This cohort study evaluates patient and treatment variables associated with use patterns of ultrahypofractionation, moderate hypofractionation, and conventional fractionation in US males with localized prostate cancer.
Key Points
Question
What is the temporal pattern of use of shorter-course radiotherapy (RT) for prostate cancer?
Findings
In this cohort study of 313 062 patients with localized prostate cancer, there was a continued increase in the use of shorter courses of RT (termed hypofractionation) for prostate cancer from 2004 to 2020 and, by 2019, conventional fractionation (ie, long-course RT) was used for a minority of patients. Multiple barriers to hypofractionation were associated with social determinants of health.
Meaning
Findings of this study suggest that realignment of value-based models to increase reimbursement for hypofractionation may reduce barriers to widespread adoption and ensure access for underserved communities.
Abstract
Importance
Randomized clinical trials have demonstrated the noninferiority of shorter radiotherapy (RT) courses (termed hypofractionation) compared with longer RT courses in patients with localized prostate cancer. Although shorter courses are associated with cost-effectiveness, convenience, and expanded RT access, their adoption remains variable.
Objective
To identify the current practice patterns of external beam RT for prostate cancer in the US.
Design, Setting, and Participants
This cohort study obtained data from the National Cancer Database, which collects hospital registry data from more than 1500 accredited US facilities on approximately 72% of US patients with cancer. Patients were included in the sample if they had localized prostate adenocarcinoma that was diagnosed between 2004 and 2020 and underwent external beam RT with curative intent. Analyses were conducted between February and March 2023.
Exposures
Radiotherapy schedules, which were categorized as ultrahypofractionation (≤7 fractions), moderate hypofractionation (20-30 fractions), and conventional fractionation (31-50 fractions).
Main Outcomes and Measures
Longitudinal pattern in RT fractionation schedule was the primary outcome. Multivariable logistic regression was performed to evaluate the variables associated with shorter RT courses. Covariables included age, National Comprehensive Cancer Network risk group, rurality, race, facility location, facility type, median income, insurance type or status, and Charlson-Deyo Comorbidity Index.
Results
A total of 313 062 patients with localized prostate cancer (mean [SD] age, 68.8 [7.7] years) were included in the analysis. There was a temporal pattern of decline in the proportion of patients who received conventional fractionation, from 76.0% in 2004 to 36.6% in 2020 (P for trend <.001). From 2004 to 2020, use of moderate hypofractionation increased from 22.0% to 45.0% (P for trend <.001), and use of ultrahypofractionation increased from 2.0% to 18.3% (P for trend <.001). By 2020, the most common RT schedule was ultrahypofractionation for patients in the low-risk group and moderate hypofractionation for patients in the intermediate-risk group. On multivariable analysis, treatment at a community cancer program (compared with academic or research program; odds ratio [OR], 0.54 [95% CI, 0.52-0.56]; P < .001), Medicaid insurance (compared with Medicare; OR, 1.49 [95% CI, 1.41-1.57]; P < .001), Black race (compared with White race; OR, 0.90 [95% CI, 0.87-0.92]; P < .001), and higher median income (compared with lower median income; OR, 1.28 [95% CI, 1.25-1.31]; P < .001) were associated with receipt of shorter courses of RT.
Conclusions and Relevance
Results of this cohort study showed an increase in the use of shorter courses of RT for prostate cancer from 2004 to 2020; a number of social determinants of health appeared to be associated with reduced adoption of shorter treatment courses. Realignment of reimbursement models may be necessary to enable broader adoption of ultrahypofractionation to support technology acquisition costs.
