Abstract
Objective
The Yasui operation was introduced in 1987 for patients with 2 adequate ventricles, a ventricular septal defect, and aortic atresia or interrupted aortic arch. Despite promising early outcomes, left ventricular outflow tract obstruction (LVOTO) remains a long-term concern. The purpose of this study is to report our institutional experience with the Yasui operation.
Methods
We retrospectively reviewed all patients undergoing the Yasui operation between 1989 and 2021. Results are reported as median with interquartile range (IQR).
Results
Twenty-five patients underwent a Yasui operation (19 primary), at 11 days (IQR, 7-218 days) of life and weight of 3 kg (IQR, 2.8-4.1 days). Fundamental diagnosis was ventricular septal defect/interrupted aortic arch in 11 patients and ventricular septal defect/aortic atresia in 14. Follow-up was 96% (24 out of 25) at 5 years (IQR, 1.4-14.7) with 92% survival. Freedom from LVOTO reoperation was 91% at late follow-up with 2 patients requiring baffle revision at 6 and 9 years. Latest echocardiogram showed 100% of patients had normal biventricular function and 87% (20 out of 23) less than mild LVOTO at 5 years (IQR, 2.3-14.9). Diagnosis, aortic valve morphology, and material used were not predictors of LVOTO. Freedom from right ventricle-to-pulmonary artery conduit reoperation was 48% at a median of 5 years (IQR, 1.4-14.7). Conduit type was not a predictor of reintervention.
Conclusions
The Yasui operation can be performed with low morbidity and mortality in patients with 2 acceptable-size ventricles and aortic atresia or interrupted aortic arch with severe LVOTO. Despite some burden of reoperation, midterm reoperation for LVOTO is not common and ventricular function is preserved.
Key Words: Yasui, biventricular repair, aortic atresia, interrupted aortic arch, left ventricular outflow tract obstruction
Graphical abstract
Graphical Abstract summarizing the findings of this single institutional experience with the Yasui operation over the past 30 years.


Freedom from reoperation for LVOTO was 91% a median of 5 years after the Yasui operation.
Central Message.
The Yasui operation can be performed with low morbidity and mortality, despite some burden of conduit reoperation, in patients with 2 acceptable-size ventricles and AA or IAA with severe LVOTO.
Perspective.
This is the largest series of Yasui operations with the longest follow-up. A rarely performed repair, unfamiliarity with this approach hinders its widespread application as the first-choice solution to LVOTO, VSD, and 2 adequately sized ventricles. Concerns persist regarding its safety and long-term durability. We report a 92% survival with a 91% freedom from LVOTO reintervention at 5 years.
The Yasui operation was introduced in 1987 for patients with 2 adequate ventricles, a ventricular septal defect (VSD), and aortic atresia (AA) or interrupted aortic arch (IAA).1 Neonatal single-stage biventricular repair for IAA/VSD with diminutive aortic valves was plagued by the late development of left ventricular outflow tract obstruction (LVOTO) with nearly half of patients requiring reintervention.2 Surgical solutions to this unique patient population included-single stage palliation with the Norwood operation or biventricular repair with a Ross/Konno/Arch repair.3 The former commits a patient to a shunted-single ventricle physiology when he or she has 2 adequate ventricles, whereas the later has a high early mortality rate (33%).4
The Yasui operation was developed to address this patient population by creating a Damus-Kaye-Stansel connection between the atretic ascending aorta and the pulmonary trunk then baffling the left ventricular outflow through the VSD to the systemic circulation. Pulmonary blood flow is established with a right ventricle to pulmonary artery (RV-PA) conduit. The Yasui procedure offers the advantage of a primary or staged biventricular repair, with no shunting and a reliable source of fully oxygenated systemic blood flow. It has been shown to have lower operative mortality in comparison to the Norwood,5 and equivocal long-term survival ranging from 58% at 5 years to 88% at 10 years.5,6 Despite promising early outcomes, LVOTO remains a long-term concern as does the need for conduit revision.7, 8, 9 Some centers advocate a staged approach because a larger-size conduit can be placed and the postoperative course is more stable,6,8 whereas other centers advocate establishment of 2-ventricle circulation as early as possible.5 The purpose of this study is to report our institutional experience with the Yasui operation in the hopes of clarifying some of the ongoing controversies.
