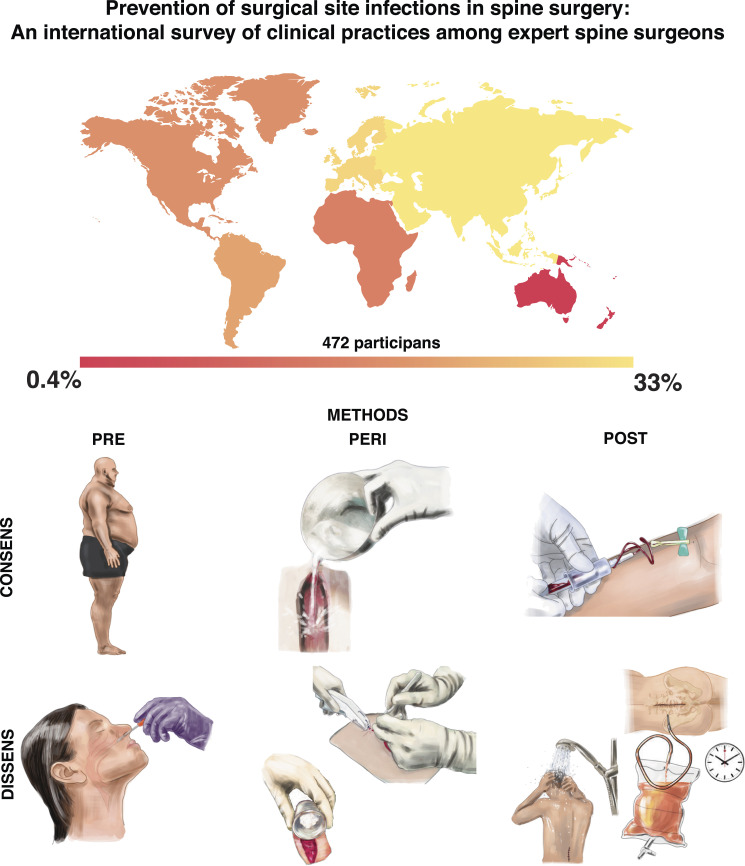
Abstract
Study Design
Questionnaire-based survey.
Objectives
Surgical site infection (SSI) is a common complication in spine surgery but universal guidelines for SSI prevention are lacking. The objectives of this study are to depict a global status quo on implemented prevention strategies in spine surgery, common themes of practice and determine key areas for future research.
Methods
An 80-item survey was distributed among spine surgeons worldwide via email. The questionnaire was designed and approved by an International Consensus Group on spine SSI. Consensus was defined as more than 60% of participants agreeing to a specific prevention strategy.
Results
Four hundred seventy-two surgeons participated in the survey. Screening for Staphylococcus aureus (SA) is not common, whereas preoperative decolonization is performed in almost half of all hospitals. Body mass index (BMI) was not important for surgery planning. In contrast, elevated HbA1c level and hypoalbuminemia were often considered as reasons to postpone surgery. Cefazoline is the common drug for antimicrobial prophylaxis. Alcohol-based chlorhexidine is mainly used for skin disinfection. Double-gloving, wound irrigation, and tissue-conserving surgical techniques are routine in the operating room (OR). Local antibiotic administration is not common. Wound closure techniques and postoperative wound dressing routines vary greatly between the participating institutions.
Conclusions
With this study we provide an international overview on the heterogeneity of SSI prevention strategies in spine surgery. We demonstrated a large heterogeneity for pre-, peri- and postoperative measures to prevent SSI. Our data illustrated the need for developing universal guidelines and for testing areas of controversy in prospective clinical trials.
Graphical Abstract.
Introduction
Health care-associated infections (HAI) with estimated point-prevalence of 721 000 in the United States (US) and estimated annual number of HAI of 4 422 629 in the European Union place a heavy burden on patients, healthcare stakeholders and society.1,2 Surgical site infections (SSI) are ranked the number one type of HAI accounting for 21.8% of all HAI in the US with estimated cost attributed to SSI averaged at $US20.785 and length of hospital stay of 11.2 days.1,3 Postoperative SSI is one of the most common complications after instrumented spinal fusion. The complication rate ranges from 0.2% to 16.7% depending on predictive variables including patient characteristics, operative procedures, and other perioperative interventions.4-6
Different organizations are aiming to improve healthcare and patient safety such as the National Quality Forum, the World Health Organization (WHO), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), or The Joint Commission. They are trying to tackle the preventable harm of HAI with evidence-based prevention strategies. These organizations suggest education of healthcare workers and patients on SSI prevention, measure strategies for regular monitoring and report of SSI rates, implementation of evidence-based SSI prevention actions, administration of perioperative antibiotics and several other pre-, peri- and postoperative strategies to avert SSI.7-13
Currently published prevention strategies in spine surgery include a variety of measures with different levels of evidence and acceptance in the clinical routine (i.e. chlorhexidine gluconate bathing, Staphylococcus aureus (SA) nasal screening and decolonization, surgical dressing care, awareness intervention, self-preparation with chlorhexidine gluconate swabs, storage optimization of supplies, preoperative antibiotics administration algorithm, staff training on betadine scrub and paint, application of vancomycin in instrumented cases, postoperative early mobilization, and wound checks 2 and 6 weeks postoperatively).14-22
The objectives of this study were to depict a global status quo on actually implemented prevention strategies in spine surgery, detect fields of dissence/controversy and form suggestions for future research on this topic by using an international survey.
