Abstract
Study Design
Cadaver study.
Objectives
Assess the feasibility of robot-assisted cervical pedicle screw (RA-CPS) placement and understand the anatomical considerations of this technique.
Methods
Four cadaver specimens free from bony pathology were acquired. Anatomical considerations, such as pedicle width (PW) and height (PH), transverse pedicle angle (TPA), and maximal screw length (MSL), were recorded from preoperative computational tomography (CT) scans. Intraoperative cone-beam CT was acquired and registered to the robotic system. After cervical levels were segmented, screw sizes and trajectories were planned, and RA-CPS were placed. Accuracy was assessed using Gertzbein and Robbin’s classification on postoperative CT scans.
Results
Thirty-five RA-CPS were placed. Major breaches (≥Grade C) occurred in 28.57% screws. Grade A or B accuracy was found in 71.43% of screws, with the most common direction of breach being medial (81.3%). The greatest proportion of breach per level occurred in the upper subaxial levels, (C3:71.4%, C4 66.6%, C5:50%) which had the smallest PW (C3: 4.34 ± .96 mm, C4: 4.48 ± .60, C5: 5.76 ± 1.11). PH was greatest at C2 (8.14 ± 1.89 mm) and ranged subaxial from 6.36 mm (C3) to 7.48 mm (C7). The mean PW was 5.37 mm and increased caudally from 4.34 mm (C3) to 6.31 mm (C7). The mean TPA was 39.9° and decreased moving caudally 46.9°) to C7 (34.4°). The MSL was 37.1 mm and increased from C2 (26.3 mm) to C7 (41.0 mm).
Conclusion
RA-CPS has the potential to be feasible, but technological and instrument modifications are necessary to increase the accuracy in the cervical region.
Keywords: robot-assisted, cervical pedicle screw, anatomical, technical, cadaver
Introduction
Robotic and navigational advancements have improved the accuracy and safety of pedicle screw placement.1-3 Robot-assisted (RA) pedicle screw insertion can be performed via an open or a percutaneous approach with similar accuracy.4,5 Over the last decade, the integration of intraoperative computed tomography (CT) navigational technology has improved the rate of screw malposition by allowing for greater visualization of spinal anatomy.6,7 The robotic system is able to stabilize and limit the surgeon’s arm to 4 of the 6 degrees of freedom to achieve submillimeter accuracy.6,8 Spinal robotic technology has primarily been studied in the thoracolumbar region. 3 Enhancing visualization through navigation and limiting unwanted movement may facilitate the accurate placement of cervical pedicle screws (CPS).6,8
CPS are used generally for weak bone, revision of lateral mass screws (LMS) or in the setting of a bony defect. 9 Subaxial CPS placement has gained recent attention.10-17 CPS offer a significantly greater biomechanical advantage, achieve greater bony purchase, and have higher strength in fatigue and lower rates of loosening at the bone-screw interface than LMS.18,19 Studies have reported safe and accurate freehand placement of subaxial CPS and its ability to stabilize multilevel constructs.9,11-13 Clinical adoption of CPS has been limited due to the potential challenges, which include small cervical pedicle diameter, the highly cortical nature of cervical pedicles, relatively greater mobility of the cervical spine, and close proximity to adjacent neurovascular structures.14,20-23 Experience with RA placement of cervical instrumentation is limited.17,24 Nevertheless, there is a drive to use newer technologies to improve the accuracy and, thus, the safety of CPS placement. 16
The purpose of this cadaveric study was to assess the feasibility of RA-CPS placement, understand anatomical considerations of this technique associated with the dimensions of the pedicles, and comment on technical challenges that were encountered and potentially how to overcome them.
Materials and Methods
Specimens and Anatomical Considerations
Four fresh-frozen cephalic-to-torso cadaver specimens were acquired. Preoperative CT was performed on all cadavers to ensure the absence of spinal pathology. Linear and angular anatomical parameters associated with cervical vertebral levels were measured bilaterally using Horos Version 3.3.6 open-source software (www.horosproject.org) from preoperative three-dimensional (3D) CT scans. Pedicle width (PW, the mediolateral diameter of the pedicle isthmus), maximal screw length (MSL, distance along the axis of the pedicle from the posterior cortex of the lateral mass to the anterior wall of the vertebral body), and transverse pedicle angle (TPA, the angle between a line drawn from the axis of the pedicle and a line drawn through the midline of the vertebral body) were collected on the axial CT image. 25 Pedicle height (PH, the rostrocaudal diameter of the pedicle) was collected on the sagittal image. 25
Because this study was a case series of cadavers, an institutional board review was deemed unnecessary.
