Abstract
Study Design
Retrospective database study.
Objective
Navigation has been increasingly used to treat degenerative disease, with positive radiographic and clinical outcomes and fewer adverse events and reoperations, despite increased operative time. However, short-term analysis on treating adult spinal deformity (ASD) surgery with navigation is limited, particularly using large nationally represented cohorts. This is the first large-scale database study to compare 30-day readmission, reoperation, morbidity, and value-per-operative time for navigated and conventional ASD surgery.
Methods
Adults were identified in the National Surgical Quality Improvement Program (NSQIP) database. Multivariate regression was used to compare outcomes between navigated and conventional surgery and to control for predictors and baseline differences.
Results
3190 ASD patients were included. Navigated and conventional patients were similar. Navigated cases had greater operative time (405 vs 320 min) and mean RVUs per case (81.3 vs 69.7), and had more supplementary pelvic fixations (26.1 vs 13.4%) and osteotomies (50.3 vs 27.7%) (P <.001).
In univariate analysis, navigation had greater reoperation (9.9 vs 5.2%, P = .011), morbidity (57.8 vs 46.8%, P = .007), and transfusion (52.2 vs 41.8%, P = .010) rates. Readmission was similar (11.9 vs 8.4%). In multivariate analysis, navigation predicted reoperation (OR = 1.792, P = .048), but no longer predicted morbidity or transfusion. Most reoperations were infectious and hardware-related.
Conclusions
Despite controlling for patient-related and procedural factors, navigation independently predicted a 79% increased odds of reoperation but did not predict morbidity or transfusion. Readmission was similar between groups. This is explained, in part, by greater operative time and transfusion, which are risk factors for infection. Reoperation most frequently occurred for wound- and hardware-related reasons, suggesting navigation carries an increased risk of infectious-related events beyond increased operative time.
Keywords: computer assisted navigation, spinal navigation, deformity, fusion, readmission, reoperation, morbidity, complications, infection
Introduction
The frequency of adult spinal deformity (ASD) surgery has been increasing due to technological improvements patient safety and outcomes.1,2 The intricate anatomy of the spine along with the additional complexities associated with spinal deformity surgery has led to the development of new techniques designed to minimize adverse events.2-4 Computer assisted navigation is one such technique. Navigation provides live intraoperative feedback of anatomic features, enabling precise placement of spinal instrumentation through different trajectories. This is particularly beneficial when operating on patients with distorted anatomy, such as is the case with revision surgery, where rates of pedicle screw malpositioning are often as high as 42%.
Currently, navigation is primarily used for pedicle screw placement and evaluation of deformity correction, with the goal of minimizing complications such as infection, excessive blood loss and transfusion, and hardware failure.2,5,6 However, this comes at the cost of an increased operative time as registration is a rigorous and time-consuming step in navigation, requiring meticulous soft tissue dissection and bony landmark exposure necessary for accurate point and surface matching. Moreover, previous studies have shown mixed findings regarding the potential impact of navigation on complications and need for revisions.2,7 Given the consistent rise in use of navigation in treating ASD, and an increasing number of surgeons performing ASD surgeries due to technological advancements, evaluation of its benefits and safety is paramount.8,9
While current research has demonstrated positive clinical satisfaction and radiographic accuracy with the use of navigation for treating degenerative disease, there is a paucity in the literature with respect to short-term outcomes in treating adult deformity, particularly in large, nationally represented cohorts. 10 Current literature is largely limited to preliminary data. 7 Therefore, the purpose of this study was to compare 30-day readmission, reoperation, and morbidity rates, as well as value-per-operative time, for adults undergoing navigated and conventional posterior-only deformity surgery. This is the first large-scale database study to compare short-term outcomes in ASD surgery with and without navigation.
Materials and Methods
Study Design and Population
This study is a retrospective analysis of data from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) database, from 2005 to 2018. The NSQIP database began in 1994 as an initiative to improve the quality of surgical care in the Veterans Administration and later expanded to all participating hospitals in the United States. At present, there are over 680 participating hospitals with standardized clinical reviewers and routine site audits to ensure reliable data. The database has frequently been used in the spine literature. This project is exempt from IRB review as this database is de-identified and no direct patient involvement occurred.
Adult patients ≥18 years old who underwent posterior deformity surgery were identified using Current Procedural Terminology (CPT) codes 22800–22804. Patients were stratified into groups with and without navigation using CPT code 61873. Patients were excluded if they had anterior fusion, nonelective, or lesion-related CPT codes (Supplementary Appendix A).
Outcomes and Variables
Primary outcomes were 30-day readmission, reoperation, overall morbidity, and specific complications. Readmission includes any inpatient stay to the same or another hospital related to the surgical procedure. Reoperation includes all major surgical procedures requiring return to the operating room for intervention of any kind. Morbidity includes infectious, pulmonary, cardiac, renal, neurological, hematologic, and thromboembolic complications reported in the ACS-NSQIP dataset. In addition, reasons for reoperation obtained from NSQIP-provided ICD 9, ICD 10, and CPT codes and were compared between navigated and conventional groups.
