Abstract
Study Design
Retrospective cohort study
Objective
Substantial variability in both the measurement and classification of subsidence limits the strength of conclusions that can be drawn from previous studies. The purpose of this study was to precisely characterize patterns of cervical cage subsidence utilizing computed tomography (CT) scans, determine risk factors for cervical cage subsidence, and investigate the impact of subsidence on pseudarthrosis rates.
Methods
We performed a retrospective review of patients who underwent one- to three-levels of anterior cervical discectomy and fusion (ACDF) utilizing titanium interbodies with anterior plating between the years 2018 and 2020. Subsidence measurements were performed by two independent reviewers on CT scans obtained 6 months postoperatively. Subsidence was then classified as mild if subsidence into the inferior and superior endplate were both ≤2 mm, moderate if the worst subsidence into the inferior or superior endplate was between 2 to 4 mm, or severe if the worst subsidence into the inferior or superior endplate was ≥4 mm.
Results
A total of 51 patients (100 levels) were included in this study. A total of 48 levels demonstrated mild subsidence (≤2 mm), 38 demonstrated moderate subsidence (2-4 mm), and 14 demonstrated severe subsidence (≥4 mm). Risk factors for severe subsidence included male gender, multilevel constructs, greater mean vertebral height loss, increased cage height, lower Taillard index, and lower screw tip to vertebral body height ratio. Severe subsidence was not associated with an increased rate of pseudarthrosis.
Conclusion
Following ACDF with titanium cervical cages, subsidence is an anticipated postoperative occurrence and is not associated with an increased risk of pseudarthrosis.
Keywords: anterior cervical discectomy and fusion, titanium cage, interbody, subsidence, pseudarthrosis, risk factors, computed tomography
Introduction
Interbody subsidence is defined as settling of an interbody into the adjacent vertebral bodies and is a known complication of their utilization throughout the spine.1-5 While some subsidence is anticipated, severe subsidence leads to increased rates of local kyphosis,6,7 reoperation,3,4 pseudarthrosis, 8 and recurrence of preoperative symptoms. 9 Many potential modifiable risk factors for subsidence have been proposed, including graft position,2,8 bone mineral density (BMD), 3 excessive end plate removal, 10 levels of interbody insertion,2,8,11 smoking status, 11 graft height,8,12,13 and smaller graft to endplate surface area ratio.8,13-15 However, as pointed out in a recent systematic review, substantial variability in both the measurement and classification of subsidence limits the strength of conclusions that can be drawn from this body of literature. 16
Traditionally, subsidence has been measured indirectly based on loss of disc height on lateral spine radiographs, which are inadequate to accurately characterize variables of small magnitude such as subsidence and fail to account for variability in rotation.8,9,11,14,17,18 The purpose of the present study was to precisely characterize patterns of cervical cage subsidence utilizing computed tomography (CT) scans. The secondary aims of this study were to determine risk factors for cervical cage subsidence and to determine the impact of subsidence on pseudarthrosis rates.
Methods
Patient Population
Following institutional review board (IRB) approval, we performed a retrospective review of prospectively maintained medical records and imaging for patients undergoing one- to three-level anterior cervical discectomy and fusion (ACDF) utilizing titanium interbody implants with anterior plating at a single institution between the years 2018 and 2020. Patients were excluded if they had previous surgery at the index level(s), underwent ACDF as part of a staged anterior–posterior approach, had greater than 3 levels of ACDF, underwent a corpectomy, underwent ACDF for nondegenerative conditions, or did not have a CT scan available for review at least 6 months from the date of surgery. Patient demographics, perioperative radiographic variables, and construct-related variables were documented. All patients included in the final analysis completed at least 6 months of follow-up from the time of surgery (mean 1.4 years, range 7 months–2.8 years).
Operative Treatment
All patients underwent a standard Smith-Robinson approach to the cervical spine. Following fluoroscopic confirmation of the intended surgical levels, a caspar cervical distractor was utilized to distract the disc space during performance of the discectomy and interbody insertion. Next, a curved cervical curette and high-speed side-cutting 2-mm matchstick burr were used to remove the remainder of the intervertebral disk, posterior annulus, osteophytes, and the endplate cartilage, while taking care not to burr into the endplates. Following completion of the decompression, rasps were used to decorticate the endplates. In this cohort, all patients then had an appropriately sized titanium cervical cage filled with morselized cancellous allograft inserted at each surgical level. Care was taken not to “overstuff” the disc space with a cage of excessive height. All patients then underwent anterior plating with a locked plate. Postoperatively, all patients were treated with a hard cervical collar for 6 weeks postoperatively.
Measuring Cage Subsidence
Superior and inferior interbody subsidence were assessed at each operative level by directly measuring the distance from the endplate-facing surface of the interbody to the endplate on both sagittal and coronal CT scans (settings: 140 kV, 280 mAs, and .75 mm slice thickness) obtained 6 months postoperatively (Figure 1). The subsidence measurements were performed by two independent reviewers, one orthopedic surgery resident and one fellowship-trained spine surgeon, and averaged to determine the final subsidence measurement. Subsidence was measured at 6 months postoperatively because it is routine within the authors’ practice to obtain CT scans at this interval to assess for fusion and also because the vast majority of subsidence occurs within the first few months postoperatively.2,8,19 Subsidence measurements in both the sagittal and coronal plane were compared to ensure the measurement was reproducible irrespective of the plane in which the measurement was performed.
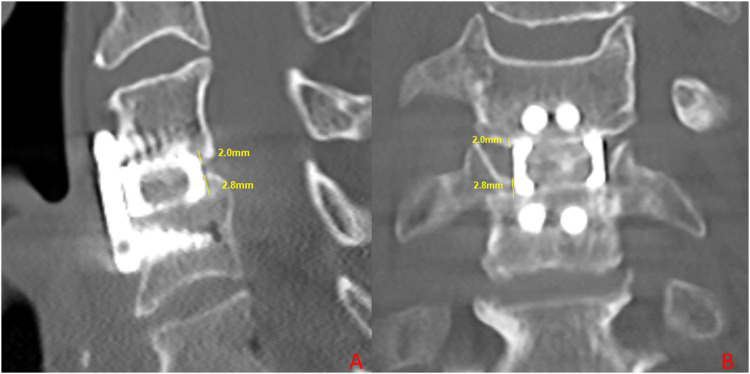
Figure 1.
Mid-sagittal (A) and coronal (B) CT images demonstrating the measurement of superior (2.0 mm) and inferior (2.8 mm) subsidence.
Interbody subsidence at each operative level was then classified as mild if subsidence into the inferior and superior endplate were both ≤2 mm, moderate if the worst subsidence into the inferior or superior endplate was between 2 to 4 mm, or severe if the worst subsidence into the inferior or superior endplate was ≥4 mm.
