 (1.39) |
32 | 3.64 (1.24) |
||||||||
| 12 | 40 | 2.80 (2.45) |
32 | 3.44 (2.76) |
37 | 3.03 (1.38) |
30 | 3.10 (1.21) |
34 | 3.35 (1.45) |
29 | 3.55 (0.91) |
35 | 0.15 (0.10) |
29 | 0.15 (0.08) |
36 | 2.11 (3.04) |
28 | 3.18 (3.30) |
Note. CGI-S = Clinical Global Impressions Scale – Self severity rating; CGI-O = Clinical Global Impressions Scale – Observer severity rating; BSCS = Brief Substance Craving Scale; ASI-Drug = Addiction Severity Index, Drug Composite Score; HAM-D-Total = Hamilton Depression Scale total score
3.4.4 Clinical Global Impression Scales
Table 3 shows the average scores from the CGI-S, which was completed by the participant, and the CGI-O, which was completed by the study physician, as a function of medication group and study week. The GEE analyses of these measures yielded similar results, with no significant Medication by Week interaction for the CGI-S (Z=−1.09, p>.05) or CGI-O (Z=−0.33, p>.05) and no significant Medication effect for CGI-S (Z=1.68, p>.05) or CGI-O (Z=1.11, p>.05). However there was a significant Week effect for the CGI-S (Z=−4.74, p<.01) and CGI-O (Z=−4.07, p<.01), suggesting that both treatment groups experienced significant improvement in global functioning during the course of the study.
3.4.5 Addiction Severity Index-Drug Composite
Average ASI drug composite score as a function of medication group and study week is provided in Table 3. A review of Table 3 might suggest that both groups improved over the course of the study; however, the GEE analysis revealed no statistically significant effects for the Medication by Week interaction (Z=−.017, p>.05), Medication (Z=0.72, p>.05) or Week (Z=−1.95, p>.05) effects.
3.5 Safety
No pattern of physical or laboratory abnormality attributable to treatment with reserpine was discovered. The GEE analysis of pulse revealed no statistically significant effects. The GEE analyses of systolic and diastolic blood pressure revealed a significant Medication effect (Z=−3.04, p<.01; Z=−2.87, p<.01); all other effects were non-significant. The regression plots (data not shown) display a significant decrease in systolic and diastolic blood pressure for the reserpine, compared to placebo, participants. The decreases were not clinically significant and were expected based on reserpine’s anti-hypertensive properties. For systolic blood pressure, the placebo participants showed an average increase of 0.84 mm/Hg (SD=6.56) while the reserpine participants had an average decrease of 3.71 mm/Hg (SD=7.76). For diastolic blood pressure, the placebo participants showed an average decrease of 0.25 mm/Hg (SD=4.98) while the reserpine participants had an average decrease of 3.42 mm/Hg (SD=6.02). The average HAM-D total score as a function of medication group and study week is provided in Table 3. A review of Table 3 might suggest that both groups improved over the course of the study; however, the GEE analysis revealed no statistically significant effects for Medication by Week interaction (Z=−.079, p>.05), Medication (Z=−0.88, p>.05) or Week (z=0.22, p>.05).
The medication was safe and well tolerated by the participants. Two serious adverse events (SAEs) were reported: a participant in the placebo group checked himself into a detoxification center and a participant in the reserpine group was admitted to the hospital for knee surgery after tripping and injuring his knee. These two SAEs were determined to be unrelated to the study medication. Five reserpine participants were discontinued from medication due to AEs: two became pregnant, one reported upper abdominal pain, one participant developed postural hypotension, and one participant developed ECG abnormalities. In the latter case, the participant’s ECG showed a ventricular conduction delay, which he had experienced a few years before at which point it had been attributed to his cocaine use. A study cardiologist determined that the ECG changes were not life threatening or an immediate emergency and that it was consistent with Wolf Parkinson White (WPW), a pre-existing congenital cardiac condition. The participant was discontinued from the study medication because of the concern about its effects on the pre-existing cardiac condition. The postural hypotension participant reported an onset of symptoms after having been on study medication for 48 days and continued to experience the hypotension until after the medication was discontinued 13 days later; the postural hypotension resolved within five days after medication discontinuation. One placebo participant was discontinued from medication due to the development of depression. Two reserpine participants had a dose reduction, one for drowsiness and one for light headedness. Fisher Exact tests revealed that reserpine, compared to placebo, participants were more likely to experience treatment-emergent respiratory (p=0.003) and nasal congestion (p=0.002) AEs.