Primary outcomes, as well as specific complications, were compared between navigated and conventional groups. Predictors of primary outcomes were analyzed among the entire cohort. Variables evaluated as potential predictors included patient demographic, comorbidity, laboratory values, and procedural factors (Table 1). Procedural factors specifically included operative time, length of hospital stay, relative value units (RVUs) per case and per minute of operative time, supplementary pelvic fixation, and osteotomy (Supplementary Appendix A). Pelvic fixation and osteotomy were evaluated as predictor variables as they are often utilized in adult deformity surgery and add an additional level of surgical invasiveness, complexity, and morbidity to the overall procedure and consequently can bias the results. Thus, the weighted odds ratios provided by the final multivariate analyses include the influence of adjunctive pelvic fixation and osteotomy on 30-day outcomes.
Table 1.
Baseline Differences in Patient Demographic, Comorbidity, Laboratory, and Procedural Factors by Presence or Absence of Computer Assisted Surgery.
| Navigated, n (%) | Conventional, n (%) | P | Cases available | |
|---|---|---|---|---|
| N = 161 (5.0%) | N = 3029 (95.0%) | 3190 | ||
| Demographics | ||||
| Mean age (years; SD) | 56.1 (18.9) | 56.2 (18.6) | .970 | 3187 |
| Nonwhite race | 12 (8.4%) | 289 (11.0%) | .323 | 2762 |
| Hispanic ethnicity | 8 (5.5%) | 109 (4.1%) | .411 | 2795 |
| Female gender | 110 (68.3%) | 1855 (61.2%) | .072 | 3190 |
| Comorbidities | ||||
| Obese | 56 (35.7%) | 1080 (36.6%) | .821 | 3111 |
| Smoker | 34 (21.1%) | 554 (18.3%) | .367 | 3190 |
| Dyspnea | 9 (5.6%) | 131 (4.3%) | .445 | 3190 |
| Diabetes mellitus | 22 (13.7%) | 401 (13.2%) | .877 | 3190 |
| COPD | 8 (5.0%) | 116 (3.8%) | .466 | 3190 |
| Heart failure | 2 (1.2%) | 6 (0.2%) | .058 a | 3190 |
| Hypertension | 73 (45.3%) | 1418 (46.8%) | .715 | 3190 |
| Disseminated cancer | 2 (1.2%) | 65 (2.1%) | .774 a | 3190 |
| Chronic steroid use | 4 (2.5%) | 167 (5.5%) | .096 | 3190 |
| Bleeding disorder | 4 (2.5%) | 75 (2.5%) | 1.000 a | 3190 |
| Preop transfusion >4 units | 4 (2.5%) | 32 (1.1%) | .105 a | 3190 |
| Discharged to rehabilitation | 61 (38.4%) | 844 (29.4%) | .017 | 3025 |
| Dependent functional status | 7 (4.4%) | 220 (7.3%) | .167 | 3172 |
| ASA-class ≥3 | 103 (64.0%) | 1646 (54.7%) | .021 | 3172 |
| Lab values (mean; SD) | ||||
| Creatinine | 0.91 (0.71) | 0.87 (0.47) | .287 | 2852 |
| White cell count | 7.40 (2.45) | 7.43 (2.62) | .887 | 2985 |
| Hematocrit | 38.98 (5.08) | 39.46 (4.95) | .267 | 3001 |
| Procedural factors | ||||
| Operative time | 405 (167) | 320 (140) | <.001 | 3183 |
| Length of stay | 7.8 (6.3) | 7.1 (6.9) | .243 | 3178 |
| Total RVUs | 88.11 (33.96) | 69.71 (35.51) | <.001 | 3190 |
| RVUs per minute | 0.24 (0.10) | 0.25 (0.17) | .491 | 3183 |
| Total RVUs minus navigation | 81.30 (33.96) | 69.71 (35.51) | <.001 | 3190 |
| Pelvic fixation | 42 (26.1%) | 407 (13.4%) | <.001 | 3190 |
| Osteotomy | 81 (50.3%) | 840 (27.7%) | <.001 | 3190 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; RVU, relative value unit; SD, standard deviation.
Bold values indicate significance (P <.05).
aFisher’s Exact Test.
Statistical Analysis
Analyses were completed in SPSS (IBM Corp.). Demographic, comorbidity, laboratory, and procedural factors were individually analyzed for baseline differences between navigated and conventional patients using Student t test for continuous and chi-squared or Fisher’s exact test for categorical variables. The above factors were also individually analyzed for association with primary outcomes using univariate logistic regression. Variables significant in the univariate analyses (P < .05), and navigated vs conventional surgery type, were then evaluated for significance (P < .05) as independent predictors and control variables in a series of multivariate logistic regression analyses of primary outcomes.
Results
Baseline Differences
There were 3190 patients included, with conventional surgery utilized in 3029 (95.0%) and navigation utilized in 161 (5.0%). Significant baseline differences were only observed in two of 21 demographic and comorbidity variables (Table 1). Patients with navigation were significantly more likely to be discharged to rehabilitation (38.4 vs 29.4%, P = .017) and to have an ASA-class ≥3 (64.0 vs 54.7%, P = .021), compared to those without navigation, respectively.