Assessment of Radiographic and Construct-Related Variables
To assess the role of BMD on interbody subsidence, Hounsfield units (HUs) were collected at the cranial, middle, and caudal aspects of the vertebral bodies directly adjacent to the interbody on preoperative axial CT (Figure 2). 20 To assess the role of cervical paraspinal sarcopenia on interbody subsidence, quantitative assessment was performed at the level of the C5-C6 interspace, including measurement of the anterior–posterior diameter, cross-sectional area, and HUs of the longus colli. To evaluate the role of excessive endplate resection on subsidence, the height of each vertebra adjacent to an interbody was measured at the anterior, middle, and posterior aspects of the vertebral body on standing lateral radiographs preoperatively and postoperatively prior to discharge from the hospital. The difference between preoperative and immediate postoperative vertebral height was calculated and used as a surrogate for intraoperative bony resection.
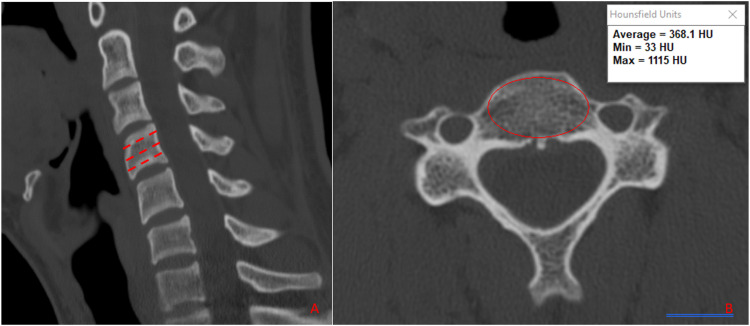
Figure 2.
Mid-sagittal (A) and axial (B) CT images demonstrating the measurement of vertebral body hounsfield units.
Operative notes were reviewed to determine construct-related variables including levels instrumented; type of cage inserted; cage height, length, and lordosis; interbody height; and use of fusion adjuncts such as morselized allograft. Cage position in the sagittal plane was assessed on postoperative lateral radiographs using the Taillard index, 2 and screw position was assessed by measuring the distance from the inferior-most screw tip to the inferior end plate on lateral radiographs (Figure 3). The cage height was compared to the preoperative disc height measured on CT scans at the anterior, middle, and posterior aspects of the disc space to determine the impact of overdistraction on subsidence.
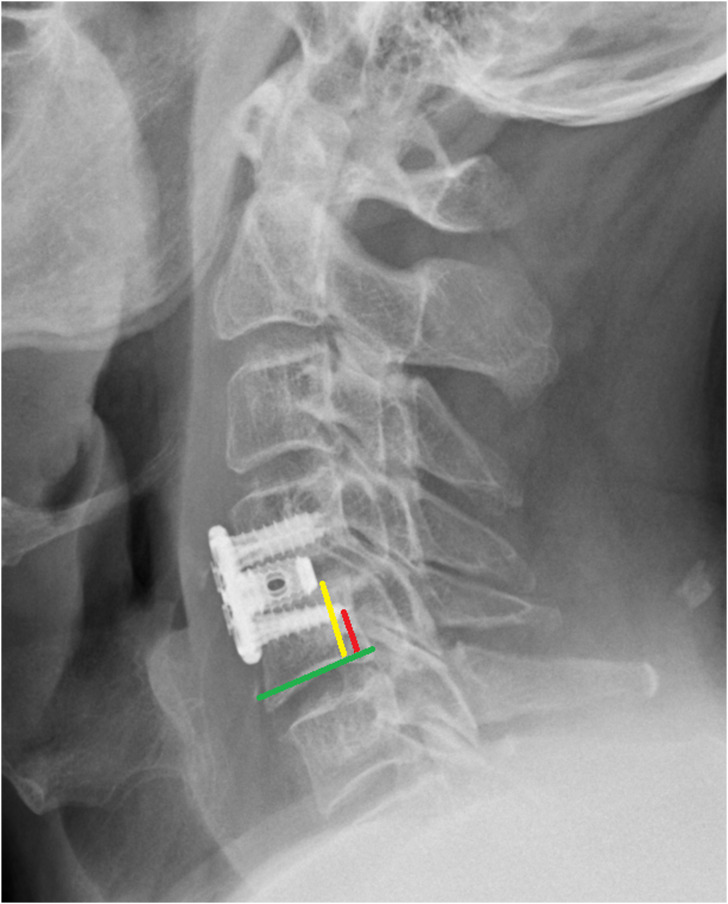
Figure 3.
Standing lateral cervical spine X-ray demonstrating measurement of screw tip height (red line) and vertebral body height (yellow line) in relation to the inferior endplate of the vertebral body (green line).
Fusion was assessed on CT scan performed at 6 months postoperatively or, preferentially, on a later CT scan if the patient had more than one CT scan available for review. We defined fusion as bony bridging between vertebrae with an absence of continuous radiolucent lines between endplates.
Statistical Analysis
Statistical analysis was conducted using the Fisher exact t-test for continuous variables and the chi-square test for categorical variables. Patients were first subdivided into two groups based upon degree of subsidence: (1) mild subsidence, which included patients with subsidence of ≤2 mm and (2) moderate or severe subsidence, which included all patients with subsidence >2 mm. Univariate analysis was then performed to assess for differences in recorded demographic, radiographic, and cage-related variables between these two subgroups. Patients were then divided into two subgroups based upon the presence of (1) severe subsidence, which included all patients with at least one level of subsidence ≥4 mm and (2) non-severe subsidence, which included all patients with <4 mm of subsidence at every operative level. A second univariate analysis was then performed to assess for differences in recorded demographic, radiographic, and cage-related variables between these subgroups. We also performed multivariable analysis to assess for independent predictors of subsidence. All statistical analyses were performed using R version 4.0.2 (R Foundation for Statistical Computing, https://www.R-project.org/) and the “rms” package. A P-value <.05 was used to determine statistical significance.
Results
We reviewed 159 patients who underwent 1–3 level ACDF with a titanium cervical cage between 2018 and 2020. After application of exclusion criteria, 51 patients (100 levels) were included in the final cohort for complete analysis. The mean age was 57.1 years, and the cohort was 54.9% women (Table 1). In the entire cohort, 16 patients (31.4%) underwent single-level ACDF, 21 patients (41.2%) underwent 2-level ACDF, and 14 patients (27.5%) underwent 3-level ACDF. Intraoperative, radiographic, and cage-related variables for the entire cohort were recorded in Table 2.
Table 1.
Demographics N = 51.
| Variable | |
|---|---|
| Age | 57.1 ± 11.7 |
| Sex | |
| Male | 23 (45.1%) |
| Female | 28 (54.9%) |
| BMI* (m/kg2) | 29.6 ± 5.7 |
| Obesity (BMI > 25) | 39 (76.5%) |
| Smoking | 5 (9.8%) |
| Diabetes | 9 (17.6%) |
| Chronic steroid use | 1 (2.0%) |
| Chronic kidney disease | 3 (5.9%) |
| Inflammatory arthritis | 2 (3.9%) |
| Number of levels fused | |
| One level | 16 (31.4%) |
| Two level | 21 (41.2%) |
| Three level | 14 (27.5%) |
*BMI: body mass index.