Procedure Details
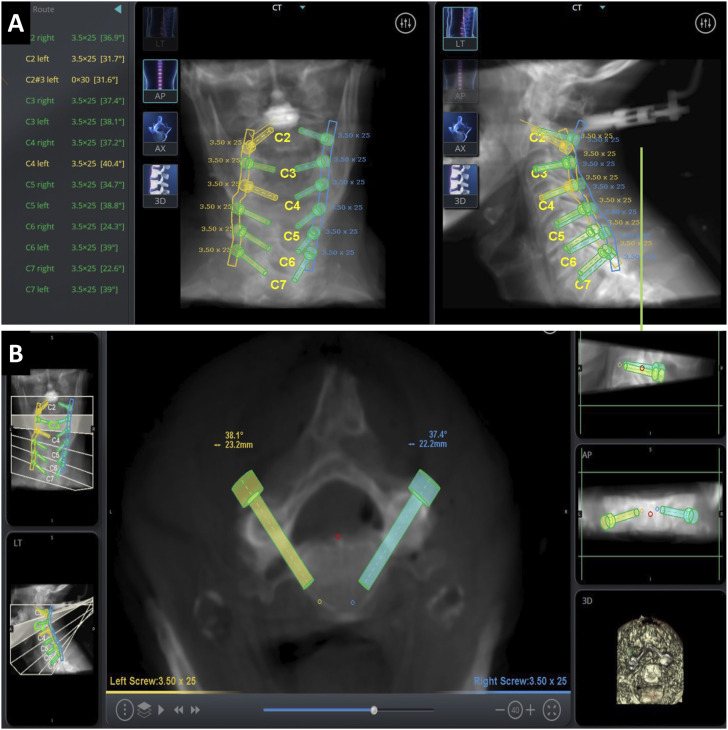
The specimens were placed in a Mayfield head holder and turned into a prone position on a Jackson table. The Mazor ™ Robotic Guidance Platform (Medtronic, Minneapolis, MN) robotic system using the intraoperative cone-beam CT scan-and-plan workflow was set up as described by O’Connor et al. 26 The robot surgical arm was docked and mounted to the Jackson table towards the caudal end of the specimen. A spinous process clamp (SPC) was attached to T1 by making a midline incision and blunt subperiosteal paraspinal muscle dissection was done to expose the spinous process. The surgical effector arm was attached to the SPC. A reference frame was attached to the lateral aspect of the robot arm. The 3D surgical work volume of the robot arm was then mapped. The surgical arm, with 2 optical cameras and 1 infrared camera, was moved in an arching motion over the region of interest to define that area. Intraoperative 3D volumetric data was acquired using cone-beam CT and transmitted to the robotic workstation. The robotic software segments each cervical level so that the surgeon can plan the pedicle screw trajectory and size (Figure 1A and B).
Figure 1.
A) Robotic segmentation of cervical vertebra and planning of cervical pedicle screw size and trajectory. B) Finalized screw placement.
The robotic percutaneous technique was adapted to insert CPS. 26 The percutaneous approach was used to reduce the traction forces from muscle seen with an open approach and to avoid intersegmental shift caused by manipulation related to paraspinal tissue dissection in an open approach.27,28 The robot effector arm was sent to the pre-planned trajectory of the first pedicle screw. A stab incision was made with a specialized scalpel through the robot’s effector arm. A cannula and navigated dilator were inserted through the effector arm and into the incision site and advanced until the bone was felt. The dilator was removed and replaced with a drill guide (Figure 2). A 3-mm navigated drill with a 20-mm positive stop was used to prevent over drilling. Drilling was done either by hand or with power. Following removal of the drill guide, a navigated tap was used through the effector arm, after which a navigated screw was placed. The robot effector arm was moved to the next trajectory, and the aforementioned steps were repeated. Once attempts were made to place CPSs bilaterally at the C2-C7 vertebral level, the robot was detached from the SPC. Postoperatively, 3D volumetric data was acquired using cone-beam CT to assess screw accuracy.
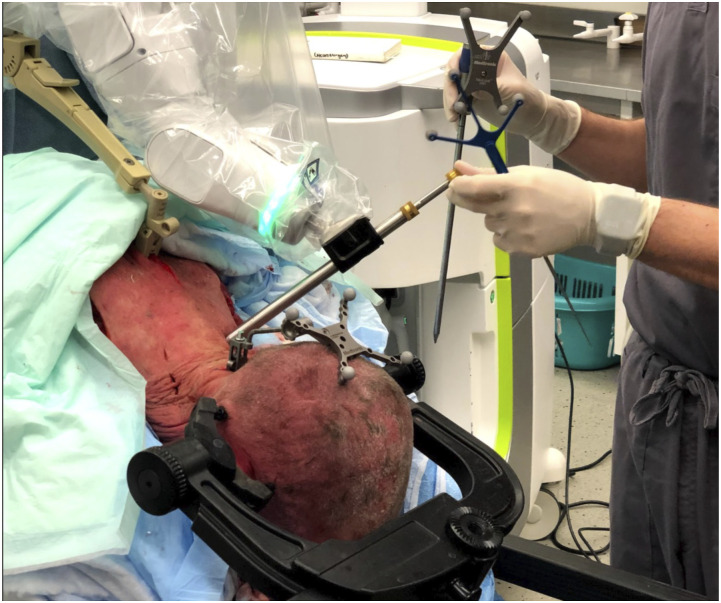
Figure 2.
Image demonstrating patient positioning. Replacement of navigated dilator with drill guide.
Accuracy and Technical Data
A spine surgeon independently graded the accuracy of screw placement using the Gertzbein and Robbins classification on the postoperative 3D-CT scan. Grade A was classified as a screw completely within the pedicle, Grade B if cortical breach was <2 mm, Grade C if cortical breach was 2 to <4 mm, grade D if cortical breach was 4 to <6 mm, and Grade E if cortical breach was ≥6 mm. 29 Major screw breach was defined as Grade C placement or worse.3,17,29 Vertebral level laterality, total time for pedicle screw insertion (from skin incision to screw placement), screw dimensions (length and width), and technical difficulties were documented.
Statistical Analysis
Continuous variables were presented as means ± standard deviation, and categorical variables are presented as frequency and percentages. For continuous parametric data, an independent T-test or analysis of variance was utilized to compare differences between means. Chi-Square testing was used to calculate P values for categorical data. A P-value of less than .05 was considered statistically significant. All statistical analyses were done using R Studio (Version 3.6.3, Vienna, Austria). Graphs were generated using Prism version 9.0 (Graphpad, San Diego, CA)
Results
Demographics
At the time of death, mean age was 75.2 ± 5.56 years old and mean body mass index was 21.4 ± 4.47 kg/m2. All cadavers were men without a history of osteoporosis or bone disease; 75% had a previous smoking history. One patient had a history of diabetes managed with weight loss (Table 1).