Patients with navigation had significantly longer mean operative times (405 vs 320 mins, P < .001) and total mean RVUs per case (88.1 vs 69.7, P < .001) but had statistically similar RVUs per minute per case (.24 vs .25, P = .491) compared to those without navigation, respectively. When subtracting for the value of RVUs for navigation, 6.81, from the cases with navigation, patients with navigation still had greater mean RVUs per case (81.3 vs 69.7, P <. 001). Cases utilizing navigation were also more likely to have adjunctive pelvic fixation (26.1 vs 13.4%, P < .001) and osteotomy (50.3 vs 27.7%, P < .001). Length of stay was similar between groups (7.8 vs 7.1 days, P = .243).
Primary Unadjusted and Adjusted Outcomes
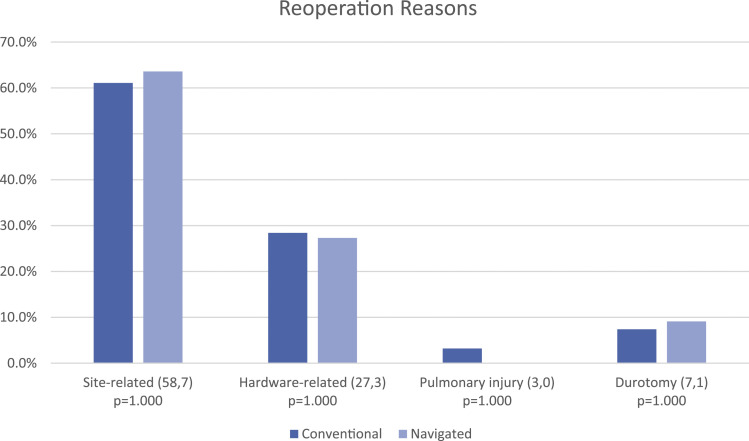
In univariate analysis (Table 2), navigation was associated with greater rates of reoperation (9.9 vs 5.2%, P = .011) and morbidity (57.8 vs 46.8%, P = .007), and specifically transfusion (52.2 vs 41.8%, P = .010). Readmission rates were similar between groups (11.9 vs 8.4%, P = .162). After adjusting for significant baseline differences and predictor variables in multivariate analysis (Tables 3-5), navigation independently predicted reoperation (OR = 1.792, P = .048, CI 95%: 1.004–3.197), but no longer predicted morbidity or transfusion. Readmission remained insignificant. The majority of reoperations were site-related, including infection, dehiscence, and hematoma, followed by hardware-related (Figure 1). There were no differences in reasons for reoperation between navigated and conventional groups.
Table 2.
Univariate and Multivariate Analyses of Primary Outcomes and Specific Complications by Presence or Absence of Computer Assisted Surgery.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Navigated, n (%) | Conventional, n (%) | P | OR (95% CI) | P | |
| (N = 161) | (N = 3029) | ||||
| Primary outcomes | |||||
| Readmission a | 16 (11.9%) | 203 (8.4%) | .162 | 1.519 (0.831, 2.775) | .174 |
| Reoperation | 16 (9.9%) | 159 (5.2%) | .011 | 1.792 (1.004, 3.197) | .048 |
| Days to reoperation | 17.9 (9.6) | 13.9 (9.0) | .112 | ||
| Morbidity | 93 (57.8%) | 1417 (46.8%) | .007 | 0.704 (0.460, 1.078) | .107 |
| Specific complications | |||||
| Wound complication | 6 (3.7%) | 117 (3.9%) | .930 | ||
| Pulmonary complication | 13 (8.1%) | 160 (5.3%) | .127 | ||
| Urinary tract infection | 7 (4.3%) | 85 (2.8%) | .228 b | ||
| Stroke | 0 | 17 (0.6%) | 1.000 b | ||
| Myocardial infarction | 3 (1.9%) | 16 (0.5%) | .068 b | ||
| Cardiac arrest | 1 (0.6%) | 16 (0.5%) | .586 b | ||
| Transfusion | 84 (52.2%) | 1267 (41.8%) | .010 | 0.656 (0.426, 1.010) | .056 |
| Deep venous thrombosis | 4 (2.5%) | 49 (1.6%) | .341 b | ||
| Sepsis/septic shock | 6 (3.7%) | 69 (2.3%) | .275 b | ||
Bold values indicate significance (P < .05). Wound complication includes superficial, deep, and organ-space wound infection and dehiscence. Pulmonary complication includes pneumonia, reintubation, pulmonary embolism, and prolonged ventilation. Data from the complete multivariate analyses for readmission, reoperation, and morbidity are provided in Tables 3–5, respectively.
aReadmission data were not collected by NSQIP until 2011; all patients included from 2011 to 2018 had readmission data: Of those 2554 patients, 135 (5.3%) had navigation and 2419 (94.7%) did not.
bFisher’s exact test.
Table 3.