Materials and Methods
Design of Questionnaire
The team of authors consisting of spinal orthopedics, neurosurgeons, and hospital hygienists designed a survey based on their clinical and scientific experience in the field of spine surgery and infection prevention strategies. Four different types of questions were used: yes-no questions, multiple-choice questions, constant sum questions, and open questions. The survey was subdivided into four sections: (1) general information, (2) preoperative management, (3) perioperative management, and (4) postoperative management. The survey was then reviewed by a clinical epidemiologist on hospital infectious disease control and prevention.
The questionnaire was evaluated by an international team of orthopedic and neurosurgeons with specialization in spine surgery with the possibility of adding or modifying the items. The team consisted of 23 neurosurgeons or orthopedists from Europe (N = 8), North America (N = 9), South America (N = 5), and Asia (N = 3) Their suggestions were implemented and once again reviewed and approved by all authors. The entire questionnaire is attached as Supplementary Material (S1).
Distribution of Questionnaire
After final approval, the questionnaire was designed using SurveyMonkey® (www.surveymonkey.com, Palo Alto, California, USA) and distributed via email to all AO Spine members worldwide, members of the Canadian Spine Society and to members of the Spine Program Surgical Faculty at the University of Toronto. AO Spine is one of the leading global academic communities for innovative education and research in spine care. The questionnaire was available from November 2019 until April 2020, response was anonymous and voluntary.
Data Analysis and Statistics
All data was checked for plausibility and then analyzed by Staburo Ltd. statistics team and descriptive statistics was provided. The answers were presented as total numbers with range or as percentages. If results were given in percentages, they refer to the number of participants who completed the question, not to the number of total responders to the survey.
Results
Response Numbers and Demographics
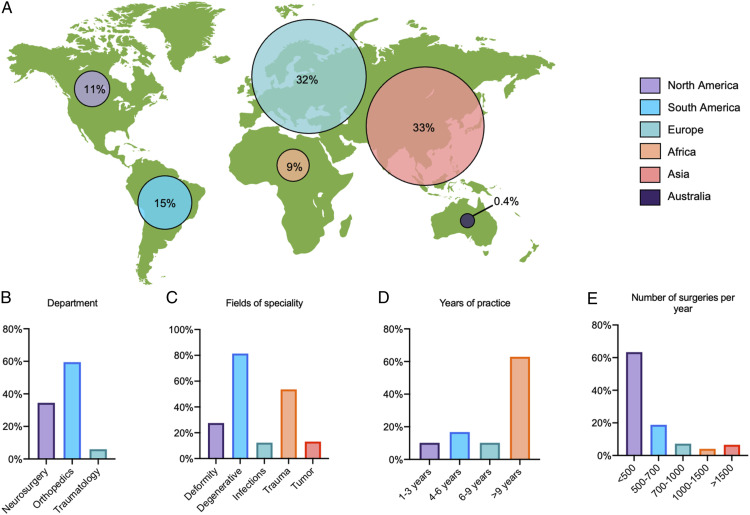
In total, 472 participants provided responses to the questionnaire. Between those participants the completion rate of the whole survey was 61% and the total number of complete responses was 288. The total number of invites was 21 755, HTML open rate was 26.97% and the click-to-open ration was 9.11%. The participants represent the global community of international spine surgery (Figure 1A). Sixty percent were orthopedic surgeons, 34% were neurosurgeons, and 6% were trauma surgeons (Figure 1B). The main field of specialty was degenerative spine surgery (81%) followed by traumatic spine surgery (54%), spine deformity surgery (28%), spinal oncology (13%), and septic spine surgery (12%, Figure 1C). A maximum of two specialties could be selected. Almost 2/3 of the surgeons had an experience of more than 9 years (63%) and 60% received fellowship training in spine surgery (Figure 1D)
Figure 1.
Illustration of demographics and fields of specialization of all participants. (A) Location of the responding spine surgeons by continent in percentages. (B) Department Affiliation of the spine surgeons. (C) Main area of specialization in the field of spine surgery (every surgeon could choose two). (D) Level of experience of the surgeon given in years of practice. (E) Number of spine surgeries performed in the institution of the surgeon per year.
The responder’s affiliations were university, public, or private hospitals in 39%, 33%, and 25%, respectively. More than 2/3 of the participants were working at teaching hospitals (76%). In most hospitals where the participants were practicing, the number of spine cases per year was below 500 (63%), open cases made up more than 80% of all cases compared to 20% of minimal invasive surgery (MIS) cases. Elective cases made up 79% of all cases compared to 21% of emergency cases (Figure 1E). Degenerative pathologies of the spine were the most common indications for surgery (50%), followed by trauma (22%), spinal oncology (13%), and deformity (12%). The SSI rate needing revision surgery was estimated to lie between 0 and 3% in 69% of the participants’ hospitals, followed by 4–7% in 25%.