Table 1.
Demographics of 4 Cadaveric Specimens.
| Characteristic | Mean ± SD or No. (%) |
|---|---|
| Age (years) | 75.2 (5.56) (range) |
| Body mass index (kg/m2) | 21.4 (4.77) |
| Sex: Male | 4 (100%) |
| Race: White | 4 (100%) |
| Smoking history | 3 (75%) |
Abbreviation: SD, standard deviation.
Anatomical Considerations
The data is provided in Table 2 and Supplementary Figure (A-D). Mean PH was 7.1 ± 1.19 mm. The PH was largest at C2 (8.14 ± 1.89 mm). Subaxial PH ranged from 6.36 mm (C3) to 7.48 mm (C7). Comparison of anatomic parameters by vertebral level demonstrated significantly larger PH at C2 than at C3 (P = . 04) and C6 (P = .01) (Supplemental Figure). Mean PW was 5.37 ± 1.21 mm. Subaxial PW increased moving caudally from 4.34 mm±.96 mm (C3) to 6.31 ± .63 mm (C7). C3 PW was significantly smaller than C6 (P = .02) and C7 (.01). C4 PW was significantly smaller than C6 (P = .048) and C7 (P = .021). Mean TPA was 39.9 ± 5.34° and decreased moving caudally from C2 (46.9 ± 4.61°) to C7 (34.4 ± 6.06°). C2 had a greater TPA compared to all other levels: C3 (P = .02), C4 (P = .01), C5 (P = .01), C6 (P = .001), and C7 (P < .0001). The mean MSL was 37.1 ± 6.67 mm overall and increased from C2 (26.3 ± 4.80 mm) to C7 (41.0 ± 1.38 mm). The MSL at C2 was significantly smaller than that at C3 (P = .001), C4 (P < .0001), C5 (P < .0001), C6 (P < .0001), and C7 (P < .0001). C3 MSL was significantly smaller than C5 (P = .03) (Supplemental Figure).
Table 2.
Cervical Anatomical Considerations.
| NO. Screws (N = 46) | C2 (N = 8) | C3 (N = 8) | C4 (N = 8) | C5 (N = 8) | C6 (N = 8) | C7 (N = 6) a | P-value | |
|---|---|---|---|---|---|---|---|---|
| PH (mm) | 7.10 (1.19) | 8.14 (1.89) | 6.36 (.88) | 7.16 (.89) | 6.97 (.66) | 6.51 (.80) | 7.48 (.95) | .009 b |
| PW (mm) | 5.37 (1.21) | 5.55 (1.21) | 4.34 (.96) | 4.48 (.60) | 5.76 (1.11) | 6.01 (1.26) | 6.31 (.63) | .001 b |
| TPA (°) | 39.9 (5.34) | 46.9 (4.61) | 40.1 (2.18) | 39.5 (3.88) | 39.6 (3.31) | 37.5 (4.02) | 34.4 (6.06) | <.001 b |
| MSL (mm) | 37.1 (6.67) | 26.3 (4.80) | 35.2 (3.30) | 38.5 (4.55) | 41.8 (4.32) | 40.7 (4.47) | 41.0 (1.38) | <.001 b |
Numbers in rows are mean and standard deviation; Abbreviations: MSL, maximal screw length; PH, pedicle height; PW, pedicle width; SPA, sagittal pedicle angle; TPA, transverse pedicle angle.
aThe C7 vertebral level was out of the plane of the preoperative scan on the one of the specimens, and anatomical measurements could not be attained.
bDenotes statistical significance.
Technical and Robotic Registration Challenges
Thirteen of 48 total potential CPSs were not placed (27.1%): 1 screw could not be placed due to a highly cortical C5 pedicle, trajectories for 6 screws could not be planned, and 6 screws could not be advanced to appropriately planned trajectories. Of the 6 screws that failed to reach the planned trajectory, 3 failures were due to the specimen’s neck size or body habitus, and the other 3 because the robotic system was docked too close to the cervical region, preventing the robot arm from reaching the appropriate trajectory. A large osteophyte caused the instrumentation to skive medially during placement of 2 screws.
Accuracy
Thirty-five CPS were placed. Mean screw placement time was 6.8 ± 3.8 minutes. Of the screws placed, 19 (54.3%) were Grade A, 6 (17.1%) were Grade B, 7 (20.0%) were Grade C, 35 (2.9%) were Grade D, and 2 (5.7%) were Grade E. Major breaches (≥Grade C) occurred in 28.6% (10/35) screws. The overall accuracy that was that was grade A or B was 71.4% (25/35 screws). The greatest breach per level was 71.4% at C3 (Table 3). Of the major pedicle breaches, 8 were medial and had entry point deviation to the lamina of the vertebra, and 2 were lateral breach and had lateral entry point deviation. The surgeons observed that the reason for the major breach was attributed to CPS skiving, cortical pedicles, large osteophytes at the entry point, and increased resistance. Most major pedicle breaches occurred at the upper subaxial cervical levels (C3: 3 screws, C4: 3 screws, C5:2) (Table 4).
Table 3.
Description of Cervical Pedicle Screws and Overall Accuracy.
| N = 35 Levels | |
|---|---|
| Vertebral level | |
| C2 | 6 (17.1%) |
| C3 | 7 (20.0%) |
| C4 | 6 (17.1%) |
| C5 | 6 (17.1%) |
| C6 | 6 (17.1%) |
| C7 | 4 (11.4%) |
| Laterality | |
| Left | 15 (42.9%) |
| Right | 20 (57.1%) |
| Screw placement time (minutes) | 6.8 (3.8) |
| Gertzbein and Robbins grade | |
| A | 19 (54.3%) |
| B | 6 (17.1%) |
| C | 7 (20.0%) |
| D | 1 (2.9%) |
| E | 2 (5.7%) |
| Breach distance (mm) | 2.8 (2.00) |
| Breach direction | |
| Lateral | 3 (18.7%) |
| Medial | 13 (81.3%) |
| Breach distribution † | |
| C2 | 1 (16.6%) |
| C3 | 5 (71.4%) |
| C4 | 4 (66.6%) |
| C5 | 3 (50.0%) |
| C6 | 2 (33.3%) |
| C7 | 1 (25.0%) |
Data is displayed as mean (standard deviation); Categorical data is displayed as the total number (percentage).
