Abstract
Study Design
Literature review and meta-analysis.
Objectives
Single-center series may be underpowered to detect whether high-dose (HD) tranexamic acid (TXA) confers a higher risk of complications. We sought to determine the safety and efficacy of HD TXA as compared to low-dose (LD) or placebo.
Methods
A systematic literature review was performed to find studies where spine surgery patients were given HD TXA (loading dose ≥30 mg/kg). Complication rates were pooled, and meta-analyses performed on outcomes of interest. Articles were evaluated for risk of bias and a strength of evidence assessment was given for each conclusion.
Results
Twenty three studies (n = 2331) were included. The pooled medical complication rate was 3.2% in pediatric patients, 8.2% in adults. Using lower dose TXA or placebo as the reference, meta-analysis showed no difference in medical complications (n = 1,723, OR 1.22 [95% CI, .78 to 1.22]; P = .388; I2 = 0%) or thrombotic events (n = 1158 patients, OR 1.27 [95% CI, .71 to 2.63]; P = .528; I2 = 0%). Compared to LD, HD TXA was associated with less intraoperative blood loss (823 patients, WMD = −285 [95% CI, −564 to −5.90]; P = .0454; I2 = 86%), fewer perioperative transfusions (n = 505, OR .28 [95% CI, .082 to .96]; P = .043; I2 = 76%) and lower perioperative transfusion volumes (n = 434, WMD −227.7 mL [95% CI, −377.3 to −78.02]; P = .0029; I2 = 0%).
Conclusion
Compared to LD TXA or placebo, there is moderate evidence that HD is not associated with an increased risk of medical complications. Compared to LD, there is moderate evidence that HD reduces transfusion requirements. High-Dose TXA can be safely utilized in healthy patients undergoing major spine surgery.
Keywords: tranexamic acid, high dose, low dose, dosing, complications, spine
Introduction
Intraoperative administration of intravenous tranexamic acid (TXA) has gained increased popularity over the last decade as a method to decrease blood loss in spine surgery.1-4 However, the optimal dose of TXA for spine surgery remains controversial. Compared to lower doses, it has been suggested that higher doses of TXA may confer additional benefits, such as lower transfusion rates and shorter operative times.2,3,5 The clinical implication is that if TXA is safe, there could be a benefit in reducing intra- and peri-operative blood loss in spine surgery. The major barrier to the use of high-dose (HD) TXA is fear of associated medical complications, particularly seizures and complications secondary to clot formation (venous thromboembolism [VTE], myocardial infarction, etc.). 6
While many series report that high dose TXA is safe, the use of HD TXA is not widely accepted.5,7-10 Given that medical complications are rare, single-center series are underpowered to detect whether HD TXA increases the rate of medical complications. This is especially true with regards to VTE, which occurs in merely 1% of cases. 11 To date, no investigators have pooled the overall medical complication rate for the many series in which HD TXA has been used. Furthermore, while several reviews have stratified the effect of TXA based on dose (ie, compared the effect of HD to placebo and low-dose [LD] to placebo), no meta-analyses have directly compared the clinical efficacy of HD to LD TXA.1-4
The definition used to differentiate HD from LD TXA is of utmost importance when asking this question. For example, Xiong Z et al. 12 performed a meta-analysis comparing dosing regimens with a threshold of 1g TXA. However, this threshold is not in line with the pharmacokinetic and cardiac surgery literature of what constitutes true “HD” TXA. The secondary effects of HD TXA do not occur until a higher dosing threshold is reached.13,14
To these ends, the primary purpose of our systematic review and meta-analysis is to test whether HD TXA is as safe as placebo or LD TXA with regards to medical, surgical complications. The secondary goal is to compare clinical measures of efficacy (blood loss, transfusion rate and volume, and operative time) between high- and LD regimens.
Methods
Literature Search
A comprehensive literature search was conducted through the PUBMED, Embase, ERIC, and MEDLINE databases using a semi-automated software (AutoLit, Nested Knowledge). 15 Nested Knowledge is an online platform that facilitates querying, screening, and data extraction for secondary analyses. Full review of the details of our data collection can be accessed on their website. De-duplication was performed, and only original articles in English were included. Further details on the methodology of our search, screening, and data extraction process are publicly available on the Nested Knowledge website.
Study Selection
Inclusion criteria for studies were as follows: (1) Patients underwent any form of spine surgery (2) TXA was administered intravenously before surgery (3) HD TXA was administered to all patients (for case series) or at least 1 treatment arm (for comparative studies) (4) Studies tracked the occurrence of one or more of the following complications: Renal, Cardiopulmonary, Gastrointestinal, Infection, Neurological, VTE, or Other. The combined cohort mean age threshold for classifying studies as “pediatric” instead of “adult” was 22 ≥. “High-dose TXA” was defined as a loading dose of ≥ 30 mg/kg or 2000 mg (29 mg/kg in a 70 kg adult), since a loading dose of 30 mg/kg with 16 mg/kg/hr maintenance is believed to maintain tissue concentrations necessary to achieve the “secondary” effects of TXA.13,14 Any other TXA dosing regimen was defined as “LD”.
Data Extraction
Predefined data, including study characteristics, group baselines, and outcomes, were extracted independently by 2 authors (Table 1). The primary outcomes of interest were the rates of medical, surgical complications, and VTE. Secondary outcomes included intra- and perioperative blood loss (mL), intra- and perioperative transfusion events (%) and volumes (mL), and operative time.
Table 1.