Materials and Methods
A retrospective chart review was performed to identify all patients who underwent a Yasui operation between September 1989 and May 2021. The study was approved by the institutional review board of Boston Children's Hospital, December 21, 2019, and patient consent was waived (IRB-P00034147). Patients were identified from the cardiac surgery and cardiology database. Inclusion criteria were all patients undergoing a Yasui operation. A staged Yasui is defined as a primary Norwood procedure followed by septation with a Yasui operation. The patient can be at any stage of the single-ventricle palliation, including bidirectional Glenn and Fontan, whereas a primary repair indicates the Yasui as the first operation. Patients being considered for the Yasui operation had 2 adequate ventricles, a VSD, and AA or IAA. There are no institutional criteria for the Yasui operation and primary repair was pursued in all patients except one who was in extremis and taken for a salvage Norwood. All other staged Yasui patients were referred after receiving the primary palliation at an outside hospital. Primary end points were survival, presence of LVOTO, number of reinterventions, and type. A detailed retrospective chart review was performed. Demographic, prenatal, postnatal, echocardiographic, catheterization, and operative data were collected from the medical record and analyzed. Follow-up for survival analysis was obtained in 91% of patients.
Surgical Technique
Although surgical technique has evolved over the 30-year study period, patients undergoing a Yasui operation were cannulated aorto bi-cavally, underwent moderate hypothermia, and received antegrade cardioplegia for a diastolic arrest. Damus-Kaye-Stansel was performed in the standard fashion. The location of the ventriculotomy determined the ease of VSD closure/baffle. The closer the ventriculotomy to the aorta, the easier the baffle, but this places the RV-PA conduit at risk directly behind the sternum. Thus, moving the incision as lateral as possible, toward the left anterior descending, is better for conduit revision but is balanced by the ease of VSD baffle. It is occasionally useful to create a larger ventriculotomy to aid baffle closure then primarily close the portion of the incision underneath the aorta/PA before implanting the conduit. Care must be taken to avoid distorting the subvalvular configuration and causing neoaortic regurgitation. If there is any concern that the VSD is small and would put the patient at risk for LVOTO, then the VSD is enlarged, even if it is a muscular defect.
Statistical Analysis
Patient and operative characteristics are represented as number (percent) for categorical variables and median and interquartile range (IQR) for continuous variables. Echocardiography measurements are summarized as mean ± SD. Sample sizes are shown for variables with missing data. Univariate Cox proportional hazard regression analysis was used to explore predictors of mortality, LVOTO, reoperation for LVOTO, and conduit reoperation. Results are presented as hazard ratios with 95% CI and P values. Multivariable modeling was not performed due to relatively small sample size and number of events. Kaplan-Meier curves were created to estimate freedom from events over time for each outcome, with 95% confidence bands obtained using Greenwood's formula. The comparison of primary and staged operations was performed using Fisher exact test for categorical variables and using the nonparametric Wilcoxon rank sum test for continuous variables. All statistical analyses were performed using Stata (version 16.1, Stata Corp LLC).
Results
Between 1989 and 2021, 25 patients underwent a Yasui operation (19 primary and 6 staged). Age at the time of operation was 11 days (IQR, 6.5-218 days) and weight was 3.1 kg (IQR, 2.8-4.2 kg). Gestational age was 39 weeks (IQR, 35-39 weeks). Fifty-six percent of patients were male (n = 14) and 24% of patients were premature (<37 weeks). Six had known genetic disorders (4 DiGeorge, 1 elastin gene defect, and 1 chromosome 5 deletion). Fundamental diagnosis was VSD/IAA in 11 patients and VSD/AA in 14 (11 hypoplastic arch and 3 double outlet right ventricle). All patients had a VSD; 92% (23 out of 25) had a posterior malalignment VSD and 2 had a muscular VSD. Additional diagnoses include subaortic stenosis (n = 7), bicuspid aortic valves (n = 6), unicuspid aortic valves (n = 3), right sided aortic arch (n = 2), and aberrant right (n = 8), and left (n = 2) subclavian arteries. Six patients had undergone the Norwood pathway with 3 having progressed to a bidirectional Glenn and 1 to a Fontan at the time of Yasui. Complete preoperative echocardiographic data was obtained in 12 patients (Table 1).
Table 1.