†Percentages calculated by number of breached screws divided by number of screws placed per level.
Table 4.
Summary of Major (Gertzbein and Robbins Grade ≥ C) Pedicle Breach.
| Level | Laterality | Accuracy | Direction | PH (mm) | TPA (°) | MSL (cm) | PW (mm) | Actual SL* (mm) | Screw width (mm) | Breech Distance (mm) | Surgeon Observations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C3 | Right | C | Medial | 6.9 | 40.8 | 33.4 | 5.1 | 22.0 | 3.5 | 2.6 | Large osteophyte |
| C4 | Right | C | Medial | 6.9 | 41.7 | 37.8 | 4.5 | 22.0 | 3.5 | 2.6 | Skive |
| C6 | Right | C | Lateral | 5.5 | 42.5 | 35.2 | 7.2 | 20.0 | 3.5 | 2.6 | Skive |
| C3 | Left | C | Medial | 6.1 | 40.3 | 35.7 | 5.7 | 22.0 | 3.5 | 3.6 | Cortical pedicle |
| C4 | Left | C | Medial | 5.7 | 43.1 | 36.1 | 5.3 | 22.0 | 3.5 | 2.5 | Cortical pedicle |
| C5 | Left | C | Medial | 7.6 | 44.9 | 42.9 | 6.7 | 22.0 | 3.5 | 2.6 | Cortical pedicle |
| C4 | Right | C | Medial | 6.9 | 41.5 | 38.6 | 5.1 | 22.0 | 3.5 | 2.2 | Resistance |
| C3 | Right | D | Medial | 5.8 | 37.5 | 37.3 | 5.5 | 22.0 | 3.5 | 4.0 | Resistance |
| C2 | Right | E | Lateral | 7.9 | 50.6 | 26.9 | 6.0 | 22.0 | 3.5 | 7.3 | Skive |
| C5 | Right | E | Medial | 7.5 | 42.6 | 49.3 | 7.5 | 20.0 | 3.0 | 7.7 | Large osteophyte |
Abbreviations: L, left; MSL, maximal screw length; PH, pedicle height; PW, pedicle width; R, right; SL, screw length; TPA, transverse pedicle angle; *Length of the screw that was used.
Discussion
Pedicle screws are routinely used in the thoracic and lumbar spine.3,16 Despite the potential benefits of subaxial CPS, they are not widely used in clinical practice due to technical difficulties and the potential for catastrophic consequences.10,22 To our knowledge, this is among the first studies to report RA-CPS placement using the intraoperative cone-beam CT scan-and-plan registration workflow. Grade A or B accuracy was achieved in 71.4% of CPS insertions. Most breaches occurred in the upper subaxial levels and in the medial direction, which had the smallest PW and greatest TPA.
In our study, all anatomical metrics differed significantly between levels. Due to anatomical variability, direct comparison to previous studies is challenging. PH dimensions were greater than PW at all levels. Although the mean PH was greater than 6 mm, the mean PW was often smaller than 6 mm. The trend of PH was greatest in C2 and remained relatively constant in the subaxial levels. The mean PW was greatest in C2. From rostral to caudal, subaxial PW increased, with the smallest PW at the upper subaxial levels (i.e., C3, C4, 5). The trend of sizes is consistent with results reported by Karaikovic et al. 30 Our mean PW (5.37 mm) was consistent with the pedicle dimensions reported by Onibokun et al, who described the overall mean PW ranging from 4.7 to 6.5 mm. 25 Variability in PW may be attributed to differences in sex: men have been reported to have larger PW. 31 Our mean PH (7.10 mm) exceeded their reported range (6.4 to 7.0 mm).25,30,32 However, our results for PH fall within the range reported by Panjabi et al. (6.7 to 11.1 mm). 33 Together, these results support that uniformly sized CPS cannot be use at every level, and care should be taken with screw size selection, particularly at the upper subaxial cervical level.25,30 Careful consideration of nearby critical structures must be considered. Although major lateral breach occurred in 2 screws on the right side due to skiving. Laterality should be taken into consideration, and great care should be taken when instrumenting left-sided pedicle screws. The vertebral artery (VA) has been reported to be left dominant in 69.3% of patients leading to greater occupancy of the transverse foramen. 31 Utilization of CT angiography can be helpful when there are enhanced concerns for breach and neurovascular injury.