4. Discussion
This 12-week double-blind, randomized clinical trial of reserpine, compared to placebo, failed to show reserpine as effective for treating cocaine dependence. These findings differ from those of an earlier, small pilot study in which a trend for reserpine’s efficacy was found (Berger et al., 2005). The present study was quite similar to the pilot study in that the same dose of reserpine was used, many of the study procedures were the same (e.g., both studies included thrice weekly visits, had similar schedules of assessments, etc.), many of the same investigators worked on both studies, and the Cincinnati site at which the pilot study was completed was a site in the present study. There were some potentially important differences between the present sample and the Berger et al. (2005) sample in terms of cocaine use, including the present sample having significantly more years of cocaine use (Mean = 13.7 (SD=7.5) compared to the Berger et al. (2005) sample (Mean=10.7 (SD=6.2), t=2.7, df=176, p<.01). In addition, there was a trend for the present sample to have fewer number of cocaine use days in the past 30 (Mean = 16.0 (SD=8.4)) compared to the Berger et al. (2005) sample (Mean = 18.5 (SD=8.3); t=−1.9, df=176, p=0.055). Perhaps differences in the study samples account for the difference in findings. Alternatively, given the fact that the earlier trial did not include a matching placebo control and included only 15 participants per arm, the trend observed in the earlier pilot study might represent a Type I error.
The present study had several strengths. First, this trial utilized a randomized, double-blind, placebo controlled design, which is the gold-standard in clinical trials. Second, power analyses conducted prior to study initiation revealed that 34 participants in each treatment group would need to complete the study in order to detect a 12% increase in the proportion of cocaine non-use days within the reserpine group compared to the placebo group with a power of 80% at a 5% Type I error rate. In the present study, 42 reserpine and 37 placebo participants completed the study and, thus, the trial was adequately powered. Third, the medication compliance rate was fairly high and even higher than the 71.5% compliance rate for the earlier pilot study. In addition, it is important to note that partial compliance with reserpine dosing might be sufficient for evaluating its efficacy since the effects of reserpine in irreversibly destabilizing CNS vesicle membranes persists for a prolonged period of time (Giachetti & Shore, 1978). For example, the effects on hypertension last from one to six weeks (Gilmore, 1971). A final strength is that the study was conducted at multiple sites, which helps to ensure the generalizability of the results.
One potential weakness of the present study was the dose of reserpine evaluated, 0.50 mg/day. In the treatment of psychiatric disorders, reserpine dose can range up to 1.0 mg depending on the patient’s response. The 0.50 mg/day dose used in the present study was selected to limit the sedative effects of reserpine. However, it is possible that reserpine at a higher dose might have evidenced efficacy for cocaine dependence.
The results of the current study suggest that reserpine is not effective for treating cocaine dependence. However, investigators might want to consider reserpine as a candidate medication for methamphetamine dependence. First, as has been noted elsewhere (Vocci & Ling, 2005), cocaine and methamphetamine have important differences in brain mechanism, with methamphetamine releasing dopamine through its action on vesicular monoamine transporters. Since reserpine binds irreversibly to a vesicular transporter protein, thereby disrupting storage of catecholamines (dopamine and norepinephrine) and serotonin (Henry et al., 1994), it might serve to block the ability of methamphetamine to release dopamine. A second interesting property of reserpine, in regard to methamphetamine, is that reserpine was one of the first antipsychotic drugs and has been found, at least in case studies, to treat PCP-induced psychosis (Berlant, 1985). This property is of interest since methamphetamine can induce psychosis, which is typically of short duration, but, in some cases, can persist (Vocci & Ling, 2005). Third, research suggests that methamphetamine may cause long-term neuronal damage (Maxwell, 2005). It has been found that methamphetamine causes the production of free radicals in rats but that this action is completely blocked by reserpine (Pubill, et al., 2005); thus, reserpine might prove to be protective against neuronal damage. Fourth, methamphetamine has been associated with severe cardiovascular events (Vocci & Ling, 2005), and methamphetamine might be even more likely than cocaine to result in cardiotoxicity and hypertension given its longer half-life (Johnson et al., 2005). The antihypertensive properties of reserpine, thus, might be of benefit for individuals who are addicted to methamphetamine. When all these qualities are considered, together with the fact that we have found reserpine to be safe for use in cocaine dependent patients in the present study as well as in our earlier study (Berger et al., 2005), we feel that it might be worthwhile to test its efficacy and safety for methamphetamine dependence.