Summary Characteristics for the 23 Included Studies.
| First Author | Country | Year | Age Group | Study Design | Sample Size (E/C) | High Dose TXA Regimen | Surgical Procedure | Levels Fused (E/C) | Follow-Up Period | Risk of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| Ahlers | USA | 2021 | Pediatric | Retrospective cohort | 106 | 50 mg/kg + 10 mg/kg/h | Deformity correction | [11] | 30 days | Low |
| Chou | Taiwan | 2021 | Pediatric | Retrospective cohort | 15/15 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 16/16 | Inpatient | High |
| DaRocha | Brazil | 2015 | Pediatric | Retrospective cohort | 21/19 | 100 mg/kg + 30 mg/kg/h | Deformity correction | 9/9 | 35 days | Low |
| Dhawale | USA | 2012 | Pediatric | Retrospective cohort | 30/40 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 16/16 | - | Low |
| Elwatidy | Saudi Arabia | 2008 | Adult | Double-blind RCT | 32/32 | 2000 mg (adults) or 30 mg/kg/h (peds) + 100 mg/h (adults) or 1 mg/kg/h (peds) | Multilevel or single-level fusion, multilevel decompression | - | 30 days | Low |
| Goobie | USA | 2018 | Pediatric | Double-blind RCT | 56/55 | 2000 mg (adults) or 30 mg/kg/h (peds) + 100 mg/h (adults) or 1 mg/kg/h (peds) | Deformity correction | [10]/[9] | 30 days | Low |
| Haddad | USA | 2020 | Adult | Retrospective cohort | 58/184 | 30 mg/kg + 3 mg/kg/h | Three-column osteotomy | 13/13 | - | High |
| Halanski | USA | 2014 | Pediatric | Double-blind RCT | 22 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 11 | Inpatient | Some concerns |
| Hasan | Malaysia | 2021 | Pediatric | Double-blind RCT | 83/83 | 30 mg/kg + 10 mg/kg/h | Deformity correction | [11]/[11] | 30 days | Low |
| Johnson | USA | 2017 | Pediatric | Retrospective cohort | 44/72 | 50 mg/kg + 5 mg/kg/h | Deformity correction | 11/11 | Inpatient | High |
| Kushioka | Japan | 2017 | Adult | Retrospective cohort | 30/30 | 2000 mg + 2000 mg 16h post-op | PLIF | - | - | Low |
| Lin | USA | 2018 | Adult | Case series | 100 | 50 mg/kg + 5 mg/kg/h | Deformity correction | 14 | - | NA |
| Lykissas | USA | 2013 | Pediatric | Retrospective cohort | 25/24 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 11/13 | - | Low |
| Ng | Hong Kong | 2015 | Pediatric | Retrospective cohort | 55/35 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 14/12 | Inpatient | High |
| Raman | USA | 2019 | Adult | Retrospective cohort | 60/258 | 40 mg/kg + 1 mg/kg/h 30 mg/kg10 mg/kg/h 50 mg/kg + 5 mg/kg/h | Deformity correction | 12/11 | 3 months | Low |
| Ramkiran | India | 2020 | Pediatric | Double-blind RCT | 12/12 | 50 mg/kg + 10 mg/kg/h | Deformity correction | Not provided | - | Some concerns |
| Sethna | USA | 2005 | Pediatric | Double-blind RTC | 23/21 | 100 mg/kg + 10 mg/kg/h | Deformity correction | [14]/[13] | - | Some concerns |
| Shapiro | USA | 2007 | Pediatric | Retrospective cohort | 20/36 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 14/14 | 2 weeks | Low |
| Shi | China | 2017 | Adult | Double-blind RCT | 50/46 | 30 mg/kg + 2 mg/kg/h | PLIF | - | Inpatient | Some concerns |
| Sui | China | 2016 | Pediatric | Retrospective cohort | 71/66 | 100 mg/kg + 10 mg/kg/h | Deformity correction | 13/13 | 90 days | Low |
| Tumber | USA | 2021 | Pediatric | Retrospective cohort | 126/97 | >30 mg/kg + 10 mg/kg/h | Deformity correction | 11/11 | - | Low |
| Xie | China | 2015 | Pediatric | Retrospective cohort | 26/33 | 100 mg/kg + 10 mg/kg/h | Vertebral column resection | 13/12 | - | High |
| Zhang | China | 2021 | Pediatric | Double-blind RCT | 108 | 50 mg/kg + 10 mg/kg/h + oral TXA | Deformity correction | 10 | - | Some concerns |
E/C = experimental (high dose)/control (low dose or placebo). Mean surgical levels fused is rounded to a whole number, median values are shown as [n]. TXA regimen are given as pre-op loading dose + continuous infusion. Values that were not provided are shown as `-`. Fields that are not applicable are shown as ‘NA’. Risk of bias was assessed either using the Cochrane ROB2 tool for randomized trials or the Newcastle Ottawa Scale for retrospective cohort studies, with a score of 7-9 classified as low risk of bias, and a score of 4-6 as a high risk of bias (Appendix 1a/1b).
Quality Assessment and Strength of Evidence
Validated scoring systems were used to perform risk of bias assessments for each study. Namely, the Newcastle-Ottawa Scale was used to evaluate retrospective cohort studies,16,17 and the Cochrane ROB-2 tool was used for randomized controlled trials 18 (Appendix 1a/1b). Two reviewers independently judged the quality of the eligible studies. The quality of evidence regarding the effect of HD TXA on each outcome of interest was evaluated qualitatively using the GRADE approach.
Statistical Analysis
Statistical analyses were performed using RStudio 4.1.2. For primary outcomes of interest, pooled complication rates were calculated for all patients receiving HD TXA. Comparative meta-analyses were performed for outcomes reported by at least 2 comparative studies. Notably, case series and studies comparing HD TXA to other anti-fibrinolytics without a control arm were excluded from comparative analysis. Primary outcomes testing the safety of TXA (medical, surgical complications, and VTE) were compared between HD and Not High Dose (NHD, defined as LD and placebo) studies. LD and placebo were combined into the NHD group to further evidence our hypothesis that HD TXA is safe. Namely, if our analyses showed that HD TXA is safe compared to placebo and LD TXA, this would provide further support for our hypothesis, than comparing HD TXA to LD TXA alone. Our purpose was to test whether HD TXA is more clinically efficacious than LD TXA, thus secondary outcomes were only evaluated within studies comparing HD vs LD. For dichotomous outcomes, odds ratios (OR) and 95% confidence intervals (CI) were calculated as pooled metrics with the Mantel-Haenszel method. The pooled effect size for continuous outcomes was reported as a weighted mean difference (WMD) and 95% CI calculated using pooled means and standard deviations. 19 Heterogeneity was assessed using I2 statistics. If there was no evidence of substantial heterogeneity (I2 ≤ 50%), a fixed-effect model was used. For further details on statistical methods, see Appendix 2. Risk of publication bias was evaluated using a funnel plot analysis performed on the most frequently reported outcome (VTE).