In addition to anatomical variability, the cortical nature of the cervical pedicles limits CPS placement.16,30,34 Highly cortical pedicles have been reported to increase resistance during instrumentation, which has been reported as a contributor to major breaches. 30 It is possible that the added force required during instrumentation causes intersegmental motion leading to inaccuracy of the planned trajectory. In the current study, 1 screw could not be placed due to the combination of a highly cortical pedicle and the available pedicle screw size. Careful pedicle screw sizing prior to the procedure can mitigate narrow medullary canals of highly cortical pedicles. Increased resistance was noted in 2 screws, which may be due to a narrow medullary canal. In the current study, all major breaches but 1 occurred with the larger 3.5 mm screw diameter. The inner PW are often much smaller than the outer PW. The inner PW has been previously reported to be <2 mm (with means of 13.2% at C2, 72.6% at C3, 67% at C4, 62.3% at C5, 51.9% at C6, and 6.6% at C7). 30 It has been reported that some pedicles do not have a medullary canal (C2: .9%, C3 and C4: 2.8%, C5: 3.8%). 30
From rostral to caudal in the present study, TPA decreased. The mean TPA was 39.9°, ranging from 46.9° to 34.4° (C2-C7), which is consistent with previous reports.25,34-36 To avoid violation of the transverse foramen protected by a thin lateral cortex and medial spinal canal, surgeons advocate for insertion angulation to be towards the upper recommended range (45° to 50°).30,34 The acute angulation of the upper cervical region presented a challenge for the robotic system, and the trajectories for 6 screws could not be planned. The acute trajectory angulation and the sloped lamina in the cervical spine add to the likelihood of the drill guide sliding medially, which may explain the predominance of medial direction of breach. 37
There is a paucity of literature reporting experience with RA cervical instrumentation for comparison of accuracy. In the thoracic and lumbar spine, there is 4 mm of allowable medial screw encroachment (2 mm of epidural and 2 mm of subarachnoid space). 29 In the cervical region, the distance from the VA to the lateral pedicle has been reported to increase from 1.1 mm at C2, to 1.7 mm at C6. 31 Tomasino et al. report 80% of VAs were outside the transverse foramen at the level of C7, and the safe zone was .65 mm. 31 Medially, from C3-C7, the dural sac is 2.4 mm to 3.1 mm to the pedicle. 31 Based on these reported values and our experience, to avoid screw breach, there should be .5 mm of pedicle width on the medial and lateral borders of the CPS. As a part of a narrative review, Lieberman et al 38 reported the accuracy in a series of 10 patients. They found 87.2% Grade A accuracy with a mean deviation of 1.25 mm. They also report that all breaches were medial in direction, and none resulted in clinical consequences. Using a robotic system with the CT-to-fluoroscopy registration work flow, Su et al. report the initial clinical result of RA-CPS. 17 In a prospective nonrandomized control, they report a significantly greater rate of Grade A (90.6% and 71.1%; P<.001) compared to Grade A or B (97.2% and 90.7%; P = .001) in the RA group compared to the freehand group. 17 Differences between their results and the current study may be due to difference in robotic platform used, registration, and open approach. The open approach may provide greater ability to level the bony surface or visualize the tip guide as it anchors into the bone, thereby improving accuracy. 37
We believe the greatest difference between accuracy is due to differences in clinical and cadaveric study design. Similar differences in accuracy between in vivo (99.2%) and ex vivo (76.3%) studies have been seen in a meta-analysis assessing navigation-assisted CPS placement. 1 Cadaveric tissue preservation alters tissue texture and causes dehydration of discs, which reduces the vertebral motion as compared to what is seen in vivo. Guha et al. have likewise reported that this altered motility in cadaver vertebrae has limited the ability to correlate results to clinical practice. 27
Technical Challenges
Various factors along the robotic workflow may influence CPS accuracy. During CPS planning in the current study, the narrow disc spaces on the preoperative CT images caused difficulty for segmenting each cervical level with the robotic software. We found that slight angulation in the gantry angle (e.g., parallel to the inferior vertebral endplate) during image acquisition improved visualization of the disc space. Proper segmentation influences the ability to appropriately plan the screw trajectory, which is done on axial and sagittal views. 39 Because the PH is greater than the PW, there is more room for adjustment on the sagittal plane. We found the smoothest planning workflow occurred when the screws were imaged first on the axial view with fine-tuning done on the sagittal plane. The robotic software does not allow screw planning to proceed if the tulip head of the pedicle screws appear to be overlapping, which accounted for all screws that were not able to be planned in the current study. We found that moving the CPS inferiorly often moved the tulip head out of the way.
Although the robotic system is fixed onto the bed frame at one end, the robot arm is not completely resistant to motion, and will deviate when too much force is applied by the surgeon. 38 Micromotion commonly comes from the tension of the soft tissue or force during instrumentation. 37 In our study, micromotion occurred during the instrumentation of specimens, which may be due to the smaller size of the cadaveric specimen compared to the size of a patient during a live operative procedure. To minimize motion, it is advised that fixation is achieved by tapping the serrated end of the drill guide into the bone, and caution is taken during tapping because too much force may shift the starting point as the tip skives along the contour of the bone. 37 Proper drill technique ensures an accurate pedicle entry point. 40 Irregular bone surface (e.g., osseous spur), steep trajectory, tissue pressure, high drilling pressure, and a dull drill bit may all lead to skiving during drilling. 41 In the current study, we experienced greater skiving with large osteophytes on the lateral mass. We recommend a high-speed drill with a sharp drill bit to facilitate low drilling pressure. Lubricating the drill and instrumentation before placement down the drill guide and effector arm allow for smooth passage and minimize motion. 26 The size of the current instrumentation was too large and was unable to appropriately fixate to the bony anatomy without increased tapping forces. Developing cervical spine-specific instrumentation, such as dilators and drills, could increase the accuracy. The small anatomy coupled with the slopes and curvature of the cervical spine added to skiving potential. Although the robotic system uses 3-mm drill bit, the utilization of the 2.1 mm drill bit from the previous edition of the robot may have improved the accuracy. Future studies with smaller instrumentation may improve accuracy.