Table 2.
Radiographic and Cage-Related Variables for the Entire Cohort N = 100 Levels.
| Radiographic Parameter | |
|---|---|
| Levels | |
| C3-4 | 8 (8.0%) |
| C4-5 | 24 (24.0%) |
| C5-6 | 36 (36.0%) |
| C6-7 | 26 (26.0%) |
| C7-T1 | 2 (2.0%) |
| T1-2 | 1 (1.0%) |
| Cage inserted | |
| Depuy conduit | 25 (25.0%) |
| Stryker tritanium C | 35 (35.0%) |
| K2M cascadia | 37 (37.0%) |
| Medtronic titan | 3 (3.0%) |
| HU* superior VB* | |
| Cephalad | 336.8 ± 87.6 |
| Middle | 344.8 ± 91.5 |
| Caudal | 365.0 ± 105.0 |
| HU inferior VB | |
| Cephalad | 304.0 ± 77.5 |
| Middle | 292.1 ± 76.8 |
| Caudal | 298.7 ± 81.7 |
| Vertebral body HU | 323.6 ± 74.4 |
| Longus colli diameter | 8.0 ± 2.5 |
| Longus colli area | 60.7 ± 29.0 |
| Longus colli HU | 56.8 ± 13.4 |
| Cage-related parameters | |
| Graft height (mm) | |
| 5 mm | 10 |
| 6 mm | 13 |
| 7 mm | 47 |
| 8 mm | 23 |
| >8 mm | 7 |
| Mean | 7.07 ± 1.1 |
| Preoperative disc space height at index level (mm) | |
| Anterior | 3.6 ± 1.5 |
| Middle | 4.4 ± 1.6 |
| Posterior | 3.2 ± 1.6 |
| Mean | 3.7 ± 1.14 |
| Cage height : index disc space height ratio | 2.2 ± 1.2 |
| Cage height : superior native disc height ratio | 1.6 ± 0.5 |
| Cage height : inferior native disc height ratio | 1.9 ± 0.8 |
| Mean cage length (mm) | 12.9 ± 1.0 |
| Distance from posterior VB* to cage (mm) | 3.7 ± 1.8 |
| VB length | 19.7 ± 2.5 |
| Cage : VB length ratio | 0.68 ± 0.09 |
| Taillard index | 18.6 ± 8.4% |
| VB height (mm) | 14.1 ± 2.2 |
| Distance from screw tip to inferior endplate (mm) | 6.2 ± 2.5 |
| Screw height : VB height ratio | 0.44 ± 0.16 |
*HU: Hounsfield unit; VB: vertebral body.
Characterizing Subsidence
On sagittal CT scan for all 100 levels, the mean superior subsidence (into the inferior endplate of the cranial vertebra) was 1.33 ± .98 mm, inferior subsidence (into the superior endplate of the caudal vertebra) was 1.91 ± 1.25 mm, and cumulative subsidence (sum of the inferior and superior subsidence) was 3.27 ± 1.70 mm (Table 3). A total of 48 levels demonstrated mild subsidence (≤2 mm), 38 demonstrated moderate subsidence (2-4 mm), and 14 demonstrated severe subsidence (≥4 mm).
Table 3.
Cage subsidence N = 100 Levels.
| Superior Endplate Subsidence (mm) | |
|---|---|
| Sagittal | 1.33 ± 0.98 |
| Coronal | 1.22 ± 0.95 |
| Inferior endplate subsidence (mm) | |
| Sagittal | 1.91 ± 1.25 |
| Coronal | 1.97 ± 1.27 |
| Cumulative endplate subsidence (mm) | |
| Sagittal | 3.27 ± 1.70 |
| Coronal | 3.21 ± 1.71 |
| Subsidence | |
| ≤2 mm (Mild)* | 48 (48.0%) |
| 2–4 mm (Moderate)+ | 38 (38.0%) |
| ≥4 mm (Severe)# | 14 (14.0%) |
*Mild = subsidence into both the inferior and superior endplate individually was < 2 mm.
+Moderate = the worst subsidence into the inferior and/or superior endplate was between 2 and 4 mm.
#Severe = The worst subsidence into the inferior and/or superior endplate was >4 mm.
The 14 severely subsided levels demonstrated mean cumulative subsidence (sum of the superior and inferior subsidence) of 5.8 ± 1.0 mm, which was significantly greater than the cohort of patients with mild or moderate subsidence (2.8 ± 1.4 mm, P < .0001) (Table 4). The direction of “worst” subsidence in the 14 severely subsided levels was superior at 4 levels (28.6%) and inferior at 10 levels (71.4%). The direction of “worst” subsidence in the 86 non-severely subsided levels (levels with mild or moderate subsidence) was superior at 34 levels (39.5%) and inferior at 52 levels (60.5%). In the direction of “worst” subsidence, the mean subsidence was 4.2 ± 0.3 mm in the severely subsided levels and 1.9 ± 0.9 mm in the non-severely subsided levels (P < .0001).
Table 4.
Comparison of Severe and Non-Severely Subsided Levels N = 100 Levels.
| <4 mm (n = 86)* | ≥4 mm (n = 14)# | P value | |
|---|---|---|---|
| Cumulative subsidence* | 2.8 ± 1.4 | 5.8 ± 1.0 | <.0001 |
| Worst subsidence+ | 1.9 ± 0.9 | 4.2 ± 0.3 | <.0001 |
| # of levels in which worst subsidence occurred superiorly | 34 (39.5%) | 4 (28.6%) | 0.43 |
| # of levels in which worst subsidence occurred inferiorly | 52 (60.5%) | 10 (71.4%) |
*Mean cumulative subsidence both superiorly and inferiorly at one level.
+Mean subsidence at each level in the direction of most significant subsidence.
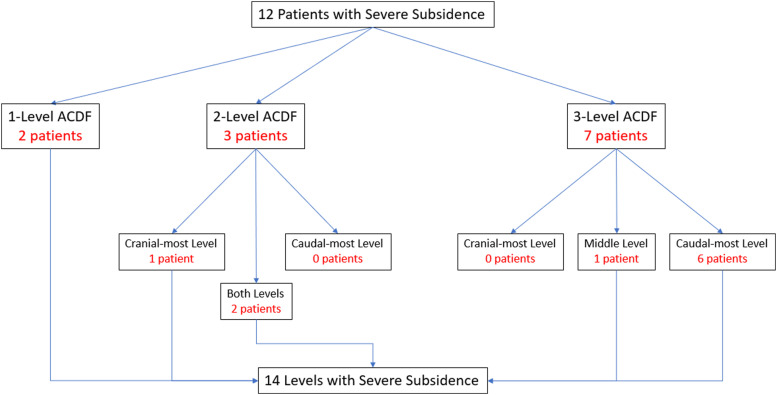
The 14 levels of severe subsidence occurred in a total of 12 patients (two patients had severe subsidence at two levels). 10 patients (83%) had multilevel constructs and 2 patients (17%) underwent single-level ACDF. Of the 10 patients with severe subsidence and multilevel constructs, 1 patient had severe subsidence at the cranial-most level of a 2 level ACDF, 2 patients had severe subsidence at both levels of an ACDF, 1 patient had severe subsidence at the middle level of a 3-level ACDF, and 6 patients had severe subsidence at the caudal-most level of a 3-level ACDF (Figure 4).