Introduction
Technological advancements in treatment planning and delivery have greatly improved the toxic effects, efficacy, and patient convenience of radiotherapy (RT) for many common cancers. External beam RT (EBRT) is an established treatment option for many individuals with localized prostate cancer. In patients with localized prostate cancer, conventional RT is delivered over 9 weeks of daily treatments or fractions. Multiple noninferiority randomized clinical trials have demonstrated the safety and efficacy of moderate hypofractionation (delivered over 4-6 weeks) and ultrahypofractionation or stereotactic body RT (SBRT; delivered over 1-2 weeks),1,2,3,4 with recent National Comprehensive Cancer Network (NCCN) guidelines endorsing both hypofractionated RT types in these settings.5
Modern EBRT is a noninvasive curative therapy with proven effectiveness and minimal impact on quality of life.6,7 However, the logistical burden of prolonged RT courses can be a barrier to receiving care, as a typical treatment regimen with conventional fractionation involves approximately 2 months of therapy for 5 days a week. Adoption of shorter courses of RT can be associated with reduced burden. However, hypofractionated RT, especially ultrahypofractionation, is often more resource intensive and may require acquisition of high-cost technology. Furthermore, longer courses of RT are reimbursed at higher rates than shorter courses.8 In this study, we evaluated EBRT practice patterns in the treatment of localized prostate cancer and defined modifiers of receiving hypofractionated therapy, with the goal of identifying the current practice patterns of EBRT for prostate cancer in the US.
Methods
In accordance with the Common Rule, this cohort study was exempt from ethics review and informed consent because it used public, nonidentifiable data. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Patients and Data Set
The data used in the study were derived from a deidentified National Cancer Database (NCDB) file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society to collect hospital registry data from more than 1500 accredited facilities. The NCDB contains data on approximately 72% of patients with cancer in the US.6
Patients were included in the sample if they were diagnosed with localized prostate adenocarcinoma from 2004 to 2020 and underwent EBRT in the absence of other radical treatment modalities (eFigure in Supplement 1). Patients with 50 or more treatments were excluded, and a histogram of remaining patients revealed clusters of patients who underwent 7 or fewer SBRTs, 20 to 30 moderate hypofractionations, and 31 to 50 conventional fractions. In the US, SBRT is defined as involving 1 to 5 fractions for billing purposes, but up to 7 treatments were included in this study given supportive clinical evidence. Patients who underwent 8 to 19 fractions were excluded as these are nonstandard treatment regimens.
Statistical Analysis
Descriptive statistics were used to characterize patient and treatment characteristics. Patients were categorized a priori based on the total number of fractions as follows: ultrahypofractionation, moderate hypofractionation, and conventional fractionation. Multivariable logistic regression was performed to evaluate the temporal use pattern of shorter RT courses (SBRT and moderate hypofractionation) and SBRT vs all other fractionation schemas. The covariables included age, NCCN risk group (low, favorable intermediate, unfavorable intermediate, or high), rurality, race (American Indian and Alaska Native, Asian or Pacific Islander, Black, White, or other/unrecorded), facility location (East North Central, East South Central, Middle Atlantic, Mountain, New England, Pacific, South Atlantic, West North Central, or West South Central), facility type (academic or research program, community cancer program, comprehensive community cancer program, or integrated network cancer program), median income (≤$57 856 or ≥$57 857), insurance type or status (Medicaid, Medicare, not insured, other government, or private insurance or managed care), and Charlson-Deyo Comorbidity Index (range: 0 for no, 1 for mild, 2 for moderate, and 3 for severe comorbidities). Race data were obtained from the NCDB and were collected in this study to examine practice patterns and their associations with race and social determinants of health.
Analyses were conducted between February and March 2023, using R, version 4.2.1 (R Foundation for Statistical Computing). Because of the large size of the data set, a 2-sided P < .001 was selected to indicate statistical significance.9
Results
Of the 316 519 patients identified based on receipt of EBRT for localized prostate cancer from 2004 to 2020, a total of 313 062 with a mean (SD) age of 68.8 (7.7) years were included in the analysis. Demographic data of the entire cohort are shown in Table 1.
Table 1. Descriptive Statistics.