Univariate and Multivariate Analysis of Predictors of Readmission.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Readmitted (N = 219) | Not Readmitted (N = 2335) | P | OR (95% CI) | P | |
| Demographics | |||||
| Mean age (years; SD) | 61 (16) | 56 (18) | <.001 | 1.010 (0.999, 1.021) | .089 |
| Nonwhite race | 18 (9.2%) | 214 (10.6%) | .549 | ||
| Hispanic ethnicity | 7 (3.6%) | 82 (4.0%) | .813 | ||
| Female gender | 123 (56.2%) | 1461 (62.6%) | .062 | ||
| Comorbidities | |||||
| Obese | 84 (39.6%) | 835 (36.5%) | .374 | ||
| Smoker | 37 (16.9%) | 436 (18.7%) | .517 | ||
| Dyspnea | 15 (6.8%) | 101 (4.3%) | .086 | ||
| Diabetes mellitus | 42 (19.2%) | 305 (13.1%) | .012 | 1.366 (0.928, 2.011) | .114 |
| COPD | 12 (5.5%) | 92 (3.9%) | .270 | ||
| Heart failure | 1 (0.5%) | 6 (0.3%) | .467 a | ||
| Hypertension | 125 (57.1%) | 1060 (45.4%) | .001 | 1.261 (0.895, 1.777) | .185 |
| Disseminated cancer | 11 (5.0%) | 48 (2.1%) | .005 | 2.334 (1.133, 4.807) | .021 |
| Chronic steroid use | 23 (10.5%) | 123 (5.3%) | .001 | 1.786 (1.093, 2.917) | .020 |
| Bleeding disorder | 12 (5.5%) | 61 (2.6%) | .015 | 1.871 (0.957, 3.658) | .067 |
| Preop transfusion >4 units | 6 (2.7%) | 25 (1.1%) | .044 a | 2.376 (0.927, 6.091) | .072 |
| Discharged to rehabilitation | 88 (40.2%) | 694 (29.8%) | .002 | 1.115 (0.793, 1.567) | .531 |
| Dependent functional status | 28 (12.8%) | 155 (6.7%) | .001 | 1.792 (1.111, 2.892) | .017 |
| ASA-class ≥3 | 147 (67.1%) | 1299 (55.9%) | .001 | 0.972 (0.687, 1.375) | .871 |
| Lab values (mean; SD) | |||||
| Creatinine | 0.96 (0.70) | 0.86 (0.41) | .003 | 1.284 (1.023, 1.612) | .031 |
| White cell count | 7.28 (2.52) | 7.49 (2.68) | .266 | ||
| Hematocrit | 38.98 (5.19) | 39.47 (4.91) | .165 | ||
| Procedural factors | |||||
| Operative time | 335 (148) | 323 (141) | .236 | 1.000 (0.999, 1.001) | .833 |
| Length of stay | 8.1 (5.7) | 7.1 (7.0) | .042 | 1.003 (0.983, 1.024) | .774 |
| Total RVUs | 76.42 (40.12) | 71.59 (34.88) | .053 | 1.002 (0.997, 1.007) | .483 |
| Pelvic fixation | 41 (18.7%) | 335 (14.3%) | .081 | 1.251 (0.823, 1.900) | .294 |
| Osteotomy | 67 (30.6%) | 702 (30.1%) | .870 | 0.939 (0.629, 1.402) | .759 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; PF, pelvic fixation; SD, standard deviation.
Bold values indicate significance (P <.05). See Table 2 for univariate and multivariate results for computer assistance as a predictor of readmission.
aFisher’s exact test.
Table 5.
Univariate and Multivariate Analysis of Predictors of Morbidity.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Morbidity (N = 1510) | No morbidity (N = 1680) | P | OR (95% CI) | P | |
| Demographics | |||||
| Mean age (years; SD) | 58 (18) | 55 (19) | <.001 | 1.002 (0.996, 1.008) | .474 |
| Nonwhite race | 150 (11.6%) | 151 (10.3%) | .255 | ||
| Hispanic ethnicity | 50 (3.8%) | 67 (4.5%) | .336 | ||
| Female gender | 1010 (66.9%) | 955 (56.8%) | <.001 | 1.335 (1.098, 1.624) | .004 |
| Comorbidities | |||||
| Obese | 533 (36.1%) | 603 (36.9%) | .656 | ||
| Smoker | 224 (14.8%) | 364 (21.7%) | <.001 | 0.649 (0.512, 0.824) | <.001 |
| Dyspnea | 82 (5.4%) | 58 (3.5%) | .006 | 1.128 (0.726, 1.752) | .593 |
| Diabetes mellitus | 206 (13.6%) | 217 (12.9%) | .546 | ||
| COPD | 65 (4.3%) | 59 (3.5%) | .247 | ||
| Heart failure | 4 (0.3%) | 4 (0.2%) | 1.000 a | ||
| Hypertension | 753 (49.9%) | 738 (43.9%) | .001 | 1.006 (0.813, 1.243) | .959 |
| Disseminated cancer | 38 (2.5%) | 29 (1.7%) | .120 | ||
| Chronic steroid use | 90 (6.0%) | 81 (4.8%) | .154 | ||
| Bleeding disorder | 51 (3.4%) | 28 (1.7%) | .002 | 1.575 (0.866, 2.864) | .136 |
| Preop transfusion >4 units | 29 (1.9%) | 7 (0.4%) | <.001 | 1.780 (0.655, 4.837) | .258 |
| Discharged to rehabilitation | 604 (42.2%) | 301 (18.9%) | <.001 | 1.503 (1.206, 1.872) | <.001 |
| Dependent functional status | 146 (9.7%) | 81 (4.8%) | <.001 | 1.631 (1.124, 2.367) | .010 |