Results
Search Outcomes
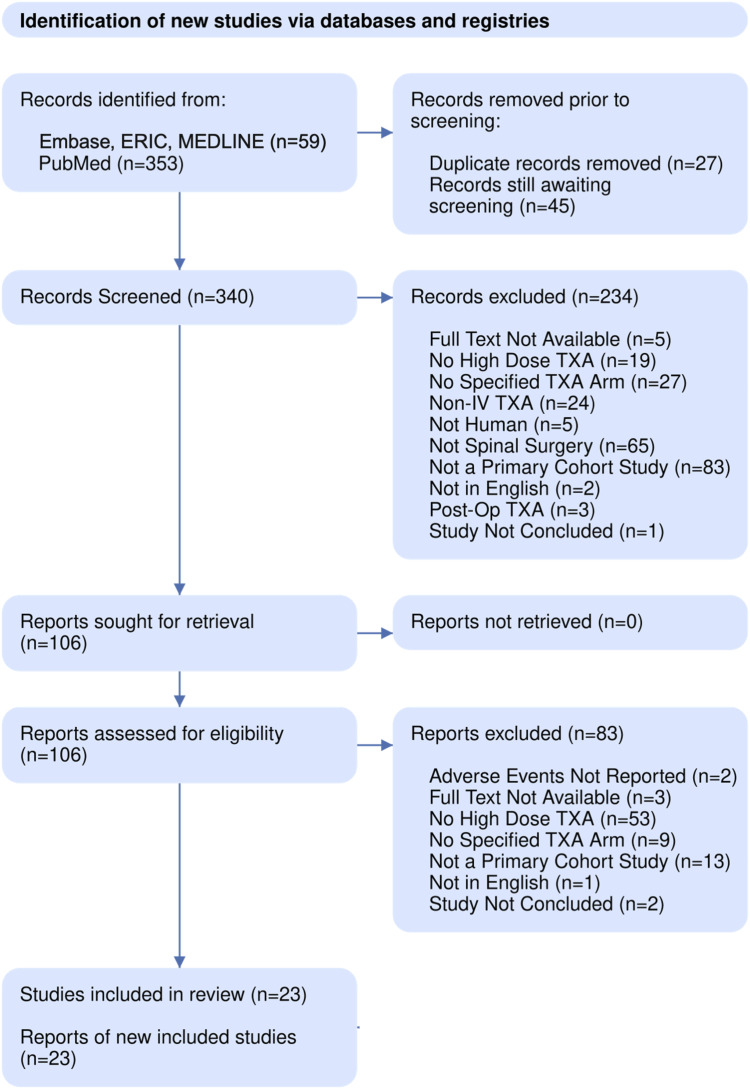
Database queries retrieved a total of 367 results. No further studies were identified through other sources and 27 duplicates were removed. After abstract and full-text screening, a total of 23 studies and 2331 patients were included in the present review. Figure 1 details our PRISMA screening process.
Figure 1.
PRISMA diagram showing the literature search and screening process.
Study Characteristics
Included studies were either retrospective cohort reviews (n = 14), double blinded RCTs (n = 8), or case series (n = 1), published between 2005 and 2021, with sample sizes ranging from 22 to 318 total participants (Table 1). Of the 23 studies, 1 was a case series of HD interventions, 20 1 compared various HD regimens to each other, 7 4 compared HD and LD cohorts,9,21-23 15 compared HD to placebo controls, and 2 compared HD to other anti-fibrolytics.24,25 Funnel plot analysis was performed using VTE as the outcome of interest, the funnel plot showed some asymmetry with a moderate risk of publication bias (Appendix 3).
Primary Outcomes
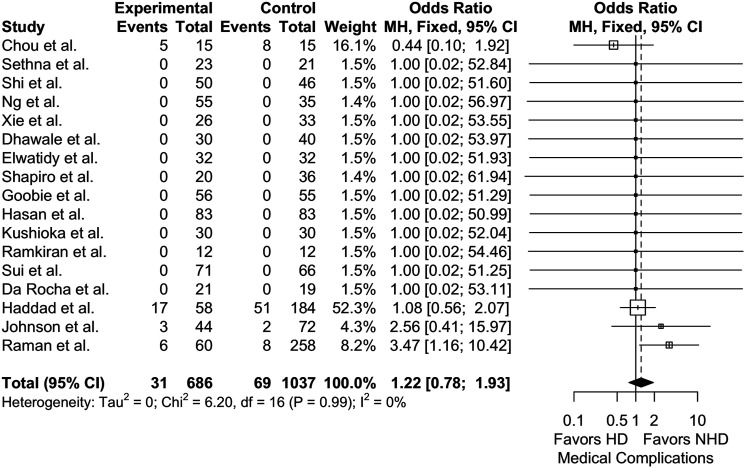
The pooled medical complication rate for patients receiving HD TXA was 4.7% (21 studies, 1022 patients). This rate was lower in pediatric patients (3.18%, 15 studies, 692 patients) than in adults (8.19%, 6 studies, 330 patients) (Incidence rate difference = 5%, P = .0006, Incidence rate ratio = 2.57, P = .0011). Meta-analysis of 17 studies, using NHD as the reference group, showed no significant difference in medical complication rates between HD and NHD TXA (1723 patients; P = .388) (Figure 2). Similarly, meta-analyses within adult (n = 5) and pediatric (n = 12) study subgroups showed no significant differences in medical complication rates (780 patients, OR = 1.37 [95% CI, .802 to 2.37]; P = .246; I2 = 0% and 943 patients, OR = .932 [95% CI, .401 to 2.13]; P = .867; I2 = 0%, respectively).