| Characteristic | Patient or treatment, No. (%) |
|---|---|
| All patients, No. | 313 062 |
| Age, y | |
| Mean (SD) | 68.8 (7.7) |
| Median (range) | 69.0 (25.0-90.0) |
| Area of residence | |
| Metropolitan | 257 598 (82.3) |
| Rural | 5671 (1.8) |
| Urban | 41 630 (13.3) |
| Missing data | 8163 (2.6) |
| Racea | |
| American Indian and Alaska Native | 686 (0.2) |
| Asian or Pacific Islander | 6856 (2.2) |
| Black | 54 042 (17.3) |
| White | 245 748 (78.5) |
| Other or unrecordedb | 5730 (1.8) |
| Facility location | |
| East North Central | 58 529 (18.7) |
| East South Central | 18 084 (5.8) |
| Middle Atlantic | 61 741 (19.7) |
| Mountain | 10 385 (3.3) |
| New England | 25 949 (8.3) |
| Pacific | 33 865 (10.8) |
| South Atlantic | 67 339 (21.5) |
| West North Central | 21 109 (6.7) |
| West South Central | 16 037 (5.1) |
| Missing data | 24 (0) |
| Median income, US $ | |
| ≤57 856 | 106 013 (33.9) |
| ≥57 857 | 170 264 (54.4) |
| Missing data | 36 785 (11.8) |
| Insurance type or status | |
| Medicaid | 9319 (3.0) |
| Medicare | 189 234 (60.4) |
| Not insured | 3799 (1.2) |
| Other government | 11 234 (3.6) |
| Private insurance or managed care | 93 980 (30.0) |
| Unknown | 5496 (1.8) |
| Facility type | |
| Academic or research programc | 93 412 (29.8) |
| Community cancer program | 23 320 (7.4) |
| Comprehensive community cancer program | 133 911 (42.8) |
| Integrated network cancer program | 62 395 (19.9) |
| Missing data | 24 (0) |
| NCCN risk group | |
| Low | 47 590 (15.2) |
| Favorable intermediate | 64 108 (20.5) |
| Unfavorable intermediate | 99 550 (31.8) |
| High | 88 652 (28.3) |
| Missing data | 13 162 (4.2) |
| Charlson-Deyo Comorbidity Indexd | |
| 0 | 263 639 (84.2) |
| 1 | 36 815 (11.8) |
| 2 | 8291 (2.6) |
| 3 | 4317 (1.4) |
Abbreviation: NCCN, National Comprehensive Cancer Network.
Race data were obtained from the National Cancer Database.
Other data not provided in the National Cancer Database.
Included National Cancer Institute–designated comprehensive cancer centers.
Charlson-Deyo Comorbidity Index range: 0 for no, 1 for mild, 2 for moderate, and 3 for severe comorbidities.
During the study period, there was a significant increase in the proportion of patients who were treated with moderate hypofractionation (Figure; eTable 1 in Supplement 1) from 22.0% in 2004 to 45.0% in 2020 (P for trend <.001). In 2019, for the first time, the proportion of patients who underwent treatment with conventional fractionation was smaller than other hypofractionated treatment schedules (46.0%), which was further reduced in 2020 to 36.6% (P for trend <.001). Ultrahypofractionation use increased 9-fold during this period from 2.0% in 2004 to 18.3% in 2020 (P for trend <.001). Similar patterns were observed by NCCN risk group, with increases in hypofractionation across each risk group (Figure; eTable 2 in Supplement 1). The low-risk group comprised more than 20% of patients who underwent RT until 2009 and subsequently diminished year after year. In 2020, the low-risk group was composed of only 7.4% of patients who had RT (eTable 3 in Supplement 1).
Figure. Temporal Pattern of External Beam Radiotherapy (RT) Fractionation Schedule Use by National Comprehensive Cancer Network Risk Group.
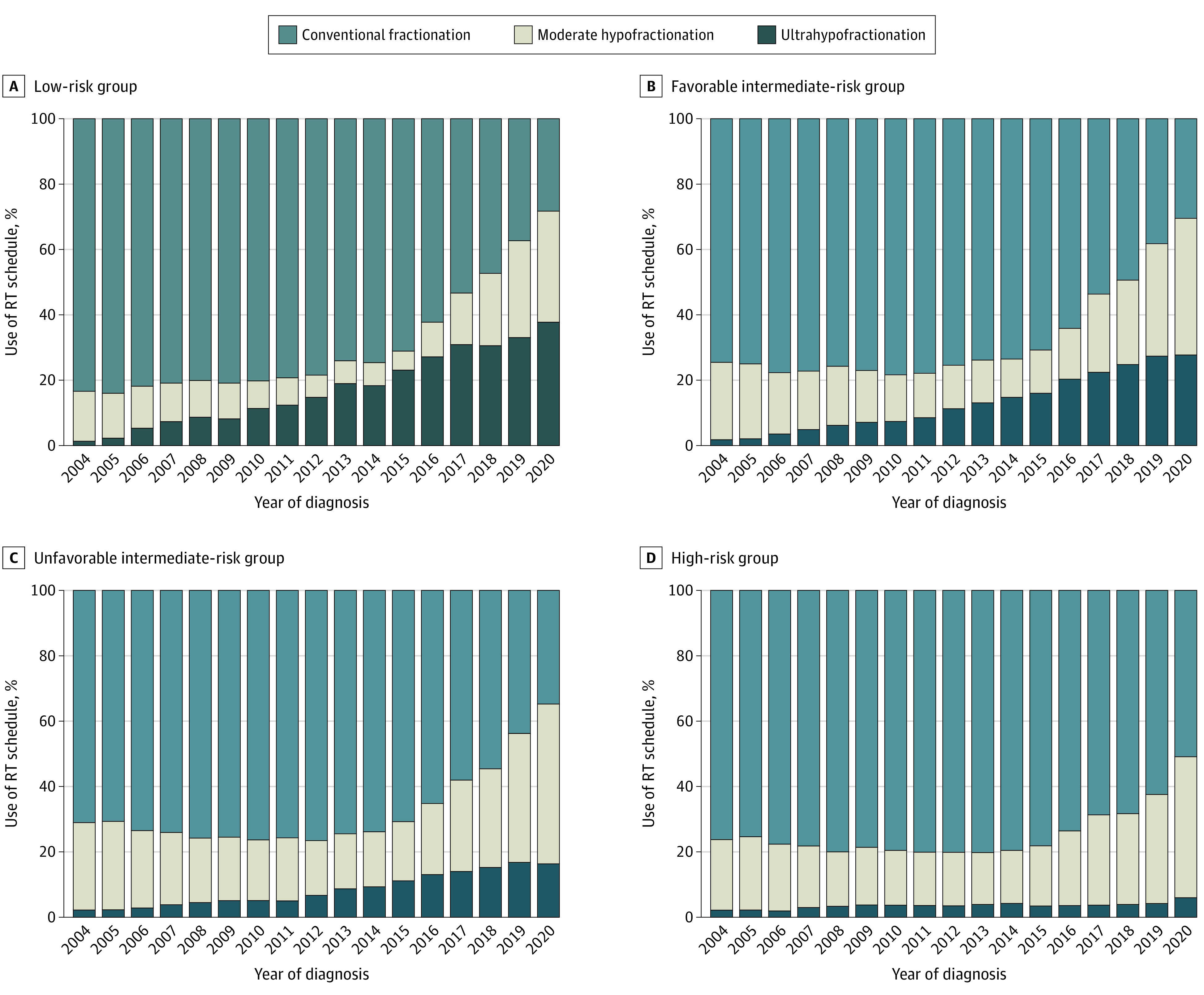
Multivariable logistic regression evaluated the associations of shorter RT courses and showed increased odds with the following variables: every 5 additional years after year of diagnosis (odds ratio [OR], 1.59; 95% CI, 1.58-1.61; P < .001), Medicare and private insurance or managed care (compared with Medicaid; OR, 1.54 [95% CI, 1.45-1.62]; P < .001), and higher median income of the area of residence (compared with lower median income; OR, 1.28 [95% CI, 1.25-1.31]; P < .001) (Table 2). Variables that were significant for an inverse association with shorter RT use were every 10 years’ increase in age (OR, 0.76; 95% CI, 0.75-0.77; P < .001), Black race (compared with White race; OR, 0.90 [95% CI, 0.87-0.92]; P < .001), and community cancer program (compared with academic or research program; OR, 0.54 [95% CI, 0.52-0.56]; P < .001). Similar patterns were seen when evaluating the associations with use of ultrahypofractionation. On multivariable analysis, treatment at a community cancer program (compared with academic or research program; OR, 0.21 [95% CI, 0.19-0.23]; P < .001), Medicaid insurance (compared with Medicare; OR, 1.58 [95% CI, 1.43-1.74]; P < .001), Black race (compared with White race; OR, 0.78 [95% CI, 0.75-0.81]; P < .001), and higher median income (compared with lower median income; OR, 1.52 [95% CI, 1.47-1.58]; P < .001) were associated with the use of ultrahypofractionation.
Table 2. Multivariable Logistic Regression: Temporal Use Patterns of Shorter-Course Radiotherapy (RT) and Ultrahypofractionated RTa.
| Variable | Shorter-course RT use | Ultrahypofractionated RT useb | ||
|---|---|---|---|---|
| OR (95% CI) | P valuec | OR (95% CI) | P valuec | |
| Year of diagnosis, every 5-y change | 1.59 (1.58-1.61) | <.001 | 2.16 (2.13-2.20) | <.001 |
| Age, every 10-y increase | 0.76 (0.75-0.77) | <.001 | 0.88 (0.86-0.90) | <.001 |
| Population size | ||||
| Metropolitan | 1 [Reference] | NA | 1 [Reference] | NA |
| Rural | 0.94 (0.88-1.01) | .09 | 1.11 (0.97-1.26) | .13 |
| Urban | 1.03 (1.00-1.06) | .03 | 1.13 (1.08-1.19) | <.001 |
| Race | ||||
| American Indian and Alaska Native | 1.23 (1.03-1.48) | .03 | 2.37 (1.83-3.07) | <.001 |
| Asian or Pacific Islander | 1 (0.94-1.06) | .97 | 0.84 (0.76-0.94) | .001 |
| Black | 0.90 (0.87-0.92) | <.001 | 0.78 (0.75-0.81) | <.001 |
| White | 1 [Reference] | NA | 1 [Reference] | NA |
| Other or unrecordedd | 0.98 (0.91-1.04) | .47 | 0.88 (0.79-0.97) | .01 |
| Median income | ||||
| ≥$57 857 | 1.28 (1.25-1.31) | <.001 | 1.52 (1.47-1.58) | <.001 |
| ≤$57 856 | 1 [Reference] | NA | 1 [Reference] | NA |
| Insurance type or status | ||||
| Medicaid | 1 [Reference] | NA | 1 [Reference] | NA |
| Medicare | 1.49 (1.41-1.57) | <.001 | 1.58 (1.43-1.74) | <.001 |
| Not insured | 0.92 (0.83-1.02) | .11 | 1.16 (0.97-1.39) | .12 |
| Other government | 1.00 (0.93-1.07) | .99 | 1.06 (0.93-1.20) | .41 |
| Private insurance or managed care | 1.54 (1.45-1.62) | <.001 | 1.62 (1.46-1.78) | <.001 |
| Unknown | 1.46 (1.34-1.60) | <.001 | 2.35 (2.06-2.70) | <.001 |
| Facility type | ||||
| Academic or research program | 1 [Reference] | NA | 1 [Reference] | NA |
| Community cancer program | 0.54 (0.52-0.56) | <.001 | 0.21 (0.19-0.23) | <.001 |
| Comprehensive community cancer program | 0.63 (0.61-0.64) | <.001 | 0.46 (0.45-0.48) | <.001 |
| Integrated network cancer program | 0.81 (0.79-0.83) | <.001 | 0.60 (0.58-0.63) | <.001 |
| Charlson-Deyo Comorbidity Indexe | ||||
| 0 | 1 [Reference] | NA | 1 [Reference] | NA |
| 1 | 1.00 (0.97-1.03) | .85 | 0.99 (0.94-1.04) | .65 |
| 2 | 0.94 (0.89-0.99) | .03 | 0.78 (0.71-0.86) | <.001 |
| 3 | 1.02 (0.95-1.10) | .62 | 0.61 (0.54-0.70) | <.001 |
| NCCN risk group | ||||
| High | 1 [Reference] | NA | 1 [Reference] | NA |
| Low | 1.06 (1.03-1.09) | <.001 | 5.31 (5.04-5.59) | <.001 |
| Favorable intermediate | 1.39 (1.36-1.43) | <.001 | 3.90 (3.71-4.09) | <.001 |
| Unfavorable intermediate | 1.46 (1.42-1.49) | <.001 | 2.41 (2.30-2.53) | <.001 |
Abbreviations: NA, not applicable; NCCN, National Comprehensive Cancer Network; OR, odds ratio.
Full multivariable model including geographic region is shown in eTable 4 in Supplement 1. Geographic variables were not included in this table because of limited space.
Comparison of ultrahypofractionation to moderate hypofractionation and conventional fractionation.
P < .001 was statistically significant.
Other data not provided in the National Cancer Database.
Charlson-Deyo Comorbidity Index range: 0 for no, 1 for mild, 2 for moderate, and 3 for severe comorbidities.
Discussion
National use of shorter RT courses for prostate cancer increased from 2004 to 2020. By 2019, patients who received conventional fractionation were in the minority and, by 2020, most patients received moderate hypofactionation. The use of ultrahypofractionation increased at a faster pace than the initial uptake of moderate hypofractionation.
Increased adoption of hypofractionation is guideline-concordant and patient centric when it is evidence-based.5 However, adoption of hypofractionation remains low in the US compared with other countries.10 Discussion of financial incentives is relevant as reductions in RT reimbursement continue despite RT objectively providing value to the health system and patients and despite RT representing a small component of annual cancer spending. In contrast, more money is spent annually on just one of many forms of immunotherapy than all radiation oncology services in the US combined. Current reimbursement models incentivize protracted treatment courses that put a greater burden on patients, especially racial and ethnic minority and underserved populations, and that fail to adequately support the increased complexity and work of deploying ultrahypofractionation.
The finding of an association between academic cancer centers and use of shorter courses of RT is consistent with results reported in the literature.11,12 The reasons for this observation are likely multifactorial and vary by center. These reasons include greater access to larger teams and peer training, onsite didactics, journal access, acquisition of capital, and potentially different financial incentives for clinicians.13,14 Other barriers to adoption include the current misalignment of financial reimbursement to support the resources required for ultrahypofractionation.8,13,15 These additional resources include but are not limited to need for optimal image guidance quality, use of 6 degrees of freedom, potential for enhanced immobilization devices, more precise quality assurance technology, time needed for image registrations to define organs at risk (ie, urethra), additional time for quality assurance, and requirement of the presence of physics and physicians for each fraction of treatment. While SBRT functionality is standard on new fully equipped linear accelerators, the mean age of active linear accelerators in the US is estimated to be 14 years, and acquisition of a new machine and construction can exceed $4 million.16
Limitations
This study is limited by its retrospective and observational nature. Additionally, the NCDB contains data on a selected group, does not cover the entire US population, and captures only patients who presented to Commission on Cancer hospital–affiliated centers. This factor may have, if anything, underestimated the disparities in access to shorter courses of RT. Other confounders existed, and selection bias was likely present.
Conclusions
This cohort study found an increase in the adoption of shorter courses of RT for prostate cancer from 2004 to 2020, particularly in the most recent years of analysis. Reduced adoption was associated with multiple social determinants of health. Realignment of value-based models to appropriately increase reimbursement for hypofractionation may reduce barriers to nationwide adoption and may ensure access to this treatment for underserved communities.
eFigure. Selection of Sample
eTable 1. Rates of Fractionation Categories Overall by Year of Diagnosis
eTable 2. Fractionation Categories by Risk Grouping and Year
eTable 3. Descriptive Data by Year of Diagnosis
eTable 4. Full Multivariable Logistic Regression: Temporal Effect on Shorter Course Radiotherapy and Ultra-Hypofractionated Radiotherapy Use
Data Sharing Statement
References
- 1.Dearnaley D, Syndikus I, Mossop H, et al. ; CHHiP Investigators . Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17(8):1047-1060. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tree AC, Ostler P, van der Voet H, et al. ; PACE Trial Investigators . Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022;23(10):1308-1320. doi: 10.1016/S1470-2045(22)00517-4 [DOI] [PubMed] [Google Scholar]
- 3.Fransson P, Nilsson P, Gunnlaugsson A, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol. 2021;22(2):235-245. doi: 10.1016/S1470-2045(20)30581-7 [DOI] [PubMed] [Google Scholar]
- 4.Niazi T, Nabid A, Malagon T, et al. Hypofractionated, dose escalation radiotherapy for high-risk prostate cancer: the safety analysis of the Prostate Cancer Study-5 (PCS-5), a GROUQ led phase III trial. Int J Radiat Oncol Biol Phys. 2023. doi: 10.1016/j.ijrobp.2023.05.014 [DOI] [PubMed] [Google Scholar]
- 5.Schaeffer EM, Srinivas S, Adra N, et al. NCCN Guidelines® insights: prostate cancer, version 1.2023. J Natl Compr Canc Netw. 2022;20(12):1288-1298. [DOI] [PubMed] [Google Scholar]
- 6.As NJV, Tree A, Ostler PJ, et al. PACE-A: an international phase 3 randomised controlled trial (RCT) comparing stereotactic body radiotherapy (SBRT) to surgery for localised prostate cancer (LPCa)—primary endpoint analysis. J Clin Oncol. 2023;41(6_suppl):298-298. doi: 10.1200/JCO.2023.41.6_suppl.298 [DOI] [Google Scholar]
- 7.Hamdy FC, Donovan JL, Lane JA, et al. ; ProtecT Study Group . Fifteen-year outcomes after monitoring, surgery, or radiotherapy for prostate cancer. N Engl J Med. 2023;388(17):1547-1558. doi: 10.1056/NEJMoa2214122 [DOI] [PubMed] [Google Scholar]
- 8.Konski A, Yu JB, Freedman G, Harrison LB, Johnstone PA. Radiation oncology practice: adjusting to a new reimbursement model. J Oncol Pract. 2016;12(5):e576-e583. doi: 10.1200/JOP.2015.007385 [DOI] [PubMed] [Google Scholar]
- 9.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 10.Pryor DI, Martin JM, Millar JL, et al. Evaluation of hypofractionated radiation therapy use and patient-reported outcomes in men with nonmetastatic prostate cancer in Australia and New Zealand. JAMA Netw Open. 2021;4(11):e2129647. doi: 10.1001/jamanetworkopen.2021.29647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang EH, Mougalian SS, Soulos PR, et al. Adoption of hypofractionated whole-breast irradiation for early-stage breast cancer: a National Cancer Data Base analysis. Int J Radiat Oncol Biol Phys. 2014;90(5):993-1000. doi: 10.1016/j.ijrobp.2014.06.038 [DOI] [PubMed] [Google Scholar]
- 12.Grant SR, Smith BD, Colbert LE, et al. National quality measure compliance for palliative bone radiation among patients with metastatic non-small cell lung cancer. J Natl Compr Canc Netw. 2021;1-6. doi: 10.6004/jnccn.2020.7688 [DOI] [PubMed] [Google Scholar]
- 13.Aneja S, Pratiwadi RR, Yu JB. Hypofractionated radiation therapy for prostate cancer: risks and potential benefits in a fiscally conservative health care system. Oncology (Williston Park). 2012;26(6):512-518. [PubMed] [Google Scholar]
- 14.Mahal BA, Chen YW, Sethi RV, et al. Travel distance and stereotactic body radiotherapy for localized prostate cancer. Cancer. 2018;124(6):1141-1149. doi: 10.1002/cncr.31190 [DOI] [PubMed] [Google Scholar]
- 15.Johnstone PAS, Peneguy S, Showalter TN, Yu JB. The case for radiotherapy in a value based environment. Rep Pract Oncol Radiother. 2019;24(2):200-203. doi: 10.1016/j.rpor.2019.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feder J. Medical equipment continues to age in the United States. June 28, 2022. Accessed August 31, 2023. https://www.oncologysystems.com/blog/medical-equipment-continues-to-age-in-the-united-states#:~:text=Linear%20accelerators%20took%20quite%20a,developed%20to%20displace%20existing%20products
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Selection of Sample
eTable 1. Rates of Fractionation Categories Overall by Year of Diagnosis
eTable 2. Fractionation Categories by Risk Grouping and Year
eTable 3. Descriptive Data by Year of Diagnosis
eTable 4. Full Multivariable Logistic Regression: Temporal Effect on Shorter Course Radiotherapy and Ultra-Hypofractionated Radiotherapy Use
Data Sharing Statement