Illustration of demographics and fields of specialization of all participants. (A) Location of the responding spine surgeons by continent in percentages. (B) Department Affiliation of the spine surgeons. (C) Main area of specialization in the field of spine surgery (every surgeon could choose two). (D) Level of experience of the surgeon given in years of practice. (E) Number of spine surgeries performed in the institution of the surgeon per year.
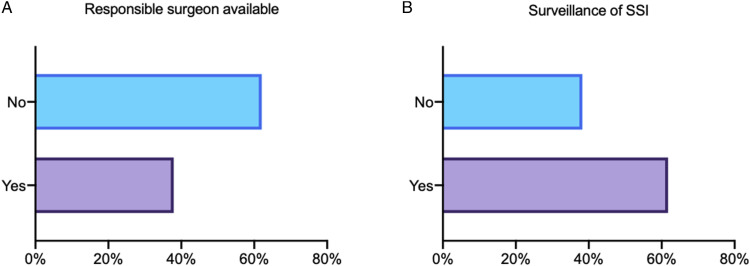
Almost 2/3 stated that there is no designated surgeon in their team who is responsible for SSI prevention issues or received special training in SSI prevention strategies (62%, Figure 2A). More than 1/3 of all responders stated that there are no internal statistics or surveillance routines on SSI rates in spine surgery in their department (38%, Figure 2B).
Figure 2.
Data on surgical site infection (SSI) surveillance and preoperative measures. (A) Is there a trained surgeon in the department dedicated to SSI prevention? (B) Did the responder’s department gather statistics on SSI?
Preoperative Management
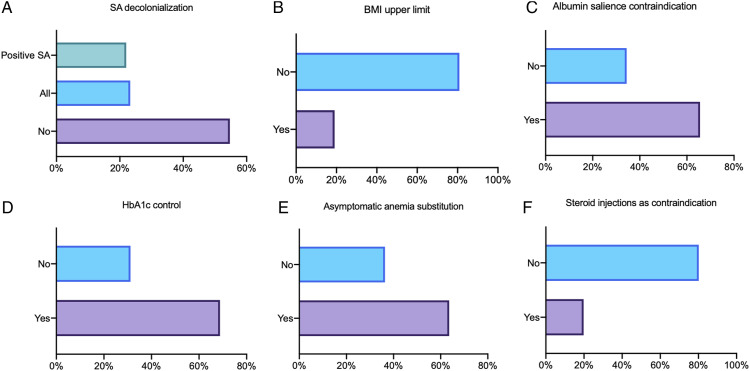
SA screening prior to elective spine surgery is performed only in 26% of cases. In the case of SA screening, 39% perform nasal swabs only and 29% perform nasal and inguinal swabs. Twenty-three percent stated that they perform decolonization in all elective spine cases without screening, whereas 22% decolonize only in case of positive SA screening results (Figure 3A). Decolonization is carried out mostly on the day before surgery plus the day of surgery (38%) at home (28%) or on ward (23%). The most frequently used agent for hair and body decolonization is chlorhexidine-based (65%), followed by beta iodine (27%). Mupirocin is most frequently used for nasal decolonization (21%).
Figure 3.
Preoperative management. (A) Is staphylococcus aureus (SA) decolonization performed prior to surgery? (B) Is there an upper body mass index (BMI) limit for spine surgery? (C) Is surgery postponed in case of albumin salience? (D) Is HbA1c controlled prior to surgery in diabetic patients? (E) Is asymptomatic anemia addressed prior to elective spine surgery? (F) Is elective surgery postponed if the patient had received steroid injections?
There was no upper BMI limit for elective spine surgeries (81%, Figure 3B). In the 19% of hospitals with a BMI limit, the average BMI for postponing elective spine surgery is >35 (range 25–55). Nutrition status is assessed in 57% of the participants’ hospitals and surgery is postponed if albumin level is salient in 66% (Figure 3C). HbA1c level is controlled prior to surgery in patients with known diabetes mellitus in 69% of all participants’ hospitals and in 68% of all participants’ hospitals, surgery is postponed if HbA1c level is salient (Figure 3D). In 64% of all participants’ hospitals, asymptomatic anemia is an indication for substitution prior to elective spine surgery (Figure 3E).
Seventy percent discontinue the administration of immunosuppressive drugs before elective spine surgery if medically justifiable. On average, immunosuppressants are discontinued 12 days prior to surgery (range 1–90 days). In contrast, local steroid injections are a reason to postpone surgery only in 20% of the hospitals. These participants then aim at a three-week steroid injection-free interval (range 1–180 days, Figure 3F).
The most favored method for hair removal is shaving with a razor prior to surgery (47%) followed by electric clipping prior to surgery (21%) and shaving with razor the day before surgery (14%). In 14% of the hospitals, no hair removal is performed.