Figure 2.
Meta-analysis with a fixed effects model of studies reporting medical complications for high-dose (HD) vs not high-dose (NHD = placebo or low-dose) cohorts across all age groups. OR = odds ratio, MH = Mantel-Haenszel, df = degrees of freedom.
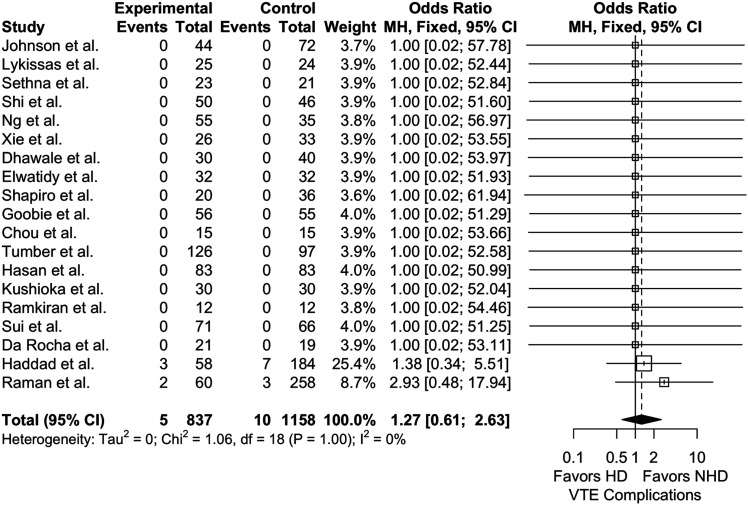
The pooled rate of VTE in HD patients was .682% (23 studies, 1173 patients), again, lower in pediatric patients (0%, 17 studies, 843 patients) compared to adults (2.42%, 6 studies, 330 patients) (Incidence rate difference = 2.4%, P < .0001, Incidence rate ratio = n/a). Using NHD as the reference, there was no appreciable difference in our 17 study meta-analyses between HD and NHD groups (1158 patients, P = .528) (Figure 3). Subgroup analysis of adult (n = 5) and pediatric (n = 14) studies also showed no significant differences in VTE outcomes (780 patients, OR = 1.58 [95% CI, .583 to 4.26]; P = .369; I2 = 0% and 1215 patients, OR = 1.00 [95% CI, .345 to 2.90]; P = 1.00; I2 = 0%, respectively).
Figure 3.
Meta-analysis with a fixed effects model of studies reporting VTE complications for high-dose (HD) vs not high-dose (NHD = placebo or low-dose) cohorts across all age groups. OR = odds ratio, MH = Mantel-Haenszel, df = degrees of freedom.
Analyzing the 3 comparative studies21,26,27 that reported surgical outcomes also did not reveal any appreciable difference between HD and NHD groups (590 patients, OR = 1.23 [95% CI, .530 2.86]; P = .629; I2 = 0%).
Secondary Outcomes
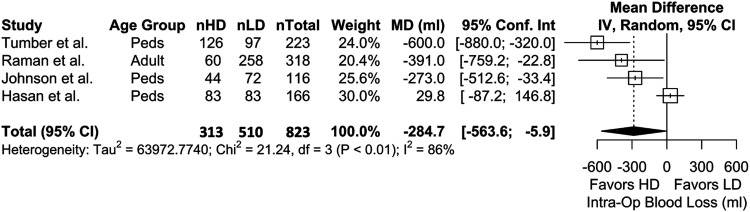
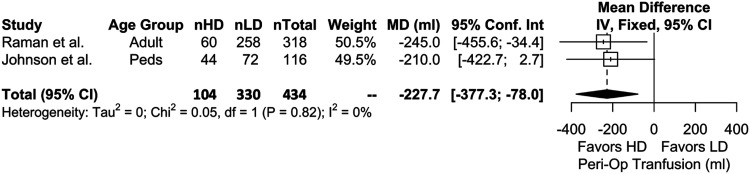
Four studies were included in the meta-analysis for intraoperative blood loss which showed that, with LD as the reference, HD TXA is significantly more effective in reducing blood loss (P = .0454) (Figure 4). Meta-analysis with 2 studies on perioperative allogenic transfusion volumes also showed that HD is more effective than LD (P = .0029) (Figure 5). Results for intraoperative transfusion volumes similarly showed significant results favoring HD (2 studies, 434 patients, WMD = −228 [95% CI, −360 to −95.1]; P = .0008; I2 = 0%).
Figure 4.
Meta-analysis with a random effects model comparing intraoperative blood loss (ml) between highdose (HD) and low-dose (LD) cohorts across all age groups. nHD = number of HD patients, nLD = number of LD patients, nTotal = total patients. MD = mean difference, IV = inverse variance, df = degrees of freedom.
Figure 5.
Meta-analysis with a fixed effects model comparing perioperative RBC transfusions (ml) between high-dose (HD) and low-dose (LD) cohorts across all age groups. nHD = number of HD patients, nLD = number of LD patients, nTotal = total patients. MD = mean difference, IV = inverse variance, df = degrees of freedom.