Preoperative echocardiographic measurements
| Mean ± SD | Mean z score ± SD | |
|---|---|---|
| Aortic valve annulus | 4.1 ± 2.3 | −4.2 ± 2.0 |
| Mitral valve annulus | 11 ± 0.34 mm | 0.68 ± 1.6 |
| Left ventricle mass | 7.7 ± 6.8 g | −1.96 ± 1.88 |
| Aortic root diameter | 0.7 ± 0.3 mm | −1.8 ± 2.2 |
| Ascending aorta diameter | 0.4 ± 0.8 mm | −2.9 ± 3.7 |
Cardiopulmonary bypass time was 264 minutes (IQR, 191-297 minutes), crossclamp time was 195 minutes (IQR, 110-221 minutes), circulatory arrest time was 18 minutes (IQR, 16-39 minutes), selective antegrade perfusion time was 90.5 minutes (IQR, 66-115 minutes), and patient temperature was 18 °C (IQR, 18-23 °C). The VSD was enlarged in 4 patients when there was concern for LVOTO. VSD/baffle was constructed of autologous pericardium (52%) or Dacron (DuPont) (20%) most commonly. Aortic arch reconstruction was performed in 16 (64%) patients and was most often constructed of thick pulmonary homograft (n = 7), aortic homograft (n = 4), or polyethylene terephthalate (n = 3). All patients had an RV-PA homograft conduit (12 pulmonary and 13 aortic) with a median size of 11 mm (IQR, 9-12 mm). Immediate postbypass echocardiogram showed no residual LVOTO. All but 2 patients were found to have good biventricular function. The chest was left open in 19 out of 23 (83%) patients and closed at 4 days postoperatively (IQR, 3-6 days). Two patients required extracorporeal membrane oxygenation (ECMO) in the perioperative period. One had a protamine reaction with arrest and was transitioned to ECMO in the operating room. The second bled postoperatively and was transitioned to ECMO for recovery of his metabolic status. Early reoperation occurred in 7 patients: 3 for bleeding, 2 for residual VSD, 1 for wound infection, and 1 for pacemaker placement. Time to extubation was 7 days (IQR, 5-16 days), intensive care unit length of stay was 18 days (IQR, 7-33 days), and total hospital length of stay was 26 days (IQR, 18-46 days).
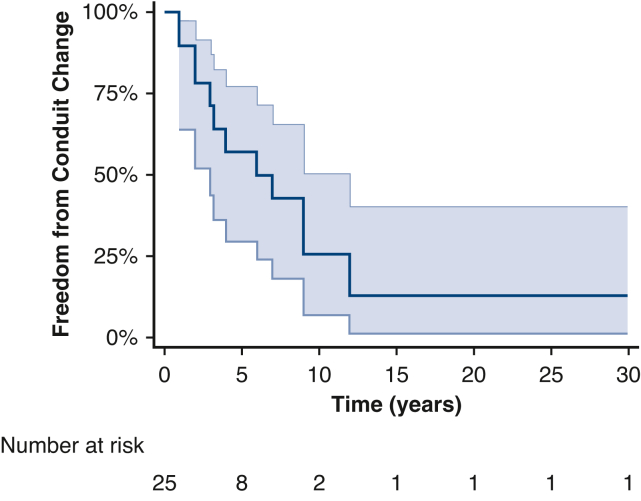
Follow-up was obtained in 96% (24 out of 25) of patients at a median of 5 years (IQR, 1.4-14.7 years) with 92% survival (Figure 1). There was 1 in-hospital death from multiorgan failure and 1 patient died of an unknown cause 1.5 years postoperatively. Freedom from LVOTO reoperation was 91% (21 out of 23) at last follow-up with 2 patients requiring baffle revision and VSD enlargement at 6 and 9 years (Figure 2). Original diagnosis was AA/VSD in these 2 patients. One was staged and had their VSD enlarged at the time of Yasui, whereas the other was a primary Yasui where the VSD was originally believed to be adequate. Latest follow-up echocardiogram showed 100% of patients had normal biventricular function and 87% (20 out of 23) had less than mild LVOTO at 5 years (IQR, 2.3-14.9 years) (Figure 3). On latest follow-up echocardiograph, aortic valve (AV) annular growth was observed in 78% (7 out of 9) of patients, all of whom had AA, a median of 5 years postoperatively (median z score, 5.1). Two patients with IAA had stably small AV annuli with a median z score of −4.6. No preoperative factors were associated with increased LVOTO or survival (Table 2). Freedom from RV-PA conduit reoperation was 48% at 5 years (IQR, 1.4-14.7 years) with 13 patients requiring conduit replacement at 4 years (IQR, 2-8 years) (Figure 4). Size of initial conduit replacement was 20 mm (IQR, 17-23 mm). Neither size nor type of conduit was predictive of conduit reintervention.