| ASA-class ≥3 | 965 (64.0%) | 784 (47.1%) | <.001 | 1.105 (0.905, 1.349) | .328 |
| Lab values (mean; SD) | |||||
| Creatinine | 0.88 (0.61) | 0.86 (0.32) | .399 | ||
| White cell count | 7.28 (2.80) | 7.57 (2.42) | .002 | 0.977 (0.942, 1.012) | .194 |
| Hematocrit | 38.41 (5.24) | 40.37 (4.50) | <.001 | 0.934 (0.914, 0.953) | <.001 |
| Procedural factors | |||||
| Operative time | 385 (144) | 269 (118) | <.001 | 1.006 (1.005, 1.007) | <.001 |
| Length of stay | 9.0 (7.5) | 5.5 (5.7) | <.001 | 1.079 (1.056, 1.102) | <.001 |
| Total RVUs | 81.41 (37.45) | 60.97 (30.93) | <.001 | 1.004 (1.0003, 1.007) | .033 |
| Pelvic fixation | 349 (23.1%) | 100 (6.0%) | <.001 | 1.818 (1.351, 2.445) | <.001 |
| Osteotomy | 617 (40.9%) | 304 (18.1%) | <.001 | 1.481 (1.167, 1.880) | .001 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; PF, pelvic fixation; SD, standard deviation.
Bold values indicate significance (P <.05). See Table 2 for univariate and multivariate results for computer assistance as a predictor of morbidity and specific complications.
aFisher’s exact test.
Figure 1.
Reasons for reoperation. Included in parentheses are number of instances of occurrences of stated reason for reoperation for conventional and navigated cases, respectively.
Predictor Analysis
There were 219 readmissions (8.6%) in 2554 patients. On multivariate analysis, history of cancer (OR = 2.334, P = .021), dependent functional status (OR = 1.792, P = .017), and increased creatinine (OR = 1.284, P = .031) predicted readmission (Table 3).
There were 175 reoperations (5.5%) in 3190 patients. Discharge to rehabilitation (OR = 1.852, P = .001) and length of stay (OR = 1.056, P < .001) predicted reoperation (Table 4).
Table 4.
Univariate and Multivariate Analysis of Predictors of Reoperation.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Reoperation (N = 175) | No reoperation (N = 3015) | P | OR (95% CI) | P | |
| Demographics | |||||
| Mean age (years; SD) | 58 (18) | 56 (19) | .117 | ||
| Nonwhite race | 20 (13.8%) | 281 (10.7%) | .250 | ||
| Hispanic ethnicity | 4 (2.7%) | 113 (4.3%) | .370 | ||
| Female gender | 97 (55.4%) | 1868 (62.0%) | .084 | ||
| Comorbidities | |||||
| Obese | 63 (36.8%) | 1073 (36.5%) | .927 | ||
| Smoker | 29 (16.6%) | 559 (18.5%) | .514 | ||
| Dyspnea | 10 (5.7%) | 130 (4.3%) | .379 | ||
| Diabetes mellitus | 30 (17.1%) | 393 (13.0%) | .119 | ||
| COPD | 9 (5.1%) | 115 (3.8%) | .377 | ||
| Heart failure | 1 (0.6%) | 7 (0.2%) | .364 a | ||
| Hypertension | 85 (48.6%) | 1406 (46.6%) | .617 | ||
| Disseminated cancer | 7 (4.0%) | 60 (2.0%) | .094 a | ||
| Chronic steroid use | 13 (7.4%) | 158 (5.2%) | .212 | ||
| Bleeding disorder | 11 (6.3%) | 68 (2.3%) | .003 a | 2.251 (1.123, 4.511) | .022 |
| Preop transfusion >4 units | 4 (2.3%) | 32 (1.1%) | .132 a | ||
| Discharged to rehabilitation | 81 (51.3%) | 824 (28.7%) | <.001 | 1.852 (1.287, 2.665) | .001 |
| Dependent functional status | 19 (10.9%) | 208 (6.9%) | .051 | ||
| ASA-class ≥3 | 114 (65.1%) | 1635 (54.6%) | .006 | 0.980 (0.672, 1.429) | .916 |
| Lab values (mean; SD) | |||||
| Creatinine | 0.89 (0.54) | 0.87 (0.47) | .573 | ||
| White cell count | 7.53 (2.97) | 7.43 (2.59) | .615 | ||
| Hematocrit | 38.90 (5.51) | 39.47 (4.93) | .146 | ||
| Procedural factors | |||||
| Operative time | 358 (149) | 322 (143) | <.001 | 1.000 (0.998, 1.001) | .540 |
| Length of stay | 12.9 (11.8) | 6.8 (6.3) | <.001 | 1.056 (1.039, 1.073) | <.001 |
| Total RVUs | 80.92 (38.98) | 70.04 (35.37) | <.001 | 1.002 (0.996, 1.008) | .474 |
| Pelvic fixation | 39 (8.7%) | 410 (13.6%) | .001 | 1.255 (0.808, 1.951) | .312 |
| Osteotomy | 71 (40.6%) | 850 (28.2%) | <.001 | 1.305 (0.855, 1.992) | .217 |
Abbreviations: ASA, American Society of Anesthesiologists; COPD, chronic obstructive pulmonary disease; PF, pelvic fixation; SD, standard deviation.