Figure 4.
Flow chart demonstrating the location of severe subsidence.
Risk Factors for Subsidence
A univariate analysis was performed to compare demographic, radiographic, and construct-related variables between patients with mild (≤2 mm) and moderate–severe (>2 mm) “worst” subsidence (Table 5). Patients with moderate–severe subsidence were older (60.1 ± 11.3 vs 50.4 ± 9.5, P = .004) with a higher proportion of men (54.3% vs 25%, P = .045) and rate of diabetes (25.7% vs 0%, P = .002). The moderate–severe subsidence group had lower mean vertebral body HU at the vertebral bodies adjacent to the interbody than the mild subsidence group (297.3 ± 72.6 vs 361.1 ± 59.2 mm, P = .01). There was no difference between these two subgroups in quantitative measures of the longus colli muscles or intraoperative vertebral body height loss. Patients with moderate-severe subsidence demonstrated a lower cage to vertebral body length ratio (.67 ± .8 vs .73 ± .8, P = .01), higher Taillard index (20.0 ± 8.3% vs 15.3 ± 7.8%, P = .009), and lower screw tip to vertebral body height ratio (.41 ± .15 vs .49 ± .17, P = .01). There was no difference between these two subgroups in preoperative disc space height, cage height, or cage lordosis. On multivariate analysis, only age remained predictive of moderate to severe subsidence (odds ratio 1.1, 95% CI 1.01–1.3, P = .04) (Table 6).
Table 5.
Univariate Analysis Comparing Patients with Mild and Moderate–Severe Subsidence N = 51.
| Variable | ≤2 mm (n = 16) | >2 mm (n = 35) | P-value |
|---|---|---|---|
| Demographics | |||
| Age | 50.4 ± 9.5 | 60.1 ± 11.3 | 0.004 |
| Sex (female) | 12 (75.0%) | 16 (45.7%) | 0.045 |
| BMI* (m/kg2) | 30.8 ± 6.4 | 29.0 ± 5.2 | 0.34 |
| Smoking | 4 (25%) | 1 (2.9%) | 0.07 |
| Diabetes | 0 (0%) | 9 (25.7%) | 0.002 |
| Chronic steroid use | 0 (0%) | 1 (2.9%) | 0.32 |
| Chronic kidney disease | 1 (6.3%) | 2 (5.7%) | 0.94 |
| Inflammatory arthritis | 1 (6.3%) | 1 (2.9%) | 0.63 |
| Number of levels fused | |||
| One level | 5 (31.3%) | 11 (31.4%) | 0.58 |
| Two level | 8 (50%) | 13 (37.1%) | |
| Three level | 3 (18.8%) | 11 (31.4%) | |
| Radiographic parameters | |||
| Vertebral body HU | 361.1 ± 59.2 | 297.3 ± 72.6 | 0.01 |
| Longus colli diameter (mm) | 7.1 ± 1.5 | 8.6 ± 2.9 | 0.08 |
| Longus colli area (mm2) | 57.7 ± 29.9 | 62.9 ± 28.2 | 0.62 |
| Longus colli HU | 55.8 ± 15.1 | 57.5 ± 12.0 | 0.74 |
| Mean difference in vertebral height (mm) | 1.5 ± 1.7 | 1.5 ± 1.8 | 0.93 |
| Difference VB height anterior | 1.6 ± 1.8 | 1.7 ± 1.8 | 0.84 |
| Difference VB height middle | 1.1 ± 1.6 | 1.1 ± 1.9 | 0.91 |
| Difference VB height posterior | 1.8 ± 2.2 | 1.5 ± 2.2 | 0.64 |
| Construct-related parameters | |||
| Cage lordosis | 6.9 ± 0.9 | 7.2 ± 1.4 | 0.21 |
| Cage height (mm) | 6.9 ± 1.2 | 7.2 ± 1.1 | 0.27 |
| Index disc space height (mm) | |||
| Anterior | 3.3 ± 1.2 | 3.7 ± 1.6 | 0.17 |
| Middle | 4.4 ± 1.2 | 4.4 ± 1.7 | 0.97 |
| Posterior | 3.2 ± 1.8 | 3.2 ± 1.5 | 0.86 |
| Mean | 3.7 ± 1.3 | 3.8 ± 1.5 | 0.71 |
| Cage : index disc space height ratio | 2.1 ± 0.8 | 2.3 ± 1.3 | 0.3 |
| Cage : superior native disc height ratio | 1.5 ± 0.5 | 1.6 ± 0.4 | 0.6 |
| Cage : inferior native disc height ratio | 1.8 ± 0.7 | 2.0 ± 0.8 | 0.44 |
| Cage length (mm) | 12.7 ± 0.9 | 13.0 ± 1.0 | 0.19 |
| Cage length : VB length | 0.73 ± .08 | 0.67 ± .08 | 0.01 |
| Taillard index (%) | 15.3 ± 7.8 | 20.0 ± 8.3 | 0.009 |
| Screw tip height : VB height | .49 ± .17 | .41 ± .15 | 0.01 |
*BMI: body mass index; HU: Hounsfield unit; VB: vertebral body.
Table 6.
Odds Ratios for Development of Moderate–Severe Subsidence.
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Age | 1.1 | 1.01-1.3 | 0.04 |
| Sex (female) | 3.8 | 0.4-40.6 | 0.27 |
| BMI* | 0.8 | 0.7-1.0 | 0.09 |
| Multilevel construct | 1.7 | 0.2-17.0 | 0.64 |
| Cage height (mm) | 1.1 | 0.2-5.8 | 0.92 |
| Mean index disc space height | 1 | 0.1-9.6 | 0.98 |
| Mean loss in vertebral height | 0.9 | 0.3-2.3 | 0.79 |
| Cage : index disc height ratio | 1 | 0.7-1.4 | 0.95 |
| Posterior VB* to cage distance | 0.8 | 0.4-1.6 | 0.53 |
| VB* length | 1.9 | 0.5-7.8 | 0.38 |
| Taillard index (%) | 1.7 | 0.4-7.1 | 0.45 |
*BMI: body mass index; VB: vertebral body.