Figure 1.
Survival after Yasui operation over time. Survival was 92% a median of 5 years postoperatively (interquartile range, 1.4-14.7 years). There were 2 deaths: 1 in-hospital and 1 at 1.5 years postoperatively. Shaded areas represent the 95% CI.
Figure 2.
Freedom from left ventricular outflow tract obstruction (LVOTO) reoperation over time showed 91% of patients to be free of LVOTO reintervention at a median of 5 years postoperatively. Two patients underwent LVOT reoperation at 6 and 9 years postoperatively. Shaded areas represent the 95% CI.
Figure 3.
Freedom from left ventricular outflow tract obstruction (LVOTO) over time. Eighty-six percent of patients had no LVOTO by most recent echocardiogram with three patients found to have mild LVOTO. All patients had preserved biventricular function. Shaded areas represent the 95% CI.
Table 2.
Univariate Cox regression analysis of mortality
| Variable | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Sex | |||
| Male | Reference | ||
| Female | 1.4 | 0.09-22.4 | .813 |
| Age at surgery (y) | 0.79 | 0.16-3.86 | .769 |
| Weight at surgery (kg) | 0.38 | 0.13-1.1 | .074 |
| BAV | Cannot calculate | ||
| Aortic z score | 1.08 | 0.54-2.17 | .83 |
| Mitral z score | 0.92 | 0.33-2.55 | .867 |
| LV mass (g) | 0.68 | 0.34-1.36 | .279 |
| LV mass z score | 0.81 | 0.35-1.88 | .62 |
| AA z score | 1.1 | 0.82-1.47 | .523 |
| Primary operation No. (1) | Cannot calculate | ||
| Arch reconstruction | 0.48 | 0.03-7.67 | .602 |
| RV-PA Conduit Size (mm) | 0.86 | 0.36-2.03 | .727 |
| Material RV-PA | |||
| Aortic | Reference | ||
| Pulmonary | 0.96 | 0.06-15.3 | .975 |
| CPB time (min) | 1.02 | 0.98-1.06 | .252 |
| Crossclamp time (min) | 1.04 | 0.96-1.14 | .348 |
| Circulatory arrest time (min) | 1.05 | 0.93-1.19 | .441 |
| SACP (min) | 0.99 | 0.96-1.03 | .656 |
| Cooled to (°C) | 0.87 | 0.39-1.95 | .731 |
| Days intubated | 1.3 | (0.84, 2.02) | .241 |
| ICU LOS (d) | 1 | (0.97, 1.03) | .921 |
| Total Hospital LOS (d) | 1.01 | (0.97, 1.06) | .563 |
CI, Confidence interval; BAV, bicuspid aortic valve; LV, left ventricle; AA, aortic atresia; RV-PA, right ventricle to pulmonary artery; CPB, cardiopulmonary bypass; SACP, selective antegrade cerebral perfusion; ICU, intensive care unit; LOS, length of stay.
Figure 4.
Freedom from conduit reintervention over time. Forty-eight percent of patients were found to require conduit reintervention at 5 years with 75% of patients requiring conduit reintervention by 10 years. Shaded areas represent the 95% CI.
Cohort size and event rate was insufficient to compare primary vs staged repairs for the primary outcomes. Comparison of operative and postoperative outcomes was performed and found length of open chest (17 vs 2 days; P = .042) and intensive care unit length of stay (23 vs 8 days; P = .017) to be significantly different between the 2 groups (Table 3).
Table 3.
Comparison of primary versus staged operations
| Primary (n = 19) | Staged (n = 6) | P value | |
|---|---|---|---|
| Cardiopulmonary bypass time (min) | 279 (246-334) n = 12 | 191 (151-264) n = 5 | .114 |
| Crossclamp time (min) | 201 (127-227) n = 12 | 106 (82-204) n = 5 | .114 |
| Circulatory arrest time (min) | 18 (16-47) n = 11 | 22 (5-39) n = 2 | .693 |
| Chest left open | 17 (89.5) | 2 (40) (n = 5) | .042∗ |
| Days intubated | 9.1 (5.8-18.2) n = 16 | 5.3 (4-7) n = 6 | .121 |
| Intensive care unit length of stay (d) | 23 (12-38) n = 14 | 8 (6-13) n = 6 | .017∗ |
| Total hospital length of stay (d) | 34 (19-54) n = 18 | 21 (14-26) n = 6 | .072 |
Values are presented as n (%) or median (interquartile range).