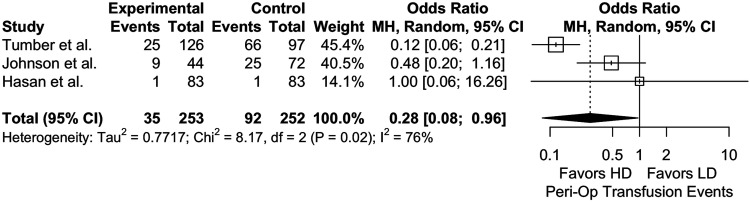
Analysis on perioperative transfusion events showed significant results favoring HD over NHD (2 studies, 505 patients, P = .043) (Figure 6) while there was a present but insignificant difference for intraoperative transfusion events (2 studies, 339 patients, OR = .244 [95% CI, .053 to 1.115]; P = .069; I2 = 88%). Lastly, 3 studies were included in the meta-analysis for surgical duration,9,21,22 which demonstrated no correlation with TXA dose (P = .904) (Appendix 4).
Figure 6.
Meta analysis with a random effects model comparing perioperative RBC transfusion events between high-dose (HD) and low-dose (LD) cohorts across all age groups. OR = odds ratio, MH = Mantel- Haenszel, df = degrees of freedom.
Discussion
In our systematic literature review we found a pooled medical complication rate after HD TXA consistent with medical complication rates in non-TXA outcomes studies.28-31 Furthermore, our meta-analyses provided high-quality evidence that HD TXA is not associated with an increased risk of medical complication after adult or pediatric spine surgery. This lack of association has been previously reported in systematic reviews on the use of TXA in pediatric spine surgery, scoliosis correction, and lumbar interbody fusion.2,32,33 Our review found only one retrospective cohort study with an increased rate of medical complications in the HD TXA cohort. Out of the 60 patients who received HD TXA, 3 patients developed postoperative atrial fibrillation (of which 1 had an NSTEMI), compared to none in the LD cohort. 10 Notably, the authors specify that these 3 patients all had a history of cardiac disease and/or diabetes mellitus and that all patients returned to sinus rhythm while inpatient or at first follow-up.
Regarding specific medical complications, we found a pooled VTE rate of 2.4% in adults, similar to reported single- and multicenter rates of VTE in non-TXA focused studies. 11 Meta-analysis revealed high-quality evidence that HD TXA did not confer an increased risk of VTE compared to NHD. However, our findings regarding medical complications and VTE must be interpreted with caution. First, except for a few patients in the case series by Xie et al, 7 no adult patient received a TXA regimen higher than 50 mg/kg loading with 5 mg/kg/h maintenance, so we cannot comment on the safety of regimens higher than this for adults. Second, most studies were retrospective, introducing selection bias in terms of which patients received HD TXA. Third, all randomized trials excluded patients with thromboembolic disorders, and several others also excluded patients with hepatic, renal, or cardiac disease.34-38 Thus, while HD TXA seems to be safe in patients without a significant medical history, the risk-benefit relationship in patients with cardiac, renal, hematologic, or neurologic conditions requires further investigation.
A special note must be made regarding seizures. Within the field of cardiac surgery, TXA has shown a dose-dependent relationship with risk of seizures. 6 However, this association has not borne out in other fields in which TXA is used and was not demonstrated in our meta-analysis.6,39 Out of the 1173 patients receiving HD TXA in our meta-analysis, no seizure occurred, and in NHD patients, only 1 seizure was reported. 21 This finding may be secondary to 2 differences. First, the maintenance dose used in the cardiac surgery literature (16 mg/kg/h) is higher than that reported in spine surgery (Table 1).6,13,40 Second, use of cardiopulmonary bypass is unique to cardiac surgery. Pharmacokinetics and tissue concentrations of TXA may vary based on these factors. Future studies on in vivo pharmacokinetics are needed to understand the TXA blood concentrations achieved by varying dosage regimens during spine surgery.
We found low-quality evidence that the rate of surgical complications is not decreased by the use of HD TXA. Our quality of evidence assessment for this conclusion is lower because this outcome was heterogeneously defined and reported by comparatively fewer studies.10,20,27,41 Given that rates of surgical complications are highly variable among procedures, defining this outcome will require procedure-specific trials.
The clinical benefit of HD TXA is dependent on its ability to reduce blood loss by a greater extent than that of LD regimens. Physiologically, TXA at low tissue concentrations (10 µg/mL) inhibits fibrinolysis by approximately 80% in animal models. 42 Higher concentrations (126-152 µg/mL) have the potential to further inhibit fibrinolysis and enhance hemostasis by increasing thrombin formation, improving platelet function.9,13,14,43 There may be an anti-inflammatory effect as well through its action on cytokine production. 39
Our analysis provides moderate evidence that there is a clinical benefit (in terms transfusion requirements and blood loss) to the use of HD over LD TXA in spine surgery. Past meta-analyses have indirectly supported this notion, finding that the effect of HD TXA vs placebo is larger than that of LD vs placebo with regards to transfusion rate.1,2,4 We showed that intraoperative blood loss and both perioperative allogeneic transfusion rate and volume was lower with HD regimens. 44 Notably, the effect size was greatest in studies with the largest blood loss.9,10 For example, Tumber et al reviewed 223 patients with AIS, with an average EBL per vertebral body fused (EBL/VB) of 160 and 104 mL in the LD and HD cohorts, respectively. 9 They found a statistically significant 48% reduction in pRBC transfusion rate (67% LD vs 19% HD, P < .001). On the other hand, Johnson et al also examined 116 AIS patients, reporting an average EBL/VB of 87 and 63, respectively. 45 However, while they reported a lower transfusion volume in the HD cohort (.4 unit pRBC LD vs 1 unit pRBC HD, P = .04), rates of transfusion were comparable (35% LD vs 21% HD, P = .1). Thus, it appears that the clinical benefit (ie, reduction of allogeneic transfusion rates) of high-vs LD TXA is likely only realized in spine surgeries with the greatest expected blood loss.
Theoretically, a reduction in blood loss could translate to a clearer surgical field with subsequent improvement in operative efficiency. However, we found low-quality evidence that HD TXA does not reduce operative time. Though Hui et al reported a reduction in operative time with any TXA vs placebo, the mean difference was likely clinically insignificant (−4.7 min, 95% CI −8.8 to −.7)4. To this end, any conclusions regarding the effect of TXA on operative time would be premature.