A univariate analysis was then performed to compare patients with severe subsidence (≥4 mm) and non-severe subsidence (<4 mm) based upon demographic, radiographic, and construct-related parameters (Table 7). The severe subsidence group had a higher proportion of men (75 vs 35.9%, P = .02). The severe subsidence group also had a greater number of multilevel constructs (83.3 vs 64.1%), which was especially evident when comparing the number of 3-level constructs (58.3 vs 17.9%, P = .02). In comparison to the non-severe subsidence group, patients with severe subsidence demonstrated greater mean vertebral height loss from preoperative to immediate postoperative (2.0 ± 1.6 vs .9 ± 2.9, P = .02). There was no difference between these subgroups in mean adjacent vertebral body HU or quantitative measures of the longus colli muscles. Patients with severe subsidence had taller cages inserted (7.4 ± .9 vs 6.9 ± 1.2, P = .03), lower Taillard index (21.4 ± 8.0% vs 17.4 ± 8.3%, P = .03), and lower screw tip to vertebral body height ratio (.39 ± .16 vs .46 ± .16, P = .03). On multivariate analysis, only older age (odds ratio 1.6, 95% CI 1.03–2.5, P = .03) and the presence of a multilevel construct (odds ratio 421.3, 95% CI 1.005–176 660, P = .049) remained predictive of severe subsidence (Table 8).
Table 7.
Univariate Analysis Comparing Patients with Severe and Non-Severe Subsidence N = 51.
| Variable | <4 mm (n = 39) | ≥4 mm (n = 12) | P-value |
|---|---|---|---|
| Demographics | |||
| Age | 55.7 ± 11.6 | 61.4 ± 10.7 | 0.14 |
| Sex (female) | 25 (64.1%) | 3 (25%) | 0.02 |
| BMI* (m/kg2) | 29.4 ± 6.2 | 30.1 ± 3.2 | 0.64 |
| Active smoking | 5 (12.8%) | 0 (0%) | 0.02 |
| Diabetes | 6 (15.4%) | 3 (25.0%) | 0.51 |
| Chronic steroid use | 1 (2.6%) | 0 (0%) | 0.32 |
| Chronic kidney disease | 2 (5.1%) | 1 (8.3%) | 0.72 |
| Inflammatory arthritis | 2 (5.1%) | 0 (0%) | 0.16 |
| Lowest BMD | 0.92 ± 1.2 | 0.94 ± | 1.0 0.87 |
| Number of levels fused | |||
| One level | 14 (35.9%) | 2 (16.6%) | 0.02 |
| Two level | 18 (46.2%) | 3 (25.0%) | |
| Three level | 7 (17.9%) | 7 (58.3%) | |
| Radiographic parameters | |||
| Vertebral body HU | 321.8 ± 77.3 | 331.7 ± 58.5 | 0.75 |
| Longus colli diameter (mm) | 7.8 ± 2.4 | 8.8 ± 3.2 | 0.56 |
| Longus colli area (mm2) | 61.3 ± 28.0 | 58.0 ± 33.1 | 0.84 |
| Longus colli HU | 55.2 ± 12.1 | 64.1 ± 16.4 | 0.29 |
| Mean difference in vertebral height (mm) | 0.9 ± 2.9 | 2.0 ± 1.6 | 0.02 |
| Difference VB height anterior | 1.4 ± 2.1 | 2.3 ± 1.6 | 0.03 |
| Difference VB height middle | 1.0 ± 2.0 | 1.4 ± 1.8 | 0.36 |
| Difference VB height posterior | 1.0 ± 3.2 | 2.3 ± 2.1 | 0.02 |
| Construct-related parameters | |||
| Cage lordosis (deg) | 7.2 ± 1.4 | 7.1 ± 0.9 | 0.72 |
| Cage Height (mm) | 6.9 ± 1.2 | 7.4 ± 0.9 | 0.03 |
| Index disc space height (mm) | |||
| Anterior | 3.4 ± 1.4 | 4.0 ± 1.7 | 0.15 |
| Middle | 4.3 ± 1.4 | 4.6 ± 1.8 | 0.38 |
| Posterior | 3.2 ± 1.5 | 3.2 ± 1.7 | 0.9 |
| Mean | 3.6 ± 1.3 | 3.9 ± 1.6 | 0.38 |
| Cage : index disc space height ratio | 2.1 ± 0.9 | 2.4 ± 1.7 | 0.46 |
| Cage : superior native disc height ratio | 1.6 ± 0.5 | 1.5 ± 0.3 | 0.41 |
| Cage : inferior native disc height ratio | 1.9 ± 0.8 | 1.9 ± 0.6 | 0.85 |
| Cage length (mm) | 12.8 ± 1.0 | 13.3 ± 1.0 | 0.08 |
| Cage length : VB length | .69 ± .09 | .66 ± 0.9 | 0.27 |
| Taillard index (%) | 17.4 ± 8.3 | 21.4 ± 8.0 | 0.03 |
| Screw tip height : VB height | 0.46 ± 0.16 | 0.39 ± 0.16 | 0.03 |
*BMI: body mass index; HU: Hounsfield unit; VB: vertebral body.
Table 8.
Odds Ratios for Development of Severe Subsidence.
| Variable | Odds Ratio | 95% Confidence Interval | P-value |
|---|---|---|---|
| Age | 1.6 | 1.03-2.5 | 0.03 |
| Sex (Female) | 3.0 | 0.3-27.7 | 0.1 |
| BMI | 1.5 | 0.8-2.9 | 0.21 |
| Multilevel Construct | 4.2 | 1.1-17.6 | 0.049 |
| Cage Height (mm) | 5.1 | 0.6-46.1 | 0.15 |
| Mean index disc space height | 0.03 | 0.0-3.6 | 0.15 |
| Mean loss in vertebral height | 1.8 | 0.5-6.5 | 0.36 |
| Cage : index disc height ratio | 0.6 | 0.3-1.3 | 0.19 |
| Posterior VB to cage distance | 2.9 | 0.9-9.5 | 0.08 |
| VB length | 0.05 | 0.002-1.04 | 0.053 |
| Taillard index (%) | 0.1 | 0.01-1.3 | 0.09 |
*BMI: body mass index; VB: vertebral body.
Complication and Fusion Rates
A complete overview of complications experienced by this cohort is documented in Table 9. Ten patients (19.6%) experienced postoperative dysphagia, eight of whom had complete resolution of their symptoms by 6 months postoperatively and two of whom required persistent diet alterations at final follow-up. Six patients (11.8%) experienced new postoperative neurologic deficits, including five patients with isolated C5 radiculopathies (two of which persisted at 1 year postoperatively and were treated nonoperatively) and 1 patient who developed progressive gait imbalance beginning at 6 months postoperatively with new C7-T1 spondylolisthesis and cord compression requiring posterior cervical decompression and fusion. Four patients (7.8%) developed a pseudarthrosis at 5 levels (one patient had a pseudarthrosis at both levels of a 2-level ACDF), all of which were identified on CT scans obtained at a mean of 13.3 months postoperatively (range 11.5-15.5 months). Three of the four patients with pseudarthroses were asymptomatic and were treated nonoperatively. A total of four patients in this cohort required reoperation, including one patient with a symptomatic pseudarthrosis who underwent posterior cervical decompression and fusion 16 months after their index ACDF. Three patients underwent reoperations due to adjacent segment disease, including one single-level cervical disc arthroplasty 2 years after the index ACDF, one single-level ACDF 1 year after the index ACDF, and 1 C3–T2 posterior cervical fusion 17 months after the index ACDF. No patients in this cohort experienced a postoperative infection.