General Perioperative Management
The most frequently used antibiotics for antimicrobial prophylaxis (AMP) are cefazolin, cefuroxime, and ceftriaxone. Vancomycin, clindamycin, and ceftriaxone are the most frequently used alternatives in case of known allergies (Table 1). AMP is mostly administered 30 min (65%) before skin incision and in 66% the anesthesiologist is responsible for AMP administration. Re-administration of AMP is carried out in 86% of all hospitals. Most frequent AMP re-administration is carried out after 4 hours of surgery (57%). If continued on the ward, AMP is stopped in a time-dependent way (49%) or after removal of drains (23%).
Table 1.
List of Top 10 First Choice of Antibiotics for Perioperative Antimicrobial Prophylaxis.
| Rank | Choice of antibiotics | n | % |
|---|---|---|---|
| 1 | Cefazolin | 166 | 57.2 |
| 2 | Cefuroxime | 52 | 17.9 |
| 3 | Ceftriaxone | 34 | 11.7 |
| 4 | Cefalexin | 9 | 3.1 |
| 5 | Cefotaxime | 7 | 2.4 |
| 6 | Cefalotine | 6 | 2.1 |
| 7 | Ceftazidime | 5 | 1.7 |
| 8 | Amoxicillin/Clavulanic acid | 4 | 1.4 |
| 9 | Vancomycin | 4 | 1.4 |
| 10 | Cefoperazon/Sulbactam | 3 | 1.0 |
Team-time-out is performed in 86% of all hospitals. In only 18% of all hospitals, an additional infections prevention protocol is utilized and checked off in the operating room (OR). Blood sugar, body temperature, and hemoglobin level are routinely controlled in most of the hospitals (77%, 81%, 83%, respectively). In 51% of all hospitals, the number of persons in the OR is limited to 6, in 44%, the number is limited to 10. Most of the hospitals have no limitation for OR door openings (68%). Laminar air flow is available in 62% of all hospitals.
Surgical Aspects of Perioperative Management
Seventy-nine percent of the surgeons use skin incision markers, 40% non-sterile prior to skin disinfection and 39% sterile. Alcohol-based chlorhexidine is the most frequently used agent for skin disinfection (44%), followed by alcohol-based iodine (20%) and non-alcoholic iodine (17%).
Double gloving is implemented by 76% of all surgeons. In most cases (77%), the scrub nurse is dressing the surgeon with gloves. Gloves are changed routinely by 73% of all surgeons during the procedure. Fifty-four percent of the surgeons change the gloves before implant contact or opening of the dura. Eighty percent make sure that the implants stay packed sterile until shortly before use. Routine repositioning of surgical retractors (78%), as well as cutting out scar tissue (in revision cases) and wound margins (67%) is carried out by most of the spine surgeons. Monopolar cutting is used by 91% of the surgeons (epidermal 12%, subcutaneous 64%, and subfascial 59%). Special hemostatic agents are used by 65% of all surgeons.
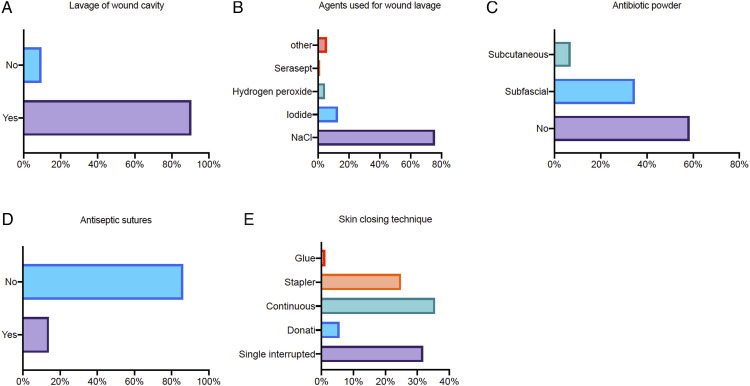
Ninety-one percent of all surgeons conduct routine lavage of the wound cavity before closing (Figure 4A). While doing so, saline is most frequently used (76%), followed by iodide (13%, Figure 4B). Most surgeons fill the cavity with the agent and allow it to soak (44%), 34% use continuous irrigation, 20% use pulsed irrigation. Wound drainage placement is done routinely in 71%.
Figure 4.
Perioperative measures. (A) Is routine lavage of the wound cavity performed? (B) Which agent is used for wound lavage? (C) Is antibiotic powder used and if so in which layer? (D) Are antimicrobial or impregnated antiseptic sutures used? (E) Which skin closing technique is performed?
The use of local antibiotics remains controversial, only 42% do so (Figure 4C). Thirty-five percent of surgeons apply local antibiotics subfascially and 7% subcutaneously. Antimicrobial impregnated sutures are used only by 14% of all surgeons (Figure 4D). Most surgeons use continuous sutures (36%) for skin closure followed by single interrupted sutures (32%) and the use of staplers (25%, Figure 4E).