Bold values indicate significance (P < .05). See Table 2 for univariate and multivariate results for computer assistance as a predictor of reoperation.
aFisher’s exact test.
There were 1510 patients who experienced morbidity (47%). Female gender (OR = 1.335, P = .004), rehabilitation discharge (OR = 1.503, P < .001), decreased hematocrit (OR = .934, P < .001), operative time (OR = 1.006, P < .001), length of stay (OR = 1.079, P < .001), total RVUs (OR = 1.004, P = .033), adjunctive pelvic fixation (OR = 1.818, P < .001), and osteotomy (OR = 1.481, P = .001) predicted morbidity (Table 5). Smoking history was protective of morbidity (OR = .649, P < .001).
Discussion
In the present study, navigation in posterior spinal deformity surgery was associated with a 79% increase in odds of reoperation, despite controlling for significant patient-related and procedural factors, including case complexity. Navigation no longer predicted morbidity or transfusion after controlling for significant factors, while readmission remained statistically similar between groups. Our reoperation and complication rates are in line with longer-term data in navigated cohorts. Smith et al. followed 291 ASD surgery patients over 2 years and found 28.2% of patients required at least one revision, which was associated with an additional 38 perioperative complications. There were also 199 delayed complications, with 42.6% of patients having at least one complication. 11
In the present study, cases utilizing navigation appeared to be more complex and larger than conventional cases. This is evidenced by a greater number of navigated patients being discharged to rehabilitation, a longer duration of hospital stay, and a greater proportion of adjunctive pelvic fixations and osteotomies being performed on the navigation cases, despite similar overall baseline patient characteristics. The cases utilizing navigation also had greater mean RVUs per case, even after subtracting-out the RVUs for navigation, which is a surrogate for case complexity. Therefore, the greater rates of reoperation seen with the navigation cases is likely explained, in part, by the longer operative times and greater numbers of transfusions being performed. Both increased operative time and transfusion are known risk factors for infection.12,13 Therefore, optimization of operative techniques is crucial for reducing repetition of surgical steps, thereby reducing operation time and improving safety. Cawley et al. outlined suggested measures for improving navigation accuracy, including positioning, dissection, reference frame, grip, and angle of attack. 14 Future studies should evaluate the effects of implementing these measures on outcomes.
The reoperation reason results provided in Figure 1 suggest infection played the largest role in reoperation. Infectious-related events may also be explained by potential accidental self-contamination during complex equipment setups or when relocating behind lead shields or into sub-sterile rooms during intraoperative O-arm spins, which may be greater with navigation. Likewise, navigation requires more operating room traffic and personnel, which may also contribute to higher infection rates. However, these points cannot be evaluated with the NSQIP dataset.
Further, the advent of navigation can allow surgeons to perform procedures that they might have otherwise not felt comfortable attempting prior to the availability of such technology. Consequently, less experienced surgeons performing more complex cases could be contributing to the similar rate of early hardware related failures observed as reasons for reoperation in both the navigated and conventional groups. While the NSQIP dataset is unable to directly evaluate a learning curve–related hypothesis, evidence exists when comparing operative times for navigated versus conventional surgeries. Patients operated on utilizing navigation had significantly longer operative times, possibly stemming from increased case complexity as well as an increase in time needed for robot setup and usage compared to freehand techniques. Keric et al. found a freehand technique to be approximately 15 minutes shorter than robot assisted transpedicular instrumentation. 15 Lonjon et al. found robot-assisted surgery to take over 2 hours longer on average compared to freehand. 16 Kim et al. reported freehand pedicle screw placement to be 30 minutes shorter compared to navigated screw placement. 17 On the contrary, Solomiichuk et al. found navigation to be 20 minutes shorter compared to conventional fluoroscopic technique. 18 The discrepancy of these studies suggests that surgeon experience and learning curve may play a substantial role in operative time. The associated learning curve has been a criticism of intraoperative navigation since its inception, with studies showing inverse relationship between experience and pedicle breach rate.19-21
Last, we identified specific comorbidities that independently predicted poorer outcomes. Identification of such predictors can allow surgeons to identify and potentially target interventions for patients who are at risk. Of note, the protective effect of a smoking history against morbidity is most likely related to the procoagulant properties of nicotine, thereby reducing transfusion and, consequently, overall case morbidity. Other studies had similar findings or did not identify smoking status to have an association with perioperative or delayed complications. 11 Previous research has been done on preoperative risk stratification and planning to minimize postoperative complications such as reoperation, infection, and rod failure.22-25 Preoperative predictive models have been developed to help stratify patients at risk for postoperative complications.23,26,27
Limitations
The NSQIP database offers thousands of standardized patients representing multiple institutions with data collected by trained reviewers. However, limitations exist. The database does not provide patient-reported outcomes or radiographic parameters or perioperative antibiotic data. Having these variables would provide additional points of discussion but ultimately would not impact the specific outcomes or conclusions outlined in our study. The NSQIP dataset also does not provide level-specific surgical details. While there is some evidence of increased infection risk when instrumentation crosses the L5-S1 level, long-construct adult deformity fusions are more-often-than-not reinforced with pelvic instrumentation, which was adequately controlled for in our multivariate analyses.