There were several limitations to this investigation. First, medical complication rates after spine surgery are widely variable and procedure dependent.28-31 There was a wide variety of procedures both within and among the trials. Despite this variability, the finding was consistent in nearly every study, regardless of the dose or population. For this reason, we believe there is still high quality evidence supporting this finding. Second, follow-up times for our primary outcome varied among studies, with some reporting complication rates but not specifying follow up times.7,9,20,26,40,46-49 However, regardless of follow-up time, our findings were largely consistent, leading us to maintain our conclusions. Third, there may have been reporting bias among the studies in how complications were defined. This was especially relevant for studies which reported “complications related to TXA” without giving further detail.25,46,47 To this end, our overall pooled rate of complications likely underestimates the true rate. However, the comparative analyses are still valid given that each study definition was applied to both the HD and NHD group. Finally, there were not enough comparative studies that reported perioperative blood loss to allow meta-analyses on this outcome.
Conclusions
With regards to the primary purpose of our study, HD TXA does not appear to confer any increased risk of medical complication when given to the “right” patient (ie, those with a medical history consistent with the patients in our reviewed studies). Our analysis also provides moderate evidence that HD TXA may reduce intraoperative blood loss and allogeneic transfusions (rates and volume), a clinical advantage over LD regimens. Thus, future research should address 3 major deficiencies. First, the exact risk-benefit relationship between dosage and comorbidities must be addressed. The question still stands whether HD (or any) TXA is safe in patients with hematologic, cardiac, or epileptic conditions. Second, trials should be prioritized to target patients which stand to benefit the most, namely those undergoing surgeries with largest expected blood losses (adult deformity corrections, oncologic resections, etc.). Patients undergoing such surgeries are also often those with the largest comorbidity burden, giving further impetus to the need for an accurate understanding of risks associated with HD TXA in patients without pristine medical histories. Finally, the pharmacokinetics of TXA in humans remain unknown. HD TXA is associated with seizures in cardiopulmonary bypass but not spine surgery, suggesting that the pharmacokinetics may vary based on the patterns and timing of blood loss specific to varying surgical procedures. Pharmacokinetic data is an essential endpoint that must be included in any well-designed study of TXA, with real implications for amount and timing of dose.
Supplemental Material
Supplemental Material for Is High-Dose Tranexamic Safe in Spine Surgery? A Systematic Review and Meta-Analysis by Izzet Akosman, Francis Lovecchio, Mitchell Fourman, Manuel Sarmiento, Keith Lyons, Stavros Memtsoudis, and Han Jo Kim in Global Spine Journal
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
IRB approval statement: No institutional review board approval was necessary for this analysis.
Supplemental Material: Supplemental material for this article is available online
ORCID iDs
Izzet Akosman https://orcid.org/0000-0002-1433-9078
Francis Lovecchio https://orcid.org/0000-0001-5236-1420
Han Jo Kim https://orcid.org/0000-0003-2170-3592
References
- 1.Zhang Y, Liu H, He F, Chen A, Yang H, Pi B. Does tranexamic acid improve bleeding, transfusion, and hemoglobin level in patients undergoing multilevel spine surgery? A systematic review and meta-analysis. World Neurosurg. 2019;127(1):289-301. doi: 10.1016/j.wneu.2019.02.170 [DOI] [PubMed] [Google Scholar]
- 2.Yuan QM, Zhao ZH, Xu BS. Efficacy and safety of tranexamic acid in reducing blood loss in scoliosis surgery: a systematic review and meta-analysis. Eur Spine J. 2017;26(1):131-139. doi: 10.1007/s00586-016-4899-0 [DOI] [PubMed] [Google Scholar]
- 3.Zhao Y, Xi C, Xu W, Yan J. Role of tranexamic acid in blood loss control and blood transfusion management of patients undergoing multilevel spine surgery: a meta-analysis. Medicine. 2021;100(7):e24678. doi: 10.1097/MD.0000000000024678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui S, Xu D, Ren Z, et al. Can tranexamic acid conserve blood and save operative time in spinal surgeries? A meta-analysis. Spine J 2018;18(8):1325-1337. doi: 10.1016/j.spinee.2017.11.017 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro F, Zurakowski D, Sethna NF. Tranexamic acid diminishes intraoperative blood loss and transfusion in spinal fusions for duchenne muscular dystrophy scoliosis. Spine (Phila Pa 1976). 2007;32(20):2278-2283. doi: 10.1097/BRS.0b013e31814cf139 [DOI] [PubMed] [Google Scholar]
- 6.Goobie SM. Tranexamic acid: still far to go. Br J Anaesth. 2017;118(3):293-295. doi: 10.1093/bja/aew470 [DOI] [PubMed] [Google Scholar]
- 7.Xie J, Lenke LG, Li T, et al. Preliminary investigation of high-dose tranexamic acid for controlling intraoperative blood loss in patients undergoing spine correction surgery. Spine J. 2015;15(4):647-654. doi: 10.1016/j.spinee.2014.11.023 [DOI] [PubMed] [Google Scholar]