Postoperative Management
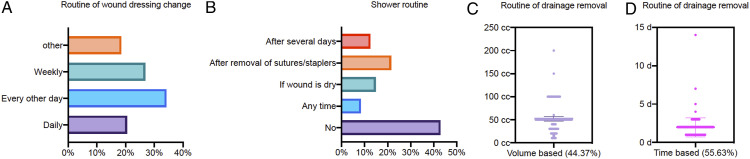
Routine postoperative check of hemoglobin is performed in 84% of institutions and asymptomatic anemia is treated with transfusion in nearly half of the hospitals (49%). Routine wound dressing change is mostly frequently every other day (34%) followed by once a week (27%) and daily (20%, Figure 5A). In almost half of all hospitals (43%), patients are not allowed to shower without covering the wound properly (Figure 5B). Standardized protocols are used for timing of wound drain removal in most hospitals (67%): in 56% based on time (Figure 5C) and in 44% based on output volume (Figure 5D).
Figure 5.
Postoperative measures. (A) How often is the wound dressing changed? (B) When are the patients allowed to shower? (C) When are the wound drains removed if based on secretion? (D) When are the wound drains removed if based on time?
The most prominent findings in the perception of SSI prevention measures are listed in Table 2.
Table 2.
Shortcomings in the Perception of SSI.
| Measure | Implication rate (%) | Proposed action |
|---|---|---|
| Surveillance of SSI | 38.3 | • Mandatory SSI surveillance • Mandatory spine surgeon responsible for analysis and control |
| BMI limitation for surgery | 19.1 | • No elective surgery on massive obese patients • Preparatory program for weight loss, including considering bariatric surgery if spine surgery is needed |
| Addressing asymptomatic anemia | 63.6 | • Abstain from administration of erythrocyte concentrates |
| Discontinuation of immunosuppressive drugs | 69.6 | • Case based decision making |
Discussion
The principal novel findings of this study are that many divergent SSI prevention procedures with different level of evidence or even without evidence (i.e.,, wound irrigation, double gloving) are implemented in spine surgery worldwide. Although responses from North America and Australia are underrepresented in this survey, the participants represent the global community of international spine surgery and almost 2/3 of the participants had a professional experience >9 years. To increase the number of participants from North America, the questionnaire was additionally to the AOSpine society distributed among Canadian Spine Society members and members of the Spine Program Surgical Faculty at the University of Toronto. As the percentage of participants from this area was still only 1/3 of the percentage of participants from Asia and Europe possible selection bias was evaluated as only marginal.
In the following, we would like to discuss measures that (i) are poorly implemented despite evidence, (ii) are frequently used without evidence, and (iii) measures with conflicting results in the literature.
i) Measures that are not implemented despite evidence
In almost 2/3 of the departments, no specifically trained surgeon was in charge of SSI surveillance and prevention despite the fact that surveillance protocols and awareness programs have been demonstrated to be effective to reduce SSI. 23 In 1/3 of the departments, there was even no surveillance protocol or regular statistical returns of SSI prevalence. Therefore, routine awareness programs and surveillance protocols should be requested.
Although Bode et al. showed a significant reduction of SSI when nasal decolonization was performed, SA screening and decolonization are not a common part of SSI prevention strategies in this study.24,25 One possible explanation for not performing this measure could be an unbearable rise in costs especially in low-income countries. Alternatively decolonizing all patients without screening could save costs but carries the risk of antimicrobial resistance. 26 Another hurdle to implementing SA decolonization could be the logistical and significant nursing overhead.
There is also clear evidence in the literature that obese patient had higher complication and reoperation rates in major spine surgeries. 27 Yet, most of the hospitals had no upper BMI limit for spine surgery. An explanation might be that the goal of preoperative weight loss cannot be achieved for many spine patients due to immobilizing pain.
There is also evidence, that normal levels of albumin and HbA1c are linked to lower risk of SSI in spine surgery. 28 In the present survey, around 2/3 of surgeons screen for elevated HbA1c levels in diabetic patients or postpone elective surgery when patients show albumin salience. Routine assessment of nutrition status and HbA1c preoperatively would contribute to identification of cases with need for optimization of such parameters. A stronger patient engagement might help improve compliance by explain the interrelationship between nutrition/HbA1c status and SSI.
ii) Measures that are frequently used without evidence
Discontinuation of immunosuppressive drugs prior to surgery represents common sense in our cohort. Interestingly, there is no sufficient evidence in literature that this measure is suitable to prevent SSI. Current studies only investigated the impact of concurrent methotrexate and tissue necrosis factor alpha-blockers on SSI rate.29,30 Thus, the decision of discontinuation of immunosuppressive drugs should be made case-based and upon discretion of the treating physicians.
Asymptomatic anemia prior to surgery was demonstrated to have no effect on postoperative SSI. 31 Additionally, blood transfusion is associated with a higher risk for SSI. 32 Nevertheless, anemia might be associated with reduced overall medical condition of the patient before surgery and preoperative anemic patients have a higher chance to require blood transfusion. This might be one explanation for the high count of surgeons addressing asymptomatic anemia prior to surgery despite the existing evidence arguing against this measure.