This study is also limited by the inability of the dataset to capture surgeon experience. It is possible that newer, less experienced surgeons are more frequently utilizing navigation, which could skew outcomes more favorably towards conventional surgery, particularly for reoperation. In addition, the dataset does not provide specific information about pedicle screw placement, such as cortical versus traditional screw fixation.
Conclusion
Computer assistance for ASD surgery has been increasing in frequency, with the goal of improving accuracy and limiting postoperative complications.1,4,28-31 On a large national scale, the results of this study failed to show that navigation achieves these goals in ASD surgery. We found that navigation was associated with a 79% increase in odds of reoperation, despite controlling for significant factors, including case complexity. Further research and large-scale long-term studies are necessary after navigation becomes more widely used to understand learning curve setbacks. This is the first large-scale database study to compare short-term outcomes in adult deformity surgery with and without navigation.
Supplemental Material
Supplemental Material, sj-pdf-1-gsj-10.1177_21925682211047551 for Impact of Navigation on 30-Day Outcomes for Adult Spinal Deformity Surgery by Austen D. Katz, Jesse Galina, Junho Song, Sayyida Hasan, Dean Perfetti, Sohrab Virk, Jeff Silber and David Essig in Global Spine Journal
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr. Silber receives teaching fees for Stryker. Dr. Essig receives consulting fees for Stryker and DePuy. For all remaining authors, none we declared
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Research Ethics: IRB approval not required for de-identified NSQIP research with no direct patient involvement. The manuscript submitted does not contain information about medical device(s)/drug(s).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Austen D. Katz https://orcid.org/0000-0003-0614-442X
Junho Song https://orcid.org/0000-0002-4853-4736
References
- 1.Safaee MM, Ames CP, Smith JS. Epidemiology and socioeconomic trends in adult spinal deformity care. Neurosurgery. 2020;87(1):25-32. [DOI] [PubMed] [Google Scholar]
- 2.Boddapati V, Lombardi JM, Urakawa H, Lehman RA. Intraoperative image guidance for the surgical treatment of adult spinal deformity. Ann Transl Med. 2021;9(1):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nooh A, Aoude A, Fortin M, et al. Use of computer assistance in lumbar fusion surgery: analysis of 15 222 patients in the ACS-NSQIP database. Global Spine J. 2017;7(7):617-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: past, present, and future: JNSPG 75th anniversary invited review article. J Neurosurg Spine. 2019;30(5):551-567. [DOI] [PubMed] [Google Scholar]
- 5.Schwab FJ, Blondel B, Bess S, et al. Radiographical spinopelvic parameters and disability in the setting of adult spinal deformity: a prospective multicenter analysis. Spine. 2013;38(13):E803-E812. [DOI] [PubMed] [Google Scholar]
- 6.Smith JS, Klineberg E, Schwab F, et al. Change in classification grade by the SRS-schwab adult spinal deformity classification predicts impact on health-related quality of life measures: prospective analysis of operative and nonoperative treatment. Spine. 2013;38(19):1663-1671. [DOI] [PubMed] [Google Scholar]
- 7.Cui G, Wang Y, Kao TH, et al. Application of intraoperative computed tomography with or without navigation system in surgical correction of spinal deformity: a preliminary result of 59 consecutive human cases. Spine. 2012;37(10):891-900. [DOI] [PubMed] [Google Scholar]
- 8.Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: past, present, and future. J Neurosurg Spine. 2019;30(5):551-567. [DOI] [PubMed] [Google Scholar]
- 9.Hussain I, Fu K-M, Uribe JS, Chou D, Mummaneni PV. State of the art advances in minimally invasive surgery for adult spinal deformity. Spine Deform. 2020;8(6):1143-1158. [DOI] [PubMed] [Google Scholar]
- 10.Strong MJ, Yee TJ, Khalsa SSS, et al. The feasibility of computer-assisted 3D navigation in multiple-level lateral lumbar interbody fusion in combination with posterior instrumentation for adult spinal deformity. Neurosurg Focus. 2020;49(3):E4. [DOI] [PubMed] [Google Scholar]
- 11.Smith JS, Klineberg E, Lafage V, et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25(1):1-14. [DOI] [PubMed] [Google Scholar]
- 12.Ferraris VA, Davenport DL, Saha SP, Austin PC, Zwischenberger JB. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg. 2012;147(1):49-55. [DOI] [PubMed] [Google Scholar]