- 8.Lin JD, Lenke LG, Shillingford JN, et al. Safety of a high-dose tranexamic acid protocol in complex adult spinal deformity: analysis of 100 consecutive cases. Spine Deform. 2018;6(2):189-194. doi: 10.1016/j.jspd.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 9.Tumber S, Bacon A, Stondell C, et al. High- versus low-dose tranexamic acid as part of a Patient Blood Management strategy for reducing blood loss in patients undergoing surgery for adolescent idiopathic scoliosis. Spine Deform. 2021;10:107-113. doi: 10.1007/s43390-021-00387-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raman T, Varlotta C, Vasquez-Montes D, Buckland AJ, Errico TJ. The use of tranexamic acid in adult spinal deformity: is there an optimal dosing strategy? Spine J. 2019;19(10):1690-1697. doi: 10.1016/j.spinee.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 11.McLynn RP, Diaz-Collado PJ, Ottesen TD, et al. Risk factors and pharmacologic prophylaxis for venous thromboembolism in elective spine surgery. Spine J. 2018;18(6):970-978. doi: 10.1016/j.spinee.2017.10.013 [DOI] [PubMed] [Google Scholar]
- 12.Xiong Z, Wu K, Zhang J, et al. Different dose regimens of intravenous tranexamic acid in adolescent spinal deformity surgery: a systematic review and meta-analysis. BioMed Res Int. 2020;2020:3101358. doi: 10.1155/2020/3101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sigaut S, Tremey B, Ouattara A, et al. Comparison of two doses of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Anesthesiology. 2014;120(3):590-600. doi: 10.1097/ALN.0b013e3182a443e8 [DOI] [PubMed] [Google Scholar]
- 14.Grassin-Delyle S, Tremey B, Abe E, et al. Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth. 2013;111(6):916-924. doi: 10.1093/bja/aet255 [DOI] [PubMed] [Google Scholar]
- 15.Nested Knowledge. Autolit: High Dose TXA Safety. Published online 2022. https://nested-knowledge.com/nest/635. Accessed January 31, 2022 [Google Scholar]
- 16.Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Published 2008. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 19, 2022. [Google Scholar]
- 17.Lau KKL, Samartzis D, To NSC, Harada GK, An HS, Wong AYL. Demographic, surgical, and radiographic risk factors for symptomatic adjacent segment disease after lumbar fusion: a systematic review and meta-analysis. J Bone Joint Surg Am. 2021;103(15):1438-1450. doi: 10.2106/JBJS.20.00408 [DOI] [PubMed] [Google Scholar]
- 18.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:1-8. doi: 10.1136/bmj.l4898 [DOI] [PubMed] [Google Scholar]
- 19.Higgins J, Thomas J, Chandler J. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Hoboken, NJ: Wiley-Blackwell; 2019. [Google Scholar]
- 20.Lin JD, Lenke LG, Shillingford JN, et al. Safety of a high-dose tranexamic acid protocol in complex adult spinal deformity: analysis of 100 consecutive cases. Spine Deform. 2018;6(2):189-194. doi: 10.1016/J.JSPD.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 21.Raman T, Varlotta C, Vasquez-Montes D, Buckland AJ, Errico TJ. The use of tranexamic acid in adult spinal deformity: is there an optimal dosing strategy? Spine J. 2019;19(10):1690-1697. doi: 10.1016/J.SPINEE.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 22.Johnson DJ, Johnson CC, Goobie SM, et al. High-dose versus low-dose tranexamic acid to reduce transfusion requirements in pediatric scoliosis surgery. J Pediatr Orthop. 2017;37(8):e552-e557. doi: 10.1097/BPO.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 23.Hasan MS, Yunus SN, Ng CC, Chan CYW, Chiu CK, Kwan MK. Tranexamic acid in pediatric scoliosis surgery: a prospective randomized trial comparing high-dose and low-dose tranexamic acid in adolescent idiopathic scoliosis undergoing posterior spinal fusion surgery. Spine (Phila Pa 1976). 2021;46(22):E1170-E1177. doi: 10.1097/BRS.0000000000004076 [DOI] [PubMed] [Google Scholar]
- 24.Halanski MA, Cassidy JA, Hetzel S, Reischmann D, Hassan N. The efficacy of amicar versus tranexamic acid in pediatric spinal deformity surgery: a prospective, randomized, double-blinded pilot study. Spine Deform. 2014;2(3):191-197. doi: 10.1016/J.JSPD.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 25.Ramkiran S, Kumar M, Krishnakumar L, Nair SG. Comparison of blood-conserving and allogenic transfusion-sparing effects of antifibrinolytics in scoliosis correction surgery. Anesth Essays Res. 2020;14(2):259. doi: 10.4103/AER.AER_59_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haddad AF, Ames CP, Safaee M, Deviren V, Lau D. The effect of systemic tranexamic acid on hypercoagulable complications and perioperative outcomes following three-column osteotomy for adult spinal deformity. Global Spine J. 2022;12:423. doi: 10.1177/2192568220953812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chou SH, Lin SY, Wu MH, et al. Reduces blood loss and transfusion volume in scoliosis surgery for spinal muscular atrophy: results of a 20-year retrospective analysis. Int J Environ Res Public Health. 2021;18:9959. doi: 10.3390/ijerph18199959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Badhiwala JH, Leung SN, Jiang F, et al. In-hospital course and complications of laminectomy alone versus laminectomy plus instrumented posterolateral fusion for lumbar degenerative spondylolisthesis: a retrospective analysis of 1804 patients from the NSQIP database. Spine (Phila Pa 1976). 2021;46(9):617-623. doi: 10.1097/BRS.0000000000003858 [DOI] [PubMed] [Google Scholar]
- 29.Yu CC, Carreon LY, Glassman SD, et al. Propensity-matched comparison of 90-day complications in robotic-assisted versus non-robotic assisted lumbar fusion. Spine (Phila Pa 1976). 2022;47(3):195-200. doi: 10.1097/brs.0000000000004288 [DOI] [PubMed] [Google Scholar]
- 30.Bauer J, Shah S, Sponseller PD, et al. Comparing short-term AIS post-operative complications between ACS-NSQIP and a surgeon study group. Spine Deform. 2020;8(6):1247-1252. [DOI] [PubMed] [Google Scholar]