Intraoperatively, double gloving and routine change of surgical gloves are implemented by almost 3/4 of the participated surgeons. Studies show that there is less perforation of the innermost gloves, which are considered the last barrier between patient and surgeon. 33 One recent review stated that glove contamination increases with length of surgery and thus routine glove change is recommended. 34 However, there is no evidence that glove change has a direct effect on SSI rates. The situation is similar when it comes to wound irrigation. There is a lack of evidence for the effectiveness of irrigation compared to no irrigation to reduce SSI. 13 Nevertheless, 91% of the surgeons perform wound irrigation. Here saline is used most frequently. One study showed that there is an advantage of using diluted betadine solution compared to saline only in spine surgery for SSI prevention. 35 Larger randomized controlled trials are needed to determine the effectiveness of the double gloving and wound irrigation as interventions to prevent SSI.
Another area of dissent and lack of evidence are wound closing techniques. About 1/4 of the surgeons use staplers for skin closing. About 3/4 use sutures in different techniques. There is no evidence whether the type of material has an influence on SSI prevalence. 36 No studies concerning spine surgery were conducted to depict whether different suture techniques influence the rate of SSI. Rationales for stapler use especially in longer skin incisions are the less time-consuming procedure and constant pressure to the wound margin reducing the risk of inflammation.
Postoperatively, there was no agreement on routine wound dressing change. There is no evidence on whether the dressing change routine has an effect on SSI prevention. There is also no conclusive evidence on whether early or late showering has an effect on SSI prevention. 37 Further studies are needed to give clear answers.
iii) Measures with conflicting results in the literature
40% of the spine surgeons do not use antibiotic powder for SSI prevention. Several studies showed that application of Vancomycin powder reduces SSI, especially in spine surgery with instrumentation.38,39 Other studies have shown no effect. 40 These contradictory results paired with fear of antimicrobial resistance might lead to extra precaution regarding the use of local vancomycin powder. The results of currently ongoing studies on efficacy and security are awaited eagerly.
Conclusion
Our results show wide international heterogeneity of pre, peri, and postoperative measures to prevent SSI. Although there are fields of universal consensus in the spine community, we demonstrate a large heterogeneity for pre, peri, and postoperative measures to prevent SSI. Our data illustrated the need for developing universal guidelines and for testing areas of controversy in prospective clinical trials. A list of debatable measures for possible future trials is given in Table 3. Future randomized clinical trials should focus on well-defined patient populations and operative strategies (i.e., open surgery for degenerative spine disease) to generate robust data on effective SSI prevention measures in spine surgery.
Table 3.
Debatable Measures for Possible Future Trials.
| Measure | Implication rate (%) | Proposed trial |
|---|---|---|
| Staph aureus screening prior to surgery | 26.2 | • RCT with strict protocol for screening and eradication |
| Staph aureus decolonization prior to surgery | 45.3 | |
| Wound cavity irrigation | 90.5 | • RCT with saline irrigation vs no irrigation • RCT with different agents |
| Nutrition status assessment | 57.2 | • Prospective trial with strict protocol for preoperative assessment • RCT to determine cut-off values for postponement of elective spine surgery due to malnutrition |
| Double-glowing | 78 | • RCT single vs double pair of gloves |
| Wound closure by stapler | 24.9 | • RCT with stapler vs sutures |
| Postoperative showering with wound coverage | 67.1 | • RCT with showering with vs without wound coverage • RCT with early vs late showering |
Supplemental Material
Supplemental material, sj-pdf-1-gsj-10.1177_21925682211068414 for Prevention of Surgical Site Infections in Spine Surgery: An International Survey of Clinical Practices Among Expert Spine Surgeons by Dimitri Tkatschenko, Sonja Hansen, Julia Koch, Christopher Ames, Michael G. Fehlings, Sigurd Berven, Lali Sekhon, Christopher Shaffrey, Justin S. Smith, Robert Hart, Han Jo Kim, Jeffrey Wang, Yoon Ha, Kenny Kwan, Yong Hai, Marcelo Valacco, Asdrubal Falavigna, Néstor Taboada, Alfredo Guiroy, Juan Emmerich, Bernhard Meyer, Frank Kandziora, Claudius Thomé, Markus Loibl, Wilco Peul, Alessandro Gasbarrini, Ibrahim Obeid, Martin Gehrchen, Andrej Trampuz, Peter Vajkoczy and Julia Onken in Global Spine Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Dimitri Tkatschenko https://orcid.org/0000-0002-4231-6789
Michael G Fehlings https://orcid.org/0000-0002-5722-6364
Han Jo Kim https://orcid.org/0000-0002-7482-6994
Kenny Kwan https://orcid.org/0000-0002-4034-8525
Asdrubal Falavigna https://orcid.org/0000-0002-0016-3198
Alfredo Guiroy https://orcid.org/0000-0001-9162-6508
Bernhard Meyer https://orcid.org/0000-0001-6486-7955
References
- 1.Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suetens C, Latour K, Kärki T, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: Results from two European point prevalence surveys, 2016 to 2017 [published correction appears in Euro Surveill. 2018 Nov;23(47). Euro Surveill. 2018;23(46):1800516. doi: 10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zimlichman E, Henderson D, Tamir O, et al. Health care-associated infections: A meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173(22):2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 4.Yao R, Zhou H, Choma TJ, Kwon BK, Street J. Surgical site infection in spine surgery: Who is at risk? Global Spine J. 2018;8(4 suppl l):5S–30S. doi: 10.1177/2192568218799056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pull ter Gunne AF, Cohen DB. Incidence, prevalence, and analysis of risk factors for surgical site infection following adult spinal surgery. Spine (Phila Pa. 1976). 2009;34(13):1422–1428. doi: 10.1097/BRS.0b013e3181a03013. [DOI] [PubMed] [Google Scholar]
- 6.Schimmel JJ, Horsting PP, de Kleuver M, Wonders G, Van Limbeek J. Risk factors for deep surgical site infections after spinal fusion. Eur Spine J. 2010;19(10):1711–1719. doi: 10.1007/s00586-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Quality Forum . Safe practices for better healthcare: 2010 update; 2010. http://www.qualityforum.org/Projects/Safe_Practices_2010.aspx. Accessed April 2, 2020.