Acknowledgments
This study was supported by the National Institutes of Health, National Institute on Drug Abuse through contract N01-DA-9-8095 (E. Somoza). We wish to thank Emily DeGarmo, BS, Jacqueline Carter, BS, R. Daniel Alfaro, M.D., Gordon Mindrum M.D., Roberto Soria, M.D., Paul Horn, Ph.D., Sara Jones, Ph.D., Frankie Kropp, M.S., Barbara Thomas, M.A., Amanda Stein, B.A., Julie Jansen, B.A., Eva Moore, Courtney Richambault B.A., Laurie Sickles R.N., Joanna Piechniczek-Buczek, M.D., and V. Chowdary Jampala, MBBS for their expert technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bant WP. Antihypertensive drugs and depression: A reappraisal. Psychol Med. 1978;8:275–283. doi: 10.1017/s003329170001432x. [DOI] [PubMed] [Google Scholar]
- Baumeister AA, Hawkins MF, Uzelac SM. The myth of reserpine-induced depression: role in the historical development of the monoamine hypothesis. J Hist Neurosci. 2003;12(2):207–20. doi: 10.1076/jhin.12.2.207.15535. [DOI] [PubMed] [Google Scholar]
- Berlant JL. Reserpine and phencyclidine-associated psychosis: three case reports. J Clin Psychiatry. 1985;46(12):542–4. [PubMed] [Google Scholar]
- Berger P, Winhusen T, Somoza E, Harrer J, Mezinskis J, Leiderman D, Montgomery M, Goldsmith R, Bloch D, Singal B, Elkashef A. A medication screening trial evaluation of reserpine, gabapentin, and lamotrigine pharmacotherapy of cocaine dependence. Addiction. 2005;100 (Suppl 1):58–67. doi: 10.1111/j.1360-0443.2005.00983.x. [DOI] [PubMed] [Google Scholar]
- Brief DJ, Bollinger AR, Horton GE, LoCastro JS. Integrated Treatment for Addictions: Education, Motivational Enhancement, and Skills Building for Alcohol & Drug Addiction. 2000 Unpublished manuscript. [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5(2):181–5. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68:134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Florin SM, Kuczenski R, Segel DS. Effects of reserpine on extracellular caudate dopamine and hippocampus norepinephrine responses to amphetamine and cocaine: mechanistic and behavioral considerations. J Pharmacol Exp Ther. 1995;274:231–241. [PubMed] [Google Scholar]
- Giachetti A, Shore PA. The reserpine receptor. Life Sci. 1978;23:89–92. doi: 10.1016/0024-3205(78)90254-0. [DOI] [PubMed] [Google Scholar]
- Gilmore HR. The treatment of chronic hypertension. Med Clin North Am. 1971;55:317–324. doi: 10.1016/s0025-7125(16)32522-6. [DOI] [PubMed] [Google Scholar]
- Goodwin FK, Bunney WE. Depressions following reserpine: A reevaluation. Semin Psychiatry. 1971;3:435–448. [PubMed] [Google Scholar]
- Gorelick DA, Wilkins JN. Bromocriptine treatment for cocaine addiction: Association with plasma prolactin levels. Drug Alcohol Depend. 2006;81:189–195. doi: 10.1016/j.drugalcdep.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Henry JP, Botton D, Sagne C, Isambert MF, Desnos C, Blanchard V, Raisman-Vozari R, Krejci E, Massoulie J, Gasnier B. Biochemistry and molecular biology of the vesicular monoamine transporter from chromaffin granules. J Exp Biol. 1994;196:251–62. doi: 10.1242/jeb.196.1.251. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Wells LT, Roache JD, Wallace C, Ait-Daoud N, Wang Y. Isradipine decreases the hemodynamic response of cocaine and methamphetamine results from two human laboratory studies: results from two human laboratory studies. Am J Hypertens. 2005;18(6):813–22. doi: 10.1016/j.amjhyper.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Maxwell JC. Emerging research on methamphetamine. Curr Opin Psychiatry. 2005;18(3):235–42. doi: 10.1097/01.yco.0000165592.52811.84. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mezinskis J, Dryenforth S, Goldsmith R, Cohen R, Somoza E. Craving and withdrawal symptoms for various drugs of abuse. Psychiatr Ann. 1998;28:577–583. [Google Scholar]
- Preston KL, Silverman K, Schuster CR, Cone EJ. Assessment of cocaine use with quantitative urinalysis and estimation of new uses. Addiction. 1997;92:717–727. [PubMed] [Google Scholar]
- Pubill D, Chipana C, Camins A, Pallas M, Camarasa J, Escubedo E. Free radical production induced by methamphetamine in rat striatal synaptosomes. Toxicol Appl Pharmacol. 2005;204(1):57–68. doi: 10.1016/j.taap.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell M. Timeline follow back: A technique for assessing self-reported ethanol consumption. In: Allen J, Lit-ten R, editors. Techniques to Assess Alcohol Consumption. Humana Press Inc.; New Jersey: 1992. pp. 19–28. [Google Scholar]
- Tracy K, Baker S, LoCastro J, Mezinskis J, Simon S, Somoza E. The Substance Clinical Global Impression (SCGI) Scale: measuring global functioning in substance related clinical trials. NIDA Res Monogr. 2000;180 [Google Scholar]
- Vocci F, Ling W. Medications development: successes and challenges. Pharmacol Ther. 2005;108(1):94–108. doi: 10.1016/j.pharmthera.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Wendy B. Do antihypertensive drugs really cause depression? J R Soc Med. 1974;67:919–923. doi: 10.1177/003591577406700937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmer RB. Reserpine: The maligned antihypertensive drug. J Fam Pract. 1985;20:81–83. [PubMed] [Google Scholar]
- Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–747. doi: 10.1001/archpsyc.1988.01800320058007. [DOI] [PubMed] [Google Scholar]