Table 9.
Complications in the Entire Cohort N = 51.
| Pseudarthrosis | 4 (7.8%) |
| Infection | 0 (0%) |
| New neurologic deficit | 6 (11.8%) |
| Dysphagia | 10 (19.6%) |
| Reoperation | 4 (7.8%) |
| Adjacent segment disease | 3 (5.9%) |
| Pseudarthrosis | 1 (2.0%) |
There was no difference in rates of pseudarthrosis (8.3 vs 7.7%), infection, development of new neurologic deficits, or dysphagia between patients with severe (≥4 mm) and non-severe (<4 mm) subsidence (Table 10). There were 4 reoperations in patients with non-severe subsidence and 0 reoperations in patients with severe subsidence (10.3 vs 0%, P = .04).
Table 10.
Comparison of Complications Based Upon Degree of Subsidence N = 51.
| <4 mm (n = 39) | ≥4 mm (n = 12) | P-value | |
|---|---|---|---|
| Pseudarthrosis | 3 (7.7%) | 1 (8.3%) | 0.95 |
| Infection | 0 | 0 | 1.00 |
| New neurologic deficit | 4 (10.3%) | 2 (16.7%) | 0.61 |
| Dysphagia | 6 (15.4%) | 4 (33.3%) | 0.26 |
| Reoperation | 4 (10.3%) | 0 (0%) | 0.04 |
Discussion
Characterizing Subsidence
The reported frequency of cervical cage subsidence in the literature varies from 13.2 to 42.5%.8,9,11,14,16,21,22 In the only study investigating the magnitude and direction of cervical cage subsidence, Barsa et al. reported that mean subsidence measured on lateral plain films was 2.59 mm and occurred in an inferior direction 89.5% of the time. 14 However, this study and all other previous studies investigating cervical cage subsidence utilized lateral radiographs to measure subsidence, which is both insufficient for a variable of such small magnitude and introduces significant variability due to the inaccuracy of such measurements if the vertebral end plates are not imaged “in plane.” Other studies investigating the subsidence of polyetheretherketone (PEEK) and allograft interbodies also utilized plain radiographs in the assessment of subsidence and should not be extrapolated to predict the subsidence of titanium cages given differences in their molecular properties.13,23
While the authors of the present study previously characterized allograft subsidence utilizing advanced imaging, 7 the present study is the first to characterize subsidence of cervical cages utilizing CT scans, which are far more precise than plain films and eliminate variability associated with rotation of the spine in relation to the X-ray image intensifier. In our cohort of 100 cervical cages, 100% of cages demonstrated any amount of subsidence. This finding, while much higher than previously reported rates in the literature, is unsurprising given that (1) CT scans are more sensitive for subsidence of small magnitude, (2) the interbodies were inserted while the disc space was distracted, (3) the inserted interbodies were approximately two times taller than the height of the index disc spaces in this cohort, and (4) direct axial load is applied postoperatively due to the weight of the head. Of the 100 interbodies inserted, 48% demonstrated mild subsidence (≤2 mm), 38% demonstrated moderate subsidence (2–4 mm), and 14% demonstrated severe subsidence (≥4 mm). In the 14 patients with severe subsidence, 10 (71.4%) subsided inferiorly into the superior endplate of the caudal vertebra. As a general rule, approximately 50% of all cervical cages will subside >2 mm, 15% will subside >4 mm, and severe subsidence occurs inferiorly 75% of the time.
Risk Factors for Subsidence
Numerous potential risk factors for cervical interbody subsidence have been identified, including BMD, smoking, intervertebral level of insertion, smaller interbody to endplate surface area ratio, excessive endplate removal, increased interbody height, and failure to position the graft at the anterior vertebral body line.2,3,8,10-15 Each of these risk factors was identified in studies that utilized plain radiographs to measure subsidence; consequently, no study has established associations between potential risk factors for subsidence and varying magnitudes of subsidence. The present study is the first to precisely measure cervical cage subsidence on CT scans, reliably classify it according to severity, and then identify risk factors for varying degrees of subsidence.
Univariate analysis comparing patients with mild (≤2 mm) subsidence to the combined cohort of patients with moderate and severe subsidence (>2 mm) identified older age, male sex, diabetes, mean adjacent vertebral body Hounsfield units (HUs), smaller cage length to vertebral body length ratio, increased Taillard index, and decreased screw tip height to vertebral body height ratio as risk factors in the development of >2 mm of subsidence. Mean adjacent vertebral body HU is a well-established surrogate for BMD in the lumbar spine; 20 however, its utility in the cervical spine has yet to be established. Still, it is interesting to note that patients with moderate–severe subsidence demonstrated lower mean adjacent vertebral body HU, signifying the probability that a similar relationship exists between HU and BMD in the cervical spine as is seen in the lumbar spine. Unfortunately, too few patients in this cohort had a preoperative DEXA scan to be able to draw meaningful conclusions regarding its impact on subsidence or relationship to HU. As previously postulated in the literature, patients in this study with a smaller cage to vertebral body length ratio and increased Taillard index, both of which signify undersizing the interbody length in relation to the size of the vertebral body, demonstrated increased subsidence. On multivariate analysis, only increased age was associated with an increased risk of >2 mm of subsidence.
Comparison of patients with severe (≥4 mm) and non-severe subsidence revealed that the development of severe subsidence was associated with male gender, active smoking, multilevel constructs, mean difference in vertebral height from preoperative to immediate postoperative, cage height, Taillard index, and screw tip height to vertebral body height. In this cohort, 12 of 14 (85.7%) levels of severe subsidence occurred in multilevel constructs, and 50% (7/14) of the 3-level ACDFs demonstrated severe subsidence. The increased incidence of subsidence in multilevel constructs is unsurprising given that (1) the middle vertebral levels require both superior and inferior endplate preparation, leading to increased risk of endplate penetration, (2) each additional level confers an additional risk of subsidence, and (3) the unitization of multiple vertebral segments creates a longer level arm and increases the forces disproportionately at the inferior-most level. Not coincidentally, 8 of the 12 patients (66%) with a severely subsided interbody demonstrated severe subsidence at the caudal-most level of a multilevel construct (Figure 4). Increasing cage height was also found to be associated with an increased risk of severe subsidence. In their in vitro biomechanical study, Truumees et al. 12 demonstrated that significantly higher distractive and compressive forces occurred when taller grafts were used. Subsequent clinical studies have demonstrated that increasing cage height may be a risk factor for subsidence, though these studies measured subsidence on plain radiographs and characterized subsidence dichotomously rather than on a spectrum of severity.8,13 On multivariate analysis, only older age and the presence of a multilevel construct remained predictive of increased risk of severe subsidence.