- 8.The Joint Commission . National patients safety goals; 2020. https://www.jointcommission.org/standards/national-patient-safety-goals/. Accessed April 2, 2020.
- 9.Strategien AWMF . Zur Prävention von postoperativen Wundinfektionen; 2018. https://www.mhp-medien.de/fileadmin/MHP/Zeitschriften/Hygiene_Medizin/AWMF/2014_HM4_wundinfektionen.pdf. Accessed April 4, 2020.
- 10.AWMF . Perioperative antibiotikaprophylaxe; 2020. https://euprevent.eu/wp-content/uploads/2017/01/AWMF-Leitlinie-Perioperative-Antibiotikaprophylaxe.pdf. Accessed April 4, 2020
- 11.Berríos-Torres SI, Umscheid CA, Bratzler DW, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection. JAMA Surg. 2017;152(8):784–791. doi: 10.1001/jamasurg.2017.0904. [DOI] [PubMed] [Google Scholar]
- 12.Robert-Koch-Institut . Prävention postoperativer Infektion im Operationsgebiet. 2018. https://www.rki.de/DE/Content/Infekt/Krankenhaushygiene/Kommission/Downloads/Empf_postopWI.pdf?__blob=publicationFile. Accessed April 4, 2020.
- 13.WHO . Global Guidelines for the Prevention of Surgical Site Infection. 2nd ed. Geneva, Switzerland: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. https://pubmed.ncbi.nlm.nih.gov/30689333/ [PubMed] [Google Scholar]
- 14.Tomov M, Mitsunaga L, Durbin-Johnson B, Nallur D, Roberto R. Reducing surgical site infection in spinal surgery with betadine irrigation and intrawound vancomycin powder. Spine (Phila Pa 1976). 2015;40(7):491–499. doi: 10.1097/BRS.0000000000000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calderone RR, Garland DE, Capen DA, Oster H. Cost of medical care for postoperative spinal infections. Orthop Clin North Am. 1996;27(1):171–182. [PubMed] [Google Scholar]
- 16.Calderone RR, Thomas JC, Jr, Haye W, Abeles D. Outcome assessment in spinal infections. Orthop Clin North Am. 1996;27(1):201–205. [PubMed] [Google Scholar]
- 17.Lee NJ, Shin JI, Kothari P, et al. Incidence, impact, and risk factors for 30-Day wound complications following elective adult spinal deformity surgery. Global Spine J. 2017;7(5):417–424. doi: 10.1177/2192568217699378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel H, Khoury H, Girgenti D, Welner S, Yu H. Burden of surgical site infections associated with select spine operations and involvement of Staphylococcus aureus. Surg Infect (Larchmt). 2017;18(4):461–473. doi: 10.1089/sur.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petilon JM, Glassman SD, Dimar JR, Carreon LY. Clinical outcomes after lumbar fusion complicated by deep wound infection: A case-control study. Spine (Phila Pa 1976). 2012;37(16):1370–1374. doi: 10.1097/BRS.0b013e31824a4d93. [DOI] [PubMed] [Google Scholar]
- 20.El-Kadi M, Donovan E, Kerr L, et al. Risk factors for postoperative spinal infection: A retrospective analysis of 5065 cases. Surg Neurol Int. 2019;10:121. doi: 10.25259/SNI-284-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maruo K, Berven SH. Outcome and treatment of postoperative spine surgical site infections: Predictors of treatment success and failure. J Orthop Sci. 2014;19(3):398–404. doi: 10.1007/s00776-014-0545-z. [DOI] [PubMed] [Google Scholar]
- 22.Casper DS, Zmistowski B, Hollern DA, et al. The effect of postoperative spinal infections on patient mortality. Spine (Phila Pa 1976). 2018;43(3):223–227. doi: 10.1097/BRS.0000000000002277. [DOI] [PubMed] [Google Scholar]
- 23.Featherall J, Miller JA, Bennett EE, et al. Implementation of an infection prevention bundle to reduce surgical site infections and cost following spine surgery. JAMA Surg. 2016;151(10):988–990. doi: 10.1001/jamasurg.2016.1794. [DOI] [PubMed] [Google Scholar]
- 24.Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362(1):9–17. doi: 10.1056/NEJMoa0808939. [DOI] [PubMed] [Google Scholar]
- 25.Kalmeijer MD, Coertjens H, Van Nieuwland-Bollen PM, et al. Surgical site infections in orthopedic surgery: The effect of mupirocin nasal ointment in a double-blind, randomized, placebo-controlled study. Clin Infect Dis. 2002;35(4):353–358. doi: 10.1086/341025. [DOI] [PubMed] [Google Scholar]
- 26.Poovelikunnel T, Gethin G, Humphreys H. Mupirocin resistance: Clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother. 2015;70(10):2681–2692. doi: 10.1093/jac/dkv169. [DOI] [PubMed] [Google Scholar]