- 13.Cheriyan T, Maier SP, II, Bianco K, et al. Efficacy of tranexamic acid on surgical bleeding in spine surgery: a meta-analysis. Spine J. 2015;15(4):752-761. [DOI] [PubMed] [Google Scholar]
- 14.Cawley D, Dhokia R, Sales J, Darwish N, Molloy S. Ten techniques for improving navigated spinal surgery. Bone Joint J. 2020;102-b(3):371-375. [DOI] [PubMed] [Google Scholar]
- 15.Keric N, Eum DJ, Afghanyar F, et al. Evaluation of surgical strategy of conventional vs. percutaneous robot-assisted spinal trans-pedicular instrumentation in spondylodiscitis. J Robot Surg. 2017;11(1):17-25. [DOI] [PubMed] [Google Scholar]
- 16.Lonjon N, Chan-Seng E, Costalat V, Bonnafoux B, Vassal M, Boetto J. Robot-assisted spine surgery: feasibility study through a prospective case-matched analysis. Eur Spine J. 2016;25(3):947-955. [DOI] [PubMed] [Google Scholar]
- 17.Kim H-J, Jung W-I, Chang B-S, Lee C-K, Kang K-T, Yeom JS. Prospective, randomized, controlled trial of robot‐assisted vs freehand pedicle screw fixation in spine surgery. Int J Med Robot. 2017;13(3):e1779. [DOI] [PubMed] [Google Scholar]
- 18.Solomiichuk V, Fleischhammer J, Molliqaj G, et al. Robotic versus fluoroscopy-guided pedicle screw insertion for metastatic spinal disease: a matched-cohort comparison. Neurosurg Focus. 2017;42(5):E13. [DOI] [PubMed] [Google Scholar]
- 19.Härtl R, Lam KS, Wang J, Korge A, Kandziora F, Audigé L. Worldwide survey on the use of navigation in spine surgery. World Neurosurg. 2013;79(1):162-172. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzenbach O, Berlemann U, Jost B, et al. Accuracy of computer-assisted pedicle screw placement. An in vivo computed tomography analysis. Spine (Phila Pa 1976). 1997;22(4):452-458. [DOI] [PubMed] [Google Scholar]
- 21.Rivkin MA, Yocom SS. Thoracolumbar instrumentation with CT-guided navigation (O-arm) in 270 consecutive patients: accuracy rates and lessons learned. Neurosurg Focus. 2014;36(3):E7. [DOI] [PubMed] [Google Scholar]
- 22.Smith JS, Shaffrey E, Klineberg E, et al. Prospective multicenter assessment of risk factors for rod fracture following surgery for adult spinal deformity. J Neurosurg Spine. 2014;21(6):994-1003. [DOI] [PubMed] [Google Scholar]
- 23.Safaee MM, Scheer JK, Ailon T, et al. Predictive modeling of length of hospital stay following adult spinal deformity correction: analysis of 653 patients with an accuracy of 75% within 2 days. World Neurosurg. 2018;115:e422-e427. [DOI] [PubMed] [Google Scholar]
- 24.Pellisé F, Serra-Burriel M, Smith JS, et al. Development and validation of risk stratification models for adult spinal deformity surgery. J Neurosurg Spine. 2019;31(4):587-599. [DOI] [PubMed] [Google Scholar]
- 25.Yagi M, Hosogane N, Fujita N, et al. Surgical risk stratification based on preoperative risk factors in adult spinal deformity. Spine J. 2019;19(5):816-826. [DOI] [PubMed] [Google Scholar]
- 26.Scheer JK, Smith JS, Schwab F, et al. Development of a preoperative predictive model for major complications following adult spinal deformity surgery. J Neurosurg Spine. 2017;26(6):736-743. [DOI] [PubMed] [Google Scholar]
- 27.Leven DM, Lee NJ, Kothari P, et al. Frailty index is a significant predictor of complications and mortality after surgery for adult spinal deformity. Spine. 2016;41(23):E1394-E1401. [DOI] [PubMed] [Google Scholar]
- 28.Smith H, Welsch M, Ugurlu H, Sasso R, Vaccaro A. Comparison of radiation exposure in lumbar pedicle screw placement with fluoroscopy vs computer-assisted image guidance with intraoperative three-dimensional imaging. J Spinal Cord Med. 2008;31(5):532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaishnav AS, Merrill RK, McAnany SJ, et al. P 38 Intraoperative 3D navigation versus fluoroscopy: a comparison of time-demand, radiation exposure and outcomes in minimally invasive transforaminal lumbar interbody fusion (MI-TLIF). Spine J. 2019;19(9):S175-S176. [Google Scholar]
- 30.Theocharopoulos N, Perisinakis K, Damilakis J, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Occupational exposure from common fluoroscopic projections used in orthopaedic surgery. J Bone Joint Surg Am. 2003;85(9):1698-1703. [DOI] [PubMed] [Google Scholar]
- 31.Ghasem A, Sharma A, Greif DN, Alam M, Maaieh MA. The arrival of robotics in spine surgery: a review of the literature. Spine. 2018;43(23):1670-1677. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-pdf-1-gsj-10.1177_21925682211047551 for Impact of Navigation on 30-Day Outcomes for Adult Spinal Deformity Surgery by Austen D. Katz, Jesse Galina, Junho Song, Sayyida Hasan, Dean Perfetti, Sohrab Virk, Jeff Silber and David Essig in Global Spine Journal