- 31.Farahani F, Riccio A, Ramo B. Low BMI (< 10th percentile) increases complications and readmissions after posterior spinal fusion in adolescent idiopathic scoliosis. Spine Deform. 2021;9(6):1533-1540. [DOI] [PubMed] [Google Scholar]
- 32.Xiong Z, Wu K, Zhang J, Leng D, Yu Z, Zhang C, et al. Different dose regimens of intravenous tranexamic acid in adolescent spinal deformity surgery: a systematic review and meta-analysis. BioMed Res Int. 2020;2020:3101358. doi: 10.1155/2020/3101358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gong M, Liu G, Chen L, Chen R, Xiang Z. The efficacy and safety of intravenous tranexamic acid in reducing surgical blood loss in posterior lumbar interbody fusion for the adult: a systematic review and a meta-analysis. World Neurosurg. 2019;122:559-568. doi: 10.1016/j.wneu.2018.09.115 [DOI] [PubMed] [Google Scholar]
- 34.Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine (Phila Pa 1976). 2008;33(24):2577-2580. doi: 10.1097/BRS.0b013e318188b9c5 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, Wang LN, Yang X, et al. The effect of multiple-dose oral versus intravenous tranexamic acid in reducing postoperative blood loss and transfusion rate after adolescent scoliosis surgery: a randomized controlled trial. Spine J. 2021;21(2):312-320. doi: 10.1016/j.spinee.2020.10.011 [DOI] [PubMed] [Google Scholar]
- 36.Goobie SM, Zurakowski D, Glotzbecker MP, et al. Tranexamic acid is efficacious at decreasing the rate of blood loss in adolescent scoliosis surgery: a randomized placebo-controlled trial. J Bone Joint Surg Am. 2018;100(23):2024-2032. doi: 10.2106/JBJS.18.00314 [DOI] [PubMed] [Google Scholar]
- 37.Hasan MS, Yunus SN, Ng CC, Chan CYW, Chiu CK, Kwan MK. Tranexamic acid in pediatric scoliosis surgery. Spine (Phila Pa 1976). 2021;46(22):E1170-E1177. doi: 10.1097/brs.0000000000004076 [DOI] [PubMed] [Google Scholar]
- 38.Shi H, Ou Y, Jiang D, Quan Z, Zhao Z, Zhu Y. Tranexamic acid reduces perioperative blood loss of posterior lumbar surgery for stenosis or spondylolisthesis a randomized trial. Medicine (United States). 2017;96(1):1-8. doi: 10.1097/MD.0000000000005718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lei YT, Xie JW, Huang Q, Huang W, Pei FX. The antifibrinolytic and anti-inflammatory effects of a high initial-dose tranexamic acid in total knee arthroplasty: a randomized controlled trial. Int Orthop. 2020;44(6):1237-1238. doi: 10.1007/s00264-020-04554-5 [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Gao X, Ma Y, et al. Different dose regimes and administration methods of tranexamic acid in cardiac surgery: a meta-analysis of randomized trials. BMC Anesthesiol. 2019;19(1):1-16. doi: 10.1186/s12871-019-0772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haddad AF, Ames CP, Safaee M, Deviren V, Lau D. The effect of systemic tranexamic acid on hypercoagulable complications and perioperative outcomes following three-column osteotomy for adult spinal deformity. Global Spine J . 2020;12:423-431. doi: 10.1177/2192568220953812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson L, Nilsoon I, Colleen S, Granstrand B, Melander B. Role of urokinase and tissue activator in sustaining bleeding and the management thereof with EACA and AMCA. Ann N Y Acad Sci. 1968;146(2):642-658. [DOI] [PubMed] [Google Scholar]
- 43.Couturier R, Grassin-Delyle S. Tranexamic acid: more than inhibition of fibrinolysis? Anesth Analg. 2014;119(2):498-499. doi: 10.1213/ANE.0000000000000251 [DOI] [PubMed] [Google Scholar]
- 44.Woods BI, Rosario BL, Chen A, et al. The association between perioperative allogeneic transfusion volume and postoperative infection in patients following lumbar spine surgery. J Bone Joint Surg Am. 2013;95(23):2105-2110. doi: 10.2106/JBJS.L.00979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson DJ, Johnson CC, Goobie SM, et al. High-dose versus low-dose tranexamic acid to reduce transfusion requirements in pediatric scoliosis surgery. J Pediatr Orthop. 2017;37(8):e552-e557. doi: 10.1097/BPO.0000000000000820 [DOI] [PubMed] [Google Scholar]
- 46.Dhawale AA, Shah SA, Sponseller PD, et al. Are antifibrinolytics helpful in decreasing blood loss and transfusions during spinal fusion surgery in children with cerebral palsy scoliosis? Spine (Phila Pa 1976). 2012;37(9):E549-E555. doi: 10.1097/BRS.0B013E31823D009B [DOI] [PubMed] [Google Scholar]
- 47.Sethna NF, Zurakowski D, Brustowicz RM, Bacsik J, Sullivan LJ, Shapiro F. Tranexamic acid reduces intraoperative blood loss in pediatric patients undergoing scoliosis surgery. Anesthesiology. 2005;102(4):727-732. doi: 10.1097/00000542-200504000-00006 [DOI] [PubMed] [Google Scholar]
- 48.Lykissas MG, Crawford AH, Chan G, Aronson LA, Al-Sayyad MJ. The effect of tranexamic acid in blood loss and transfusion volume in adolescent idiopathic scoliosis surgery: a single-surgeon experience. J Child Orthop. 2013;7(3):245-249. doi: 10.1007/S11832-013-0486-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kushioka J, Yamashita T, Okuda S, et al. High-dose tranexamic acid reduces intraoperative and postoperative blood loss in posterior lumbar interbody fusion. J Neurosurg Spine. 2017;26(3):363-367. doi: 10.3171/2016.8.SPINE16528 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Is High-Dose Tranexamic Safe in Spine Surgery? A Systematic Review and Meta-Analysis by Izzet Akosman, Francis Lovecchio, Mitchell Fourman, Manuel Sarmiento, Keith Lyons, Stavros Memtsoudis, and Han Jo Kim in Global Spine Journal