Lower pedicle screw accuracy has been reported in the cervical region compared to the thoracic or lumbar region due to anatomical variation, steep pedicle angle, and smaller pedicle size.2,27 The use of SPC has known associations with stereotaxic errors. 42 The stability of the SPC to the mounting platform is affected by the intersegmental mobility of the cervical spine, which can shift during surgical manipulation. 27 Flexion during patient positioning may minimize intersegmental mobility. 43 Another potential source of inaccuracy is disruption of the reference array during the procedure leading to spine shift (i.e., movement of the spine in relation to the robotic system’s understanding of the position of the robot arm). Navigational error of 2 mm has been observed at 2 levels away from the reference array. 27 When misalignment was noticed, another O-arm image was acquired, the system was reregistered, and screw trajectory and size were replanned. In real life, this process adds to operating room time, and improvement in this technology will improve workflow efficiency.
Limitations
This study has some limitations. These results are limited due to the nature of a feasibility study, limited sample size, patient sex, and lack of bone pathology. Due to limitations with the robotic software, not all available pedicles could be instrumented. Accuracy with percutaneous pedicle screw placement can be influenced by minor alterations in patient positioning or from friction along the screw shaft and insertion devices from skin, fascia, or muscle. 44 Furthermore, achieving lateral exposure with a percutaneous approach is challenging. Cadaveric tissue preservation alter tissue texture and dehydration of discs lead to increased stiffness. 27 The Gertzbein and Robbins classification is commonly used for assessing PS position. However, it should be noted that the system was originally designed for the thoracolumbar regions. Further investigations are necessary for the development of a CPS placement classification. There is an inherent limitation to TPA measurement. The angle may be influenced by the slicing of the acquired volume from the 3D-CT scan.
Conclusion
Given the accuracy and breach rate associated with RA-CPS, further advancement in robotic software, instrumentation, and navigational systems is necessary for safe and accurate CPS placement. Preoperative planning for the feasibility of CPS placement is recommended because anatomical variations, such as insufficient PW of the cortical pedicles, may favor the placement of lateral mass screws instead.
Supplemental Material
Supplemental Material, sj-jpg-1-gsj-10.1177_21925682211068410 for Anatomical and Technical Considerations of Robot-Assisted Cervical Pedicle Screw Placement: A Cadaveric Study by Jennifer Z. Mao, Mohamed A.R. Soliman, Brian A. Karamian, Asham Khan, Alexander G. Fritz, Naval Avasthi, Stephen DiMaria, Bennett R. Levy, Timothy E. O’Connor, Gregory Schroeder, John Pollina, Alexander R. Vaccaro and Jeffrey P. Mullin in Global Spine Journal
Acknowledgments
The authors thank Ryan A. Rava, PhD Candidate, for his medical imaging expertise, Paul H. Dressel, BFA, for formatting the illustrations, Debra J. Zimmer for editorial assistance, and Kelly Mackey for formatting assistance.
Appendix
Abbreviations
- CAN
computer-assisted navigation
- CPS
cervical pedicle screw(s)
- CT
computed tomography
- MSL
maximal screw length
- PH
pedicle height
- PW
pedicle width
- RA
robot-assisted
- SPA
sagittal pedicle angle
- TPA
transverse pedicle angle
- VA
vertebral artery
- 3D
3-Dimensional
Footnotes
Author Contribution: Conception and design: Mao, Mullin, Acquisition of data: Mao, Interpretation of data: All authors, Drafting the manuscript: Mao, Critically revising the manuscript: All authors, Reviewed submitted version of manuscript: All authors, Statistical analysis: Mao, Study supervision: Mullin.
Declaration of Conflicting Interests: Ms. Mao: Research funding: AOSpine North America (AOSNA) for works to advance 3D printing, Dr. Soliman: None, Dr. Karamian: None, Dr. Khan: Research grant: Scoliosis Research Society to study scoliosis in Chiari patients, Mr. Fritz: None, Mr. Avasthi: None, Mr. DiMaria: None, Mr. Levy: None, Dr. O’Connor: Research funding: AOSpine Foundation as part of the Discovery and Innovation Award, Dr. Schroeder: Speaker’s Bureau/Honorarium: Zimmer, Stryker, Medtronic, AOSpine, Teladoc, Wolters Kluwer, Bioventus; Consultant: Advanced Medical, Zimmer, Stryker, Medtronic; Editorial Board: Clinical Spine Surgery; Board of Directors/Committee: Cervical Spine Research Society, Dr. Pollina: Surgical training: Medtronic; Consultant and Royalties: ATEC Spine, Dr. Vaccaro: Royalties, Financial, or Material Support: Aesculap, Atlas Spine, Elsevier, Globus, Jaypee, Medtronic, SpineWave, Stryker Spine; Taylor Francis/Hodder and Stoughton, Thieme; Stock/stock options: Advanced Spinal Intellectual Properties, Atlas Spine, Avaz Surgical, Bonovo Orthopaedics, Computational Biodynamics, Cytonics, Deep Health, Dimension Orthotics LLC, Electrocore, Flagship Surgical, FlowPharma, Globus, Innovative Surgical Design, Insight Therapeutics, Jushi, Nuvasive, Orthobullets, Paradigm Spine, Parvizi Surgical Innovation, Progressive Spinal Technologies, Replication Medica, Spine Medica, Spinology, Stout Medical, Vertiflex, ViewFi Health, and Dr. Mullin is involved with clinical research for Cerapedics. He receives research funding from AOSpine North America (AOSNA) and the Research Committee Award #87639; and from Medtronic External Research Program Health Professionals, ERP ID#2020-12271.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research support toward this study was received from Medtronic Sofamor Danek USA.
Ethical Approval: Because this study was a case series of cadavers, an institutional board review was deemed unnecessary.
Data Availability: Data that support the findings of this study are available from the corresponding author on reasonable request.
Supplemental material: Supplemental material for this article is available online.