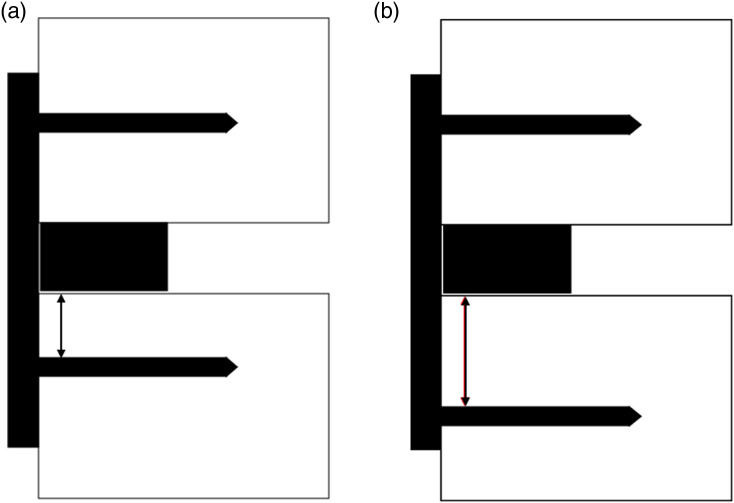
Decreased screw tip to vertebral body height ratio was associated with increased risk of both severe (≥4 mm) and moderate–severe (>2 mm) subsidence. Given that the majority of severe subsidence occurred at the caudal-most level of multilevel constructs in an inferior direction, the most likely explanation for this finding relates to the biomechanical principle of “working length.” Working length is a principle most commonly applied in orthopedic fracture care and refers to the distance between a mobile fracture site and the screw closest to the fracture that passes through a plate spanning the fracture site. Multiple biomechanical studies have established that decreased working length leads to increased construct rigidity and decreased motion at the fracture site.24-28 These principles have yet to be applied to arthrodesis of mobile spine segments, but a similar relationship can be inferred. In the present study, increasingly inferior screw placement at the caudal-most level of a multilevel construct effectively increases the working length of the construct, decreasing rigidity at that spinal segment and increasing the risk of subsidence (Figure 5).
Figure 5.
Schematic demonstrating the concept of ‘working length’ within the spine. In comparison to image (A), image (B) demonstrates a more inferior screw placement in the caudal vertebral body, leading to increased working length (red double arrow) and decreased construct rigidity.
Complications
Previous studies have not demonstrated a relationship between subsidence and pseudarthrosis.8,9,11,16,22,29 However, these studies measured subsidence on plain radiographs and utilized a combination of both CT and flexion-extension radiographs to assess fusion. In our cohort, the overall pseudarthrosis rate was 7.8% (4/51 patients). There was no difference in pseudarthrosis rate between patients with severe (8.3%) and non-severe (7.7%) subsidence. A previous study by the present authors investigating subsidence of cervical allograft interbodies demonstrated that severe subsidence was associated with a significant increase in pseudarthrosis. 7 It is the author’s experience that severe allograft subsidence leads to subsequent resorption of the allograft, leaving a void in the intervertebral space and too much ongoing motion to allow for fusion. Conversely, severe subsidence of a cervical titanium cage does not cause any loss of structural integrity of the cage, allowing the cage to settle in a subsided position while still providing the structural support necessary to restrict motion at the arthrodesis site and allow for fusion. Pardoxically, the non-severe subsidence cohort demonstrated a higher reoperation rate (10.3%) than the cohort of patients that experienced severe subsidence (0%), although this is likely related to small sample size.
Limitations
This study has several limitations. First, this study is retrospective in nature, which limits the data that can be collected for the cohort. Second, this study included only 51 patients and 100 levels, limiting the ability of the multivariate analysis to identify statistically significant risk factors for subsidence. Third, our study measured subsidence on CT scans performed at least 6 months postoperatively, which means the timing of subsidence and the progression of subsidence cannot be determined. Likewise, if subsidence is a process that progresses beyond 6 months, CTs obtained longer after surgery may be biased towards showing greater subsidence. However, the time from surgery to the CT scan measured for analysis averaged 12.2 months for those with severe subsidence vs 10.7 months for those without severe subsidence (P > .05). Prior work corroborates the assumption that the majority of subsidence occurs within the first few months after surgery.1,8,19 Future studies may consider assessing subsidence on CT scans performed at longer follow-up intervals to definitively demonstrate that subsidence is a process that occurs soon after surgery rather than progressing over the ensuing years. Fourth, fusion assessments were performed solely on CT scans, which may not be as sensitive as flexion-extension X-rays in the detection of pseudarthrosis.
Fifth, while the present study identified decreased cervical vertebral body HUs as a risk factor for subsidence, too few of the patients in the present study had a DEXA scan to draw meaningful conclusions regarding the impact of BMD on subsidence as assessed on this adjunctive imaging modality. Future studies investigating the correlation between cervical HUs and BMD assessed on DEXA scan would be clinically useful, as has been performed previously in the lumbar spine. 20 Sixth, while it is the authors’ practice to obtain CT scans at regular intervals postoperatively, it is not our express recommendation that CT scans be obtained rather than plain radiographs, especially in the absence of a formal risk-benefit or cost analysis. However, because the authors of the present study regularly obtain CT scans, this study will add to the current literature by defining potentially modifiable risk factors for subsidence in the setting of more precisely characterized subsidence. As such, the purpose of this study is not to recommend the regular utilization of CT scans in the assessment of interbody subsidence but instead to identify potentially modifiable risk factors in an effort to prevent subsidence. Future studies investigating the correlation between subsidence measurements performed on CT scans and those performed on plain radiographs may better define the accuracy of plain radiographs in accurately measuring subsidence, which is a variable of small magnitude. Finally, the main limitation of the present study is its inability to connect the impact of subsidence on patient reported outcomes. However, the purpose of the present study is simply to utilize more accurate advance imaging to characterize subsidence and identify risk factors for severe subsidence; future studies with prospectively collected patient reported outcomes may better define the impact of subsidence on clinical outcomes.
Conclusions
The present study is the first to precisely characterize patterns of cervical cage subsidence utilizing postoperative CT scans. Based upon the results of this study, approximately 50% of cervical cages will subside greater than 2 mm and 15% of interbodies will subside greater than 4 mm. When severe subsidence (≥4 mm) occurs, it will most commonly occur at the caudal-most level of a multilevel construct in an inferior direction. Potential risk factors for subsidence include older age, male gender, decreased vertebral body HUs, excessive vertebral endplate resection, decreased cage to vertebral body length ratio, increased cage height, and inferior placement of the screw caudal to the interbody. There was no relationship identified between severe subsidence and increased risk of pseudarthrosis. Additional studies are needed to further clarify the clinical sequelae of interbody subsidence.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: IRB: Approval obtained from Mayo Clinic IRB. IRB Approval Number: 10-002852.
Informed Consent: Consent was obtained from all study participants prior to inclusion in this study.