- 27.Goyal A, Elminawy M, Kerezoudis P, et al. Impact of obesity on outcomes following lumbar spine surgery: A systematic review and meta-analysis. Clin Neurol Neurosurg. 2019;177:27–36. doi: 10.1016/j.clineuro.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 28.Epstein NE. Preoperative measures to prevent/minimize risk of surgical site infection in spinal surgery. Surg Neurol Int. 2018;9:251. doi: 10.4103/sni.sni_372_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthold E, Geborek P, Gülfe A. Continuation of TNF blockade in patients with inflammatory rheumatic disease. An observational study on surgical site infections in 1,596 elective orthopedic and hand surgery procedures. Acta Orthop. 2013;84(5):495–501. doi: 10.3109/17453674.2013.842431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214–217. doi: 10.1136/ard.60.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weber WP, Zwahlen M, Reck S, et al. The association of preoperative anemia and perioperative allogeneic blood transfusion with the risk of surgical site infection. Transfusion. 2009;49(9):1964–1970. doi: 10.1111/j.1537-2995.2009.02204.x. [DOI] [PubMed] [Google Scholar]
- 32.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208(5):931–937. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Tanner J, Parkinson H. Double gloving to reduce surgical cross-infection. Cochrane Database Syst Rev. 2006;(3):CD003087. doi: 10.1002/14651858.CD003087.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim K, Zhu M, Munro JT, Young SW. Glove change to reduce the risk of surgical site infection or prosthetic joint infection in arthroplasty surgeries: A systematic review. ANZ Journal of Surgery. 2019;89:1009-1015. doi: 10.1111/ans.14936. [DOI] [PubMed] [Google Scholar]
- 35.Cheng MT, Chang MC, Wang ST, Yu WK, Liu CL, Chen TH. Efficacy of dilute betadine solution irrigation in the prevention of postoperative infection of spinal surgery. Spine (Phila Pa 1976). 2005;30(15):1689–1693. doi: 10.1097/01.brs.0000171907.60775.85. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan RJ, Crawford EJ, Syed I, Kim P, Rampersaud YR, Martin J. Is the risk of infection lower with sutures than with staples for skin closure after orthopaedic surgery? A meta-analysis of randomized trials. Clin Orthop Relat Res. 2019;477(5):922–937. doi: 10.1097/CORR.0000000000000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toon CD, Sinha S, Davidson BR, Gurusamy KS. Early versus delayed post-operative bathing or showering to prevent wound complications. Cochrane Database Syst Rev. 2015;(7):CD010075. doi: 10.1002/14651858.CD010075.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strom RG, Pacione D, Kalhorn SP, Frempong-Boadu AK. Decreased risk of wound infection after posterior cervical fusion with routine local application of vancomycin powder. Spine (Phila Pa 1976). 2013;38(12):991–994. doi: 10.1097/BRS.0b013e318285b219. [DOI] [PubMed] [Google Scholar]
- 39.Devin CJ, Chotai S, McGirt MJ, et al. Intrawound vancomycin decreases the risk of surgical site infection after posterior spine surgery: A multicenter analysis. Spine (Phila Pa 1976). 2018;43(1):65–71. doi: 10.1097/BRS.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 40.Martin JR, Adogwa O, Brown CR, et al. Experience with intrawound vancomycin powder for posterior cervical fusion surgery. J Neurosurg Spine. 2015;22(1):26–33. doi: 10.3171/2014.9.SPINE13826. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-gsj-10.1177_21925682211068414 for Prevention of Surgical Site Infections in Spine Surgery: An International Survey of Clinical Practices Among Expert Spine Surgeons by Dimitri Tkatschenko, Sonja Hansen, Julia Koch, Christopher Ames, Michael G. Fehlings, Sigurd Berven, Lali Sekhon, Christopher Shaffrey, Justin S. Smith, Robert Hart, Han Jo Kim, Jeffrey Wang, Yoon Ha, Kenny Kwan, Yong Hai, Marcelo Valacco, Asdrubal Falavigna, Néstor Taboada, Alfredo Guiroy, Juan Emmerich, Bernhard Meyer, Frank Kandziora, Claudius Thomé, Markus Loibl, Wilco Peul, Alessandro Gasbarrini, Ibrahim Obeid, Martin Gehrchen, Andrej Trampuz, Peter Vajkoczy and Julia Onken in Global Spine Journal