P values were calculated using Fisher exact test or the Wilcoxon rank sum test.
Statistically significant.
Discussion
The Yasui operation provides a 2-ventricle solution to a single ventricle physiology. The combination of AA or IAA with VSD and 2 adequately sized ventricles can be safely managed with either a single-ventricle or 2-ventricle approach with acceptable early survival. Concerns exist regarding subsequent development of LVOTO, the need for LVOT reintervention and the burden of conduit replacement. We evaluated our experience over the past 30 years with the Yasui operation to address some of these concerns.
To the authors' knowledge, this is the largest published series of Yasui operations (n = 25) with the longest follow up (33 years).4, 5, 6 A rarely performed repair, unfamiliarity with this approach hinders its widespread application as the first-choice solution to LVOTO, VSD, and 2 adequately sized ventricles and concerns persist regarding its safety and long-term durability. We report a 92% survival with a 91% freedom from LVOTO reintervention and 48% freedom from conduit reintervention at a median of 5 years (IQR, 1.4-14.7 years). This corroborates the assertion that the Yasui operation is a safe 2-ventricle solution to AA or IAA, VSD, and 2 adequately sized ventricles.5,6
Additionally, concerns regarding the need for early LVOT reintervention are alleviated. The majority of patients (86%) did not have LVOTO at 5 years, and those who did (n = 3) reported less than mild LVOTO. All patients were found to have preserved biventricular function at follow-up. The lack of LVOTO is likely attributed to careful patient selection. Although all patients had diminutive aortic annuli (median z score, −4.7) they all also had apex forming LVs and normal-sized mitral valves (median z score, −0.15). Thus, when applied to the appropriate patient population, the Yasui operation can be performed with minimal risk of recurrent LVOTO.10
The need for conduit reintervention is significant with almost half of patients requiring conduit replacement at 5 years. When evaluated against the reintervention rate for the alternative solutions (Norwood or Ross/Konno/Arch repair), the Yasui compares favorably. In the Norwood pathway, most patients will undergo a minimum of 3 operations with the first occurring as early as age 4 months. The Ross/Konno/Arch repair has the same burden of conduit replacement but has a high early mortality rate (33%).5,6,11, 12, 13 So, although there is a burden of reintervention for conduit replacement in the Yasui operation that is unavoidable, the need for reintervention is on par or less than the other surgical alternatives.
Given our small sample size of staged repairs, we were unable to compare the primary outcomes of freedom from LVOTO, freedom from reintervention for LVOTO, freedom from conduit change and survival for primary vs staged patients. We did find that patients who were initially staged had their chest closed sooner and had faster intensive care unit length of stays as suggested by Nakano and colleagues.6 This is logically attributed to the older age and greater weight at staged repair. It is erroneous to conclude that staged repair is safer or better based on these findings because the 2 patient populations have too many confounding factors precluding direct comparison. Additionally, the added risk of undergoing single ventricle palliation with the inherent interstage mortality must also be considered. Some advocate pulmonary artery banding as an alternative to the Norwood to delay decision making regarding the need for Yasui or standard biventricular repair.6 No patients in our series were managed with pulmonary artery banding. Of the 6 patients who were staged, 5 presented after undergoing single-ventricle palliation at an outside hospital and 1 underwent a salvage Norwood because the patient was in extremis. It is our assertion that if patients can be repaired safely as neonates and avoid a shunted physiology that is better for their overall prognosis.5,10
Although this study confirms the findings of previous investigations, it is a single-center retrospective review of a rare operation with a small sample size and is subject to all the biases and limitations of a retrospective chart review.
Conclusions
The Yasui operation can be performed with low morbidity and mortality in patients with 2 acceptable-size ventricles and AA or IAA with severe LVOTO. Despite some burden of reoperation, midterm reoperation for LVOTO is not common and ventricular function is preserved. A primary Yasui operation avoids the morbidity and mortality associated with a shunted single-ventricle palliation and can be performed safely with durable result in select patient populations with AA or IAA, severe LVOTO, and 2 acceptable-size ventricles (Figure 5).
Figure 5.
Graphical Abstract summarizing the findings of this single institutional experience with the Yasui operation over the past 30 years. LVOTO, Left ventricular outflow tract obstruction.
Conflict of Interest Statement
The authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Footnotes
IRB #: IRB-P00034147.