ORCID iDs
Jennifer Z. Mao, MBA https://orcid.org/0000-0001-8336-3808
Brian A. Karamian, MD https://orcid.org/0000-0003-0512-6019
John Pollina, MD https://orcid.org/0000-0002-6234-4517
Jeffrey P. Mullin, MD https://orcid.org/0000-0003-0640-2329
References
- 1.Kosmopoulos V, Schizas C. Pedicle screw placement accuracy: a meta-analysis. Spine. 2007;32(3):E111-E120. [DOI] [PubMed] [Google Scholar]
- 2.Nooh A, Lubov J, Aoude A, et al. Differences between manufacturers of computed tomography-based computer-assisted surgery systems do exist: A systematic literature review. Global Spine J. 2017;7(1):83-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fatima N, Massaad E, Hadzipasic M, Shankar GM, Shin JH. Safety and accuracy of robot-assisted placement of pedicle screws compared to conventional free-hand technique: a systematic review and meta-analysis. Spine J. 2020. [DOI] [PubMed] [Google Scholar]
- 4.Khan A, Meyers JE, Siasios I, Pollina J. Next-Generation Robotic Spine Surgery: First Report on Feasibility, Safety, and Learning Curve. Oper Neurosurg (Hagerstown). 2019;17(1):61-69. [DOI] [PubMed] [Google Scholar]
- 5.Hyun SJ, Kim KJ, Jahng TA, Kim HJ. Minimally Invasive Robotic Versus Open Fluoroscopic-guided Spinal Instrumented Fusions: A Randomized Controlled Trial. Spine. 2017;42(6):353-358. [DOI] [PubMed] [Google Scholar]
- 6.Malham GM, Wells-Quinn T. What should my hospital buy next?-Guidelines for the acquisition and application of imaging, navigation, and robotics for spine surgery. J Spine Surg. 2019;5(1):155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pechlivanis I, Kiriyanthan G, Engelhardt M, et al. Percutaneous placement of pedicle screws in the lumbar spine using a bone mounted miniature robotic system: first experiences and accuracy of screw placement. Spine. 2009;34(4):392-398. [DOI] [PubMed] [Google Scholar]
- 8.Hoeckelmann M, Rudas IJ, Fiorini P, Kirchner F, Haidegger T. Current Capabilities and Development Potential in Surgical Robotics. Int J Adv Rob Syst. 2015;12(5):61. [Google Scholar]
- 9.Steinmetz MP, Stewart TJ, Kager CD, Benzel EC, Vaccaro AR. Cervical deformity correction. Neurosurgery. 2007;60(suppl 1):S90-S97. [DOI] [PubMed] [Google Scholar]
- 10.Shimokawa N, Takami T. Surgical safety of cervical pedicle screw placement with computer navigation system. Neurosurg Rev. 2017;40(2):251-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duff J, Hussain MM, Klocke N, et al. Does pedicle screw fixation of the subaxial cervical spine provide adequate stabilization in a multilevel vertebral body fracture model? An in vitro biomechanical study. Clin Biomech. 2018;53:72-78. [DOI] [PubMed] [Google Scholar]
- 12.Jo DJ, Seo EM, Kim KT, Kim SM, Lee SH. Cervical pedicle screw insertion using the technique with direct exposure of the pedicle by laminoforaminotomy. J Korean Neurosurg Soc. 2012;52(5):459-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo Y, Lee SB, Lee BJ, et al. The Learning Curve of Subaxial Cervical Pedicle Screw Placement: How Can We Avoid Neurovascular Complications in the Initial Period? Oper Neurosurg (Hagerstown). 2019;17(6):603-607. [DOI] [PubMed] [Google Scholar]
- 14.Arab A, Alkherayf F, Sachs A, Wai EK. Use of 3D Navigation in Subaxial Cervical Spine Lateral Mass Screw Insertion. J Neurol Surg Rep. 2018;79(1):e1-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chachan S, Bin Abd Razak HR, Loo WL, Allen JC, Shree Kumar D. Cervical pedicle screw instrumentation is more reliable with O-arm-based 3D navigation: analysis of cervical pedicle screw placement accuracy with O-arm-based 3D navigation. Eur Spine J. 2018;27(11):2729-2736. [DOI] [PubMed] [Google Scholar]
- 16.Gan G, Kaliya-Perumal AK, Yu CS, Nolan CP, Oh JY. Spinal Navigation for Cervical Pedicle Screws: Surgical Pearls and Pitfalls. Global Spine J. 2020:2192568220902093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Su XJ, Lv ZD, Chen Z, et al. Comparison of Accuracy and Clinical Outcomes of Robot-Assisted Versus Fluoroscopy-Guided Pedicle Screw Placement in Posterior Cervical Surgery. Global Spine J. 2020:2192568220960406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston TL, Karaikovic EE, Lautenschlager EP, Marcu D. Cervical pedicle screws vs. lateral mass screws: uniplanar fatigue analysis and residual pullout strengths. Spine J. 2006;6(6):667-672. [DOI] [PubMed] [Google Scholar]
- 19.Jones EL, Heller JG, Silcox DH, Hutton WC. Cervical pedicle screws versus lateral mass screws. Anatomic feasibility and biomechanical comparison. Spine. 1997;22(9):977-982. [DOI] [PubMed] [Google Scholar]
- 20.Horn SR, Passias PG, Hockley A, et al. Cost-utility of revisions for cervical deformity correction warrants minimization of reoperations. J Spine Surg. 2018;4(4):702-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neo M, Sakamoto T, Fujibayashi S, Nakamura T. The clinical risk of vertebral artery injury from cervical pedicle screws inserted in degenerative vertebrae. Spine. 2005;30(24):2800-2805. [DOI] [PubMed] [Google Scholar]
- 22.Yoshihara H, Passias PG, Errico TJ. Screw-related complications in the subaxial cervical spine with the use of lateral mass versus cervical pedicle screws: a systematic review. J Neurosurg Spine. 2013;19(5):614-623. [DOI] [PubMed] [Google Scholar]