ORCID iDs
Zachariah W Pinter https://orcid.org/0000-0003-3011-0991
Mohamad Bydon https://orcid.org/0000-0002-0543-396X
References
- 1.Choi JY, Sung KH. Subsidence after anterior lumbar interbody fusion using paired stand-alone rectangular cages. Eur Spine J. 2006;15:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim M-C, Chung H-T, Cho J-L, Kim D-J, Chung N-S. Subsidence of polyetheretherketone cage after minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech. 2013;26(2):87-92. [DOI] [PubMed] [Google Scholar]
- 3.Tempel ZJ, Gandhoke GS, Okonkwo DO, Kanter AS, Impaired bone mineral density as a predictor of graft subsidence following minimally invasive transpsoas lateral lumbar interbody fusion. Eur Spine J. 2015;24(Suppl 3):414-419. [DOI] [PubMed] [Google Scholar]
- 4.Tempel ZJ, McDowell MM, Panczykowski DM, et al. Graft subsidence as a predictor of revision surgery following stand-alone lateral lumbar interbody fusion. J Neurosurg Spine. 2018;28(1):50-56. [DOI] [PubMed] [Google Scholar]
- 5.Wilke HJ, Kettler A, Goetz C, Claes L. Subsidence resulting from simulated postoperative neck movements. Spine. 2000;25(21):2762-2770. [DOI] [PubMed] [Google Scholar]
- 6.Katsuura A, Hukuda S, Saruhashi Y, Mori K. Kyphotic malalignment after anterior cervical fusion is one of the factors promoting the degenerative process in adjacent intervertebral levels. Eur Spine J. 2001;10(4):320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinter ZW, Mikula A, Shirley M, et al. Allograft subsidence decreases postoperative segmental lordosis with minimal effect on global alignment following ACDF. Global Spine J. 2021;Epub ahead of print: 2192568220988270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamagata T, Takami T, Uda T, et al. Outcomes of contemporary use of rectangular titanium stand-alone cages in anterior cervical discectomy and fusion: cage subsidence and cervical alignment. J Clin Neurosci. 2012;19(12):1673-1678. [DOI] [PubMed] [Google Scholar]
- 9.Gercek E, Arlet V, Delisle J, Marchesi D. Subsidence of stand-alone cervical cages in anterior interbody fusion: warning. Eur Spine J. 2003;12(5):513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim T-H, Kwon H, Jeon C-H, et al. Effect of endplate conditions and bone mineral density on the compressive strength of the graft-endplate interface in anterior cervical spine fusion. Spine. 2001;26(8):951-956. [DOI] [PubMed] [Google Scholar]
- 11.Bartels RHMA, Donk RD, Feuth T. Subsidence of stand-alone cervical carbon fiber cages. Neurosurgery. 2006;58(3):502-508. [DOI] [PubMed] [Google Scholar]
- 12.Truumees E, Demetropoulos CK, Yang KH, Herkowitz HN. Effects of disc height and distractive forces on graft compression in an anterior cervical discectomy model. Spine. 2002;27(22):2441-2445. [DOI] [PubMed] [Google Scholar]
- 13.Yang JJ, Yu CH, Chang B-S, Yeom JS, Lee JH, Lee C-K. Subsidence and nonunion after anterior cervical interbody fusion using a stand-alone polyetheretherketone (PEEK) cage. Clin Orthop Surg. 2011;3(1):16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barsa P, Suchomel P. Factors affecting sagittal malalignment due to cage subsidence in standalone cage assisted anterior cervical fusion. Eur Spine J. 2007;16(9):1395-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Closkey RF, Parsons JR, Lee CK, Blacksin MF, Zimmermant MC. Mechanics of interbody spinal fusion. Analysis of critical bone graft area. Spine. 1993;18(8):1011-1015. [DOI] [PubMed] [Google Scholar]
- 16.Karikari IO, Jain D, Owens TR, et al. Impact of subsidence on clinical outcomes and radiographic fusion rates in anterior cervical discectomy and fusion. J Spinal Disord Tech. 2014;27(1):1-10. [DOI] [PubMed] [Google Scholar]
- 17.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine. 2006;4(6):447-453. [DOI] [PubMed] [Google Scholar]
- 18.Weber MH, Fortin M, Shen J, et al. Graft subsidence and revision rates following anterior cervical corpectomy. Clin Spine Surg. 2017;30(9):E1239-E1245. [DOI] [PubMed] [Google Scholar]
- 19.Ordway NR, Rim BC, Tan R, Hickman R, Fayyazi AH. Anterior cervical interbody constructs: effect of a repetitive compressive force on the endplate. J Orthop Res. 2012;30(4):587-592. [DOI] [PubMed] [Google Scholar]
- 20.Mikula AL, Puffer RC, Jeor JDS, et al. Teriparatide treatment increases Hounsfield units in the lumbar spine out of proportion to DEXA changes. J Neurosurg Spine. 2019;18:1-6. [DOI] [PubMed] [Google Scholar]
- 21.Hida K, Iwasaki Y, Yano S, Akino M, Seki T. Long-term follow-up results in patients with cervical disk disease treated by cervical anterior fusion using titanium cage implants. Neurol Med -Chir. 2008;48(10):440-446. [DOI] [PubMed] [Google Scholar]
- 22.Schmieder K, Wolzik-Grossmann M, Pechlivanis I, Engelhardt M, Scholz M, Harders A. Subsidence of the wing titanium cage after anterior cervical interbody fusion: 2-year follow-up study. J Neurosurg Spine. 2006;4(6):447-453. [DOI] [PubMed] [Google Scholar]
- 23.Kao T-H, Wu C-H, Chou Y-C, Chen H-T, Chen W-H, Tsou H-K. Risk factors for subsidence in anterior cervical fusion with stand-alone polyetheretherketone (PEEK) cages: a review of 82 cases and 182 levels. Arch Orthop Trauma Surg. 2014;134(10):1343-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao P, Conrad BP, Lewis DD, Horodyski M, Pozzi A. Effect of plate working length on plate stiffness and cyclic fatigue life in a cadaveric femoral fracture gap model stabilized with a 12-hole 2.4 mm locking compression plate. BMC Vet Res. 2013;9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis T, Bourgeault CA, Kyle RF. Screw position affects dynamic compression plate strain in an in vitro fracture model. J Orthop Trauma. 2001;15(5):333-337. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmeier KL, Hofmann GO, Mückley T. Choosing a proper working length can improve the lifespan of locked plates. Clin BioMech. 2011;26(4):405-409. [DOI] [PubMed] [Google Scholar]
- 27.Kanchanomai C, Muanjan P, Phiphobmongkol V. Stiffness and endurance of a locking compression plate fixed on fractured femur. J Appl Biomech. 2010;26(1):10-16. [DOI] [PubMed] [Google Scholar]
- 28.Maxwell M, Horstman CL, Crawford RL, Vaughn T, Elder S, McLaughlin R. The effects of screw placement on plate strain in 3.5 mm dynamic compression plates and limited-contact dynamic compression plates. Vet Comp Orthop Traumatol. 2009;22(2):125-131. [DOI] [PubMed] [Google Scholar]
- 29.Yson SC, Sembrano JN, Santos ERG. Comparison of allograft and polyetheretherketone (PEEK) cage subsidence rates in anterior cervical discectomy and fusion (ACDF). J Clin Neurosci. 2017;38:118-121. [DOI] [PubMed] [Google Scholar]