References
- 1.Yasui H., Kado H., Nakano E., Yonenaga K., Mitani A., Tomita Y., et al. Primary repair of interrupted aortic arch and severe aortic stenosis in neonates. J Thorac Cardiovasc Surg. 1987;93:539–545. [PubMed] [Google Scholar]
- 2.Hirata Y., Quaegebeur J., Mosca R., Takayama H., Chen J. Impact of aortic annular size on rate of reoperation for left ventricular outflow tract obstruction after repair of interrupted aortic arch and ventricular septal defect. Ann Thorac Surg. 2010;90:588–592. doi: 10.1016/j.athoracsur.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 3.Kanter K.R., Miller B.E., Cuadrado A.G., Vincent R.N. Successful application of the Norwood procedure for infants without hypoplastic left heart syndrome. Ann Thorac Surg. 1995;59:301–304. doi: 10.1016/0003-4975(94)00944-3. [DOI] [PubMed] [Google Scholar]
- 4.Hickey E.J., Yej T., Jacobs J.P., Caldarone C.A., Tchervenkov C.I., McCrindle B.W., et al. Ross and Yasui operations for complex biventricular repair in infants with critical left ventricular outflow tract obstruction. Eur J Cardio Thorac Surg. 2010;37:279–288. doi: 10.1016/j.ejcts.2009.06.060. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo S., Mainwaring R., Schaffer J., Wright G., Maeda K., Hanley F., et al. Contemporaneous comparison of the Yasui and Norwood procedures at a single institution. J Thorac Cardiovasc Surg. 2015;149:508–513. doi: 10.1016/j.jtcvs.2014.09.120. [DOI] [PubMed] [Google Scholar]
- 6.Nakano T., Kado H., Tatewaki H., Hideki T., Hinokiyama K., Machida D., et al. The Yasui operation for patients with adequate-sized ventricles and ventricular septal defect associated with obstructions of the aortic arch and left ventricular outflow tract. Eur J Cardio Thorac Surg. 2014;45:e166–e172. doi: 10.1093/ejcts/ezt658. [DOI] [PubMed] [Google Scholar]
- 7.Kanter K.R., Kirshbom P.M., Kogon B.E. Biventricular repair with the Yasui operation (Norwood/Rastelli) for systemic outflow tract obstruction with two adequate ventricles. Ann Thorac Surg. 2012;93:1999–2006. doi: 10.1016/j.athoracsur.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 8.Alsoufi B., Schlosser B., McCracken C., Sachdeva R., Kogon B., Border W., et al. Selective management strategy of interrupted aortic arch mitigates left ventricular outflow tract obstruction risk. J Thorac Cardiovasc Surg. 2016;151:412–420. doi: 10.1016/j.jtcvs.2015.09.060. [DOI] [PubMed] [Google Scholar]
- 9.Riggs K., Tweddell J. How small is too small? Decision-making and management of the small aortic root in the setting of interrupted aortic arch. Semin Thorac Cardiovasc Surg Pediatric Card Surg Annu. 2019;22:21–26. doi: 10.1053/j.pcsu.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Nathan M., Rimmer D., del Nido P.J., Mayer J.E., Bacha E.A., Shin A., et al. Aortic atresia or severe left ventricular outflow tract obstruction with ventricular septal defect: results of primary biventricular repair in neonates. Ann Thorac Surg. 2006;82:2227–2232. doi: 10.1016/j.athoracsur.2006.05.124. [DOI] [PubMed] [Google Scholar]
- 11.Shinkawa T., Bove E.L., Hirsch J.C., Devaney E.J., Ohye R.G. Intermediate-term results of the Ross procedure in neonates and infants. Ann Thorac Surg. 2010;89:1827–1832. doi: 10.1016/j.athoracsur.2010.02.107. [DOI] [PubMed] [Google Scholar]
- 12.Maeda K., Rizal R.E., Lavrsen M., Malhotra S.P., Akram S.A., Davies R., et al. Midterm results of the modified Ross/Konno procedure in neonates and infants. Ann Thorac Surg. 2012;94:156–162. doi: 10.1016/j.athoracsur.2012.03.007. discussion 162-3. [DOI] [PubMed] [Google Scholar]
- 13.Ruzmetov M., Geiss D.M., Shah J.J., Buckley K., Fortuna R.S. The Ross-Konno is a high-risk procedure when compared with the Ross operation in children. Ann Thorac Surg. 2013;95:670–675. doi: 10.1016/j.athoracsur.2012.08.041. [DOI] [PubMed] [Google Scholar]