- 23.Yukawa Y, Kato F, Ito K, et al. Placement and complications of cervical pedicle screws in 144 cervical trauma patients using pedicle axis view techniques by fluoroscope. Eur Spine J. 2009;18(9):1293-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kostrzewski S, Duff JM, Baur C, Olszewski M. Robotic system for cervical spine surgery. Int J Med Robot. 2012;8(2):184-190. [DOI] [PubMed] [Google Scholar]
- 25.Onibokun A, Khoo LT, Bistazzoni S, Chen NF, Sassi M. Anatomical considerations for cervical pedicle screw insertion: the use of multiplanar computerized tomography measurements in 122 consecutive clinical cases. Spine J. 2009;9(9):729-734. [DOI] [PubMed] [Google Scholar]
- 26.O'Connor TE, O'Hehir MM, Khan A, et al. Mazor X Stealth Robotic Technology: A Technical Note. World Neurosurg. 2020;145:435-442. [DOI] [PubMed] [Google Scholar]
- 27.Guha D, Jakubovic R, Gupta S, et al. Intraoperative Error Propagation in 3-Dimensional Spinal Navigation From Nonsegmental Registration: A Prospective Cadaveric and Clinical Study. Global Spine J. 2019;9(5):512-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng P, Chen K, Chen H, et al. Comparison of O-arm navigation and microscope-assisted minimally invasive transforaminal lumbar interbody fusion and conventional transforaminal lumbar interbody fusion for the treatment of lumbar isthmic spondylolisthesis. J Orthop Translat. 2020;20:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gertzbein SD, Robbins SE. Accuracy of pedicular screw placement in vivo. Spine. 1990;15(1):11-14. [DOI] [PubMed] [Google Scholar]
- 30.Karaikovic EE, Daubs MD, Madsen RW, Gaines RW., Jr. Morphologic characteristics of human cervical pedicles. Spine. 1997;22(5):493-500. [DOI] [PubMed] [Google Scholar]
- 31.Tomasino A, Parikh K, Koller H, et al. The vertebral artery and the cervical pedicle: morphometric analysis of a critical neighborhood. J Neurosurg Spine. 2010;13(1):52-60. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Napolitano JT, Ebraheim NA. Systematic review of cervical pedicle dimensions and projections. Spine. 2010;35(24):E1373-E1380. [DOI] [PubMed] [Google Scholar]
- 33.Panjabi MM, Duranceau J, Goel V, Oxland T, Takata K. Cervical human vertebrae. Quantitative three-dimensional anatomy of the middle and lower regions. Spine. 1991;16(8):861-869. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto T, Neo M, Nakamura T. Transpedicular screw placement evaluated by axial computed tomography of the cervical pedicle. Spine. 2004;29(22):2510-2515. discussion 2515. [DOI] [PubMed] [Google Scholar]
- 35.Chazono M, Soshi S, Inoue T, Kida Y, Ushiku C. Anatomical considerations for cervical pedicle screw insertion: the use of multiplanar computerized tomography reconstruction measurements. J Neurosurg Spine. 2006;4(6):472-477. [DOI] [PubMed] [Google Scholar]
- 36.Lee DH, Lee SW, Kang SJ, et al. Optimal entry points and trajectories for cervical pedicle screw placement into subaxial cervical vertebrae. Eur Spine J. 2011;20(6):905-911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urakov TM, Chang KH, Burks SS, Wang MY. Initial academic experience and learning curve with robotic spine instrumentation. Neurosurg Focus. 2017;42(5):E4. [DOI] [PubMed] [Google Scholar]
- 38.Lieberman IH, Kisinde S, Hesselbacher S. Robotic-Assisted Pedicle Screw Placement During Spine Surgery. JBJS Essent Surg Tech. 2020;10(2):e0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang ZF. Freehand Pedicle Screw Placement Using a Universal Entry Point and Sagittal and Axial Trajectory for All Subaxial Cervical, Thoracic and Lumbosacral Spines. Orthop Surg. 2020;12(1):141-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan KA, Lin S, Chin BZ, Thadani VN, Hey HWD. Anatomic techniques for cervical pedicle screw placement. J Spine Surg. 2020;6(1):262-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuo KL, Su YF, Wu CH, et al. Assessing the Intraoperative Accuracy of Pedicle Screw Placement by Using a Bone-Mounted Miniature Robot System through Secondary Registration. PLoS One. 2016;11(4):e0153235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vardiman AB, Wallace DJ, Crawford NR, Riggleman JR, Ahrendtsen LA, Ledonio CG. Pedicle screw accuracy in clinical utilization of minimally invasive navigated robot-assisted spine surgery. J Robot Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anderst WJ, Donaldson WF, 3rd, Lee JY, Kang JD. Cervical motion segment percent contributions to flexion-extension during continuous functional movement in control subjects and arthrodesis patients. Spine. 2013;38(9):E533-E539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hussain I, Cosar M, Kirnaz S, et al. Evolving Navigation, Robotics, and Augmented Reality in Minimally Invasive Spine Surgery. Global Spine J. 2020;10(suppl l-2):22s-33s. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-jpg-1-gsj-10.1177_21925682211068410 for Anatomical and Technical Considerations of Robot-Assisted Cervical Pedicle Screw Placement: A Cadaveric Study by Jennifer Z. Mao, Mohamed A.R. Soliman, Brian A. Karamian, Asham Khan, Alexander G. Fritz, Naval Avasthi, Stephen DiMaria, Bennett R. Levy, Timothy E. O’Connor, Gregory Schroeder, John Pollina, Alexander R. Vaccaro and Jeffrey P. Mullin in Global Spine Journal