Abstract
BACKGROUND
The function of the thymus in human adults is unclear, and routine removal of the thymus is performed in a variety of surgical procedures. We hypothesized that the adult thymus is needed to sustain immune competence and overall health.
METHODS
We evaluated the risk of death, cancer, and autoimmune disease among adult patients who had undergone thymectomy as compared with demographically matched controls who had undergone similar cardiothoracic surgery without thymectomy. T-cell production and plasma cytokine levels were also compared in a subgroup of patients.
RESULTS
After exclusions, 1420 patients who had undergone thymectomy and 6021 controls were included in the study; 1146 of the patients who had undergone thymectomy had a matched control and were included in the primary cohort. At 5 years after surgery, all-cause mortality was higher in the thymectomy group than in the control group (8.1% vs. 2.8%; relative risk, 2.9; 95% confidence interval [CI], 1.7 to 4.8), as was the risk of cancer (7.4% vs. 3.7%; relative risk, 2.0; 95% CI, 1.3 to 3.2). Although the risk of autoimmune disease did not differ substantially between the groups in the overall primary cohort (relative risk, 1.1; 95% CI, 0.8 to 1.4), a difference was found when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis (12.3% vs. 7.9%; relative risk, 1.5; 95% CI, 1.02 to 2.2). In an analysis involving all patients with more than 5 years of follow-up (with or without a matched control), all-cause mortality was higher in the thymectomy group than in the general U.S. population (9.0% vs. 5.2%), as was mortality due to cancer (2.3% vs. 1.5%). In the subgroup of patients in whom T-cell production and plasma cytokine levels were measured (22 in the thymectomy group and 19 in the control group; mean follow-up, 14.2 postoperative years), those who had undergone thymectomy had less new production of CD4+ and CD8+ lymphocytes than controls (mean CD4+ signal joint T-cell receptor excision circle [sjTREC] count, 1451 vs. 526 per microgram of DNA [P = 0.009]; mean CD8+ sjTREC count, 1466 vs. 447 per microgram of DNA [P<0.001]) and higher levels of proinflammatory cytokines in the blood.
CONCLUSIONS
In this study, all-cause mortality and the risk of cancer were higher among patients who had undergone thymectomy than among controls. Thymectomy also appeared be associated with an increased risk of autoimmune disease when patients with preoperative infection, cancer, or autoimmune disease were excluded from the analysis. (Funded by the Tracey and Craig A. Huff Harvard Stem Cell Institute Research Support Fund and others.)
the thymus is critical for normal development of the immune system. Infants who undergo thymectomy have reduced T-cell counts that do not recover to normal levels, even after several years of follow-up,1–3 and have impaired immune responses to childhood vaccines.4,5 Children with congenital heart defects who undergo thymectomy during surgery have a reduction in naive T-cell counts that persists into young adulthood.6,7
Whether the thymus is critical for adult health is less clear, particularly since the thymus naturally involutes with age. Thymus atrophy begins in infancy and is markedly accelerated in puberty, and it results in an exponential decline in new T-cell generation.8,9 With declining central T-cell production, the body maintains the number of T cells through peripheral clonal proliferation of T-cell populations10 that have an increasingly limited T-cell receptor (TCR) repertoire11 as a result of long-term stimulation of only a fraction of TCRs.12 Age-related contraction of the TCR repertoire may hinder immune surveillance, increasing the risk of infection and cancer,13 and may be a risk factor in the development of autoimmune disease.14
Previous studies have shown that, although the thymus continues to produce T cells into adulthood, thymic activity decreases gradually with age.15,16 How important, then, is the adult thymus? We addressed this question by evaluating health outcomes among adults who had undergone thymectomy. Thymectomy is performed by means of a median sternotomy, video- or robot-assisted thoracoscopic surgery, or transcervical dissection. Therefore, patients who had undergone cardiothoracic surgery without thymectomy were chosen to control for the effect of the surgical procedure. Through epidemiologic, clinical, and immunologic analyses, we investigated the influence of thymectomy on mortality and on the risk of infection, cancer, and autoimmune disease.
METHODS
STUDIES IN HUMANS
Using the Mass General Brigham (MGB) Research Patient Data Registry, we identified all adult patients who had undergone a thymectomy procedure at Massachusetts General Hospital (MGH) between January 1, 1993, and March 1, 2020. Patients who died within 90 days after the procedure or who had nonlaparoscopic cardiac surgery within 5 years after the procedure were excluded.
Using the same database, we identified all adult patients at MGH who had undergone nonlaparoscopic cardiac surgery between January 1, 2000, and December 31, 2019, and had no history of thymectomy for inclusion in the control group. Patients who died within 90 days after the procedure, who had preoperative heart failure, or who had a second cardiac surgery within 5 years after the procedure were excluded (because preservation of the thymus is unlikely in repeat thoracic operations). For all patients, data on demographic characteristics, diagnostic history, and mortality status were obtained from the Research Patient Data Registry; the data are valid up to March 1, 2022.
The incidence of infection, cancer, and autoimmune disease (a list is provided in the statistical analysis plan [see the Supplementary Appendix, available with the full text of this article at NEJM.org]) was inferred with the use of codes from the International Classification of Diseases (ICD), 9th and 10th revisions. Postoperative infections, cancers, and autoimmune disease were defined as those that appeared solely after surgery, with no similar diagnosis occurring before the procedure. To reduce bias from delayed reporting, any infection, cancer, or autoimmune disease that occurred within 90 days after surgery was assumed to be preoperative. Thymomas were assumed to be malignant unless explicitly stated as benign in the ICD code. Given the high prevalence of benign thymomas, key results were also generated after exclusion of all patients with preoperative thymoma, whether malignant or benign.
For survival analysis, patients who had undergone thymectomy were matched to controls with respect to sex, race, age (<5-year gap), and the occurrence of preoperative infections, cancers, or autoimmune disease (separately for the three categories), without replacement of matched controls. If multiple matches were available, the patient closest in age was chosen. Greater than 1-to-1 matching was not performed in order to limit the exclusion of patients who had undergone thymectomy but did not have multiple matches. Survival analysis was performed in the matched groups with the use of Kaplan–Meier curves, with the last date of data collection (March 1, 2022) used as a censor. Mortality data are nationally linked and accurately maintained, but diagnosis data may not be for patients who have moved out of state. Given this limitation, we replicated key results with data censored on the basis of each patient’s last MGB system interaction (most recent laboratory test, procedure, hospital visit, or prescription). The significance of between-group differences in incidence was calculated with the use of a log-rank test.
To analyze the characteristics of cancers and autoimmune disease, chart review was performed for 75 patients who had undergone thymectomy and 75 control patients with postoperative cancer, as well as for 75 patients in each group who had a postoperative autoimmune disease (chart-review cohorts). A sample size of 75 patients per group was calculated to power the detection of an effect size of 0.2 diagnoses, which was reasoned to be clinically meaningful. Patients were randomly selected and their charts assessed to quantify relevant features of postoperative diagnoses (e.g., type, severity, and recurrence). We sought to investigate differences in demographic profiles between patients who had undergone thymectomy and controls who subsequently had a postoperative infection, cancer, or autoimmune disease, as well as differences in the nature of these diagnoses. Consequently, these results did not involve matching according to demographic characteristics.
To contrast mortality with that in the general population, mortality in the thymectomy group and mortality in the control group were compared with values reported for the general population by the Centers for Disease Control and Prevention (CDC).17 This analysis included data from all patients with more than 5 years of follow-up in our study, regardless of whether they had a matched control with more than 5 years of follow-up data. Age- and year-stratified mortality data from the CDC were pooled and weighted to match the distribution of surgical year and age at surgery in the thymectomy group. To account for differences between mortality in Massachusetts and the United States as a whole, the values from the CDC were reduced by 17% — that is, the difference between Massachusetts and the overall United States,18 weighted proportionally to the distribution of ages at thymectomy and years in which surgery was performed. Mortality was converted from 1 year to 5 years by assuming independence, with 5-year mortality calculated as [1 – (1 – 1-year mortality)5].
All the patients in the thymectomy and control groups were considered for follow-up study. A total of 96 health care providers were notified about their patients’ eligibility, since many patients had changed providers from MGB. Study staff obtained consent for phlebotomy from patients who gave permission to their health care providers. All consent and phlebotomy procedures were conducted in accordance with national and institutional guidelines and with the approval of the institutional review board of MGH.
ASSESSING THE SEVERITY OF CANCER
Across the chart-review cohorts, we evaluated pathology reports of breast cancer in patients in each group, and Bloom–Richardson scores were averaged and compared between the thymectomy group and the control group. Because the cancers that developed in the two groups — and therefore their American Joint Committee on Cancer (AJCC) tumor–node–metastasis (TNM) staging criteria — differed, TNM staging criteria were normalized by classifying cancers as either locoregional or widespread disease on the basis of National Comprehensive Cancer Network (NCCN) guidelines. NCCN guidelines were reviewed for each gastrointestinal, genitourinary, and hematologic cancer that was found in order to stratify them according to whether it would be treated with a conservative course or an intensive regimen involving multimodal treatment strategies.
PROCESSING OF SAMPLES FROM PATIENTS
A 20-ml sample of blood was obtained by means of peripheral blood phlebotomy at MGB facilities. Blood was separated with Ficoll and centrifugation at 500×g for 30 minutes at room temperature. The mononuclear layer was collected and resuspended in 90% fetal bovine serum (FBS, Gibco) and 10% dimethyl sulfoxide (Sigma Aldrich) for cryopreservation in liquid nitrogen. For analysis, the peripheral-blood mononuclear cells were thawed and allowed to rest in culture for 24 hours in RPMI-1640 supplemented with 10% FBS, 10-mmol-per-liter HEPES (Gibco), 1% penicillin–streptomycin (Gibco), and 1% nonessential amino acids (Gibco). After 24 hours, separation of CD4+ and CD8+ lymphocytes was performed with the use of magnetic beads (STEMCELL Technologies), and purity was determined to be above 90%.
CYTOKINE AND SJTREC ANALYSIS
For cytokine analysis, blood plasma was isolated at the time of Ficoll separation and cryopreserved before cytokine profiling with the use of the Human Cytokine/Chemokine 71-Plex Discovery Assay Array (HD71) (Eve Technologies). For the analysis of signal joint T-cell receptor excision circles (sjTRECs), genomic DNA was isolated from at least 200,000 magnetically separated CD4+ and CD8+ lymphocytes per patient with the DNeasy Blood and Tissue kit (Qiagen). The number of sjTRECs was subsequently determined with a singleplex MyTREC real-time quantitative polymerase-chain-reaction kit (GenenPlus).
TCR SEQUENCING
Genomic DNA was isolated from at least 100,000 CD4+ and CD8+ T cells (in most cases, genomic DNA was submitted from more than 200,000 cells; DNeasy Blood and Tissue Kit, Qiagen) and sent to Adaptive Biotechnologies for bulk T-cell receptor sequencing of the VJ (variable and joining) or VDJ (variable, diversity, and joining) regions of TRB, the gene encoding TCRβ, with the use of the ImmunoSEQ Human TCRB sequencing kit (Adaptive Biotechnologies). Productive rearrangements were then measured with the ImmunoSEQ Analyzer platform for analysis. Clonality of CD4+ and CD8+ T cells was assessed with a downsampled productive Simpson’s clonality index, and richness was measured with the Simpson’s evenness index.
STATISTICAL ANALYSIS
Statistical analysis was performed with RStudio software, version 1.2.5042 (Posit), and MATLAB software, version 2021b (MathWorks). Sample sizes were chosen on the basis of previous experiments, and no statistical methods were used to predetermine sample size. The significance of distributions was calculated with an unpaired two-tailed Student’s t-test (normal distributions) or Wilcoxon rank-sum test (nonnormal distributions). Unless otherwise specified, all data are expressed as effect sizes and 95% confidence intervals, and a P value of less than 0.05 was considered to indicate statistical significance. Epidemiologic and clinical findings were not adjusted for multiple comparisons, and therefore inferences drawn from confidence intervals may not be reproducible.
RESULTS
IDENTIFICATION OF PATIENTS WHO HAD UNDERGONE THYMECTOMY
Our search of the registry identified 1470 adult patients who had undergone thymectomy and 16,679 patients who had undergone cardiothoracic surgery without thymectomy; after exclusions, the numbers that remained in the study were 1420 and 6021, respectively. Of the 1420 patients in the thymectomy group, 1146 (81%) had at least one valid match to a control, and therefore the primary cohort consisted of 1146 patients who had undergone thymectomy and 1146 age-, race-, and sex-matched controls. No patients with 22q11.2 deletion syndrome (DiGeorge’s syndrome) were found among the patients in the thymectomy group. The primary indications for surgery (obtained through manual chart review) are summarized in Table S1 in the Supplementary Appendix. Half the patients in the cohort had had their thymus removed without a malignant or other definitive thymus condition. Demographic characteristics of the patients are shown in Table S2.
RISK OF DEATH
To determine the effect of thymectomy on life expectancy, all-cause mortality at 5 years after surgery was assessed in the thymectomy group and the control group. Patients who had undergone thymectomy were more than twice as likely as controls to die within 5 years (8.1% vs. 2.8%; relative risk, 2.9; 95% confidence interval [CI], 1.7 to 4.8; P<0.001) (Fig. 1A). This effect was preserved after the exclusion of patients with preoperative myasthenia gravis, patients with preoperative thymoma, or patients with preoperative cancer, as well as after the exclusion of all patients with any preoperative infection, cancer, or autoimmune disease taken together19–24 (Fig. 1A). Kaplan–Meier analysis over a period of 20 years after surgery also showed significantly higher mortality among patients who had undergone thymectomy (hazard ratio, 1.5; 95% CI, 1.3 to 1.7; P = 0.001) (Fig. 1B). This signal was consistent after the exclusion of patients with preoperative myasthenia gravis (Fig. S1A), thymoma (Fig. S1B), or cancer (Fig. S1C); after the exclusion of all patients with a preoperative history of infection, cancer, or autoimmune disease (Fig. 1C); when controlling for the incidence of postoperative infection, cancer, and autoimmune disease (Fig. S2); and when only patients who had undergone a total thymectomy were included (Fig. S5; a superset analysis excluding all patients with preoperative infections, cancers, or autoimmune disease or with benign or malignant thymoma is shown in Fig. S6).
Figure 1. Effect of Thymectomy on Long-Term Mortality.
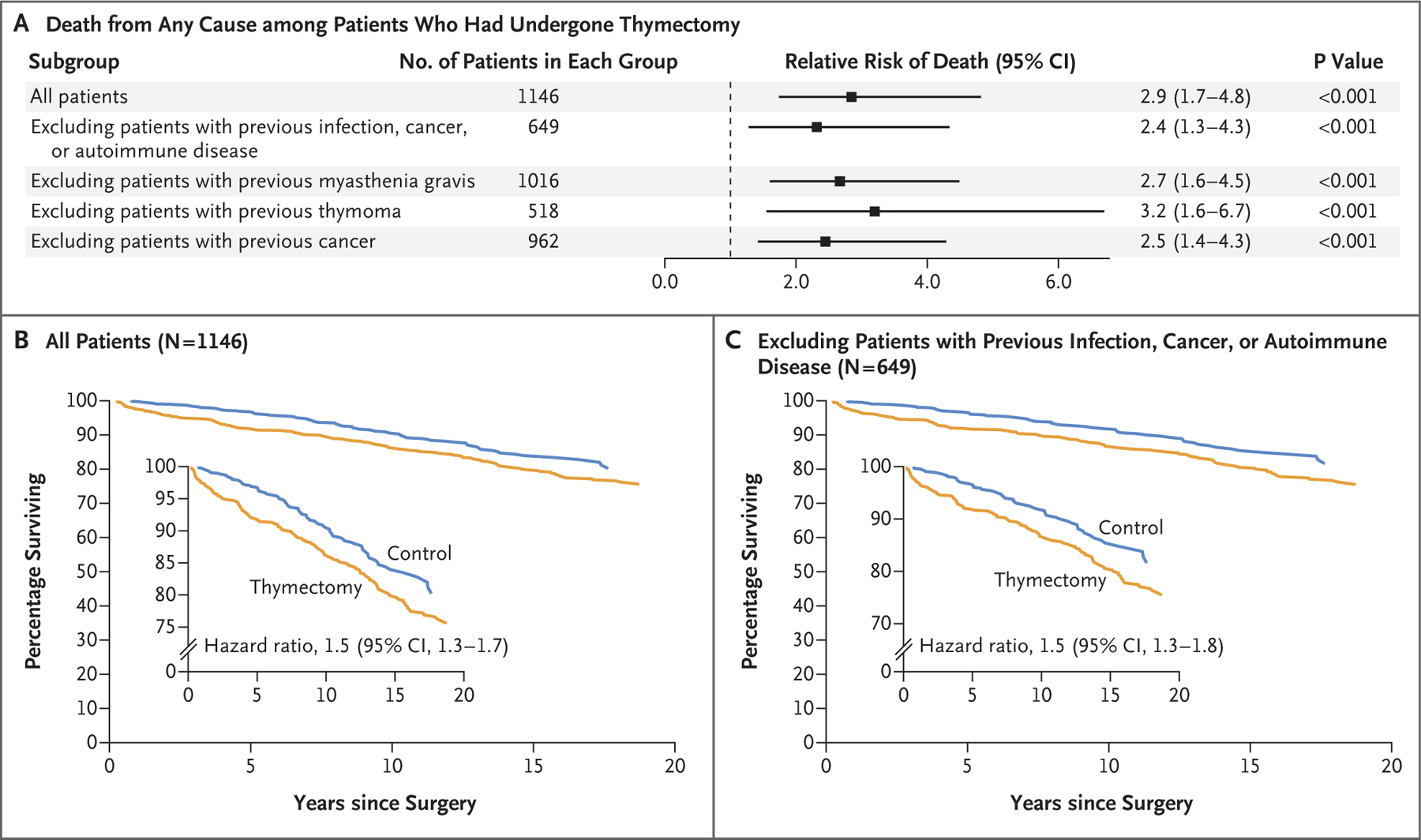
Panel A shows the relative risk of death from any cause among patients who had undergone thymectomy as compared with age-, race-, and sex-matched controls in the first 5 years after surgery, both in the overall study population and in subgroups. The relative risk of death from any cause within 5 years was increased in patients who had undergone thymectomy, regardless of whether they had a preoperative history of infection, cancer, or autoimmune disease. Panels B and C show the percentages of patients who survived over a period of 20 years after surgery in the group with no exclusions (Panel B) and in the subgroup in which patients with a preoperative history of infection, cancer, or autoimmune disease were excluded from the analysis. The insets show the same data on an expanded y axis.
We also performed an analysis to compare mortality over the 5 years after surgery (reflecting data from all patients with >5 years of follow-up) with mortality reported for the United States by the CDC,17 after adjustment for age, year, and U.S. state.18 All-cause mortality at 5 years in this analysis was higher among patients who had undergone thymectomy than in the general population for each age group and in aggregate (9.0% vs. 5.2%; relative risk, 1.7; 95% CI, 1.4 to 2.1) (Fig. S3).
RISK OF CANCER
To determine the effect of thymectomy on the risk of cancer, we assessed the relative risk of cancer at 5 years after surgery in the control group as compared with the thymectomy group. Patients who had undergone thymectomy were more likely than controls to have cancer within 5 years after surgery (7.4% vs. 3.7%; relative risk, 2.0; 95% CI, 1.3 to 3.2); the results were robust to the exclusion of patients with preoperative myasthenia gravis, patients with preoperative thymoma, or patients with preoperative cancer, as well as to the exclusion of all patients with preoperative infection, cancer, or autoimmune disease (Fig. 2A).
Figure 2. Effect of Thymectomy on the Long-Term Risk of Cancer.
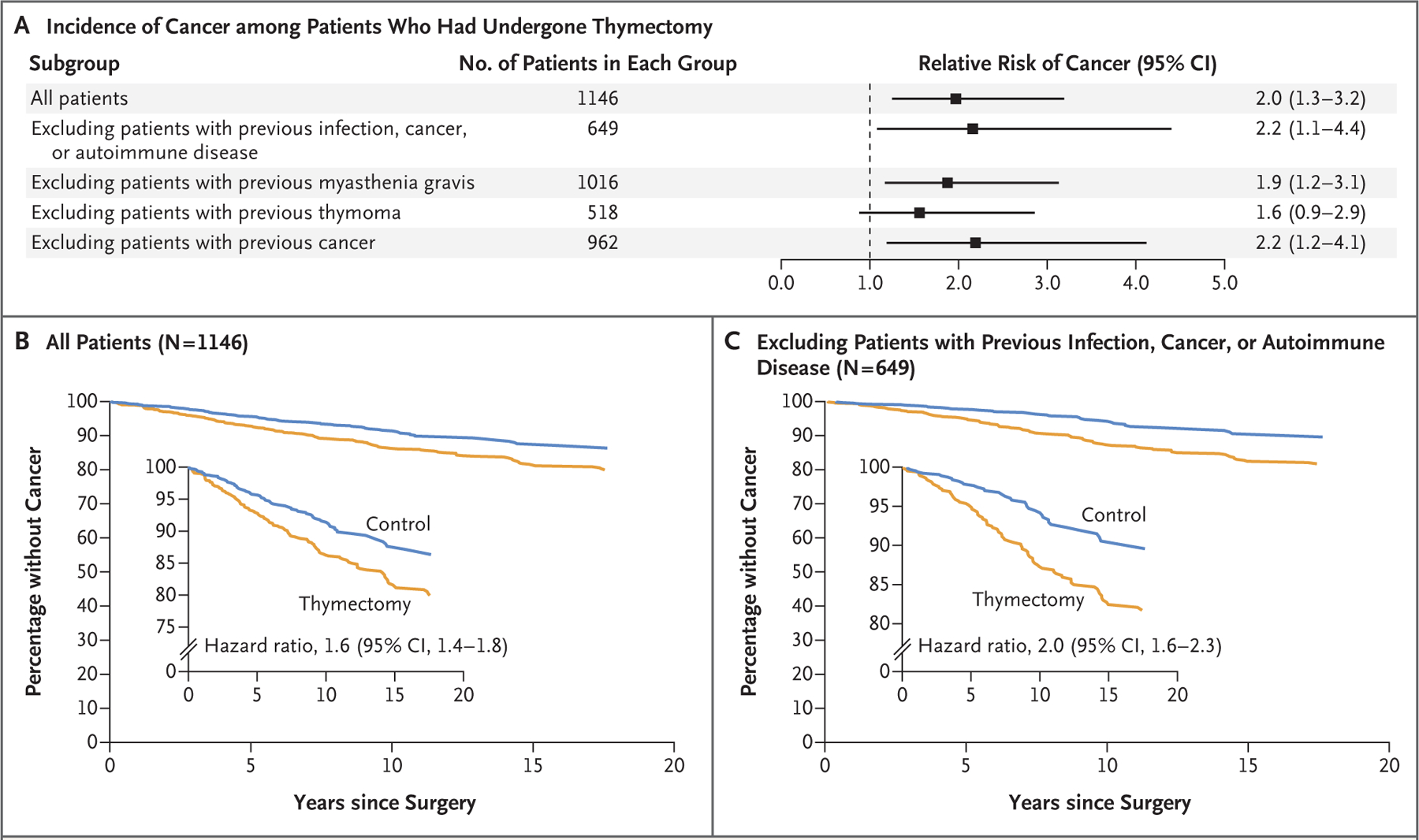
Panel A shows the relative risk of cancer among patients who had undergone thymectomy as compared with age-, race-, and sex-matched controls in the first 5 years after surgery, both in the overall study population and in subgroups. The relative risk of cancer within 5 years was increased in patients who had undergone thymectomy, regardless of whether they had a preoperative history of infection, cancer, or autoimmune disease. Panels B and C show the percentages of patients who survived without cancer over a period of 20 years after surgery in the group with no exclusions (Panel B) and in the subgroup in which patients with a preoperative history of infection, cancer, or autoimmune disease were excluded from the analysis. The insets show the same data on an expanded y axis.
In an analysis comparing our data (from all patients with >5 years of follow-up) with data from the National Cancer Institute, the 5-year incidence of cancer among controls (3.9%) was similar to that among similarly aged members of the general U.S. population (0.8% per year for persons 55 to 59 years of age, or 3.9% cumulatively over 5 years25), whereas the incidence in the thymectomy group was higher than that in the general population (7.0%; relative risk, 1.8; 95% CI, 1.2 to 2.7).
In a Kaplan–Meier analysis over the 20 years after surgery, the risk of cancer among patients who had undergone thymectomy was also higher than that among controls (hazard ratio, 1.6; 95% CI, 1.4 to 1.8) (Fig. 2B), even after the exclusion of patients with preoperative myasthenia gravis (Fig. S1D), thymoma (Fig. S1E), or cancer (Fig. S1F) and after the exclusion of all patients with preoperative infection, cancer, or autoimmune disease (Fig. 2C), as well as when the analysis was limited to patients who had undergone total thymectomy (Fig. S5; a superset analysis excluding patients with any infection, cancer, or autoimmune disease or with benign or malignant thymoma is shown in Fig. S6). In an analysis of data censored on the basis of each patient’s last MGB system interaction (most recent laboratory test, procedure, hospital visit, or prescription), the findings were similar to those in the main analysis (Fig. S7).
CHARACTERISTICS OF POST-THYMECTOMY CANCER
Detailed medical record reviews were performed for 75 patients who had undergone thymectomy and 75 controls randomly chosen from the subgroup of patients with postoperative cancer (Table S6). The patients who had undergone thymectomy had more cancers per patient (1.7 vs. 1.2; difference, 0.43; 95% CI, 0.21 to 0.65) (Fig. 3A), regardless of preoperative cancer history (number of postoperative cancers per patient among those with a preoperative history of cancer, 1.9 vs. 1.2; difference, 0.68; 95% CI, 0.34 to 1.02) (Fig. 3B). Cancers among patients who had undergone thymectomy were more diverse than those among controls. Skin cancers — the most frequent cancers in the United States26,27 and those most commonly associated with immune suppression28,29 — were unexpectedly less frequent in the thymectomy group than in the control group (46.5% vs. 64.9% of cancers; relative risk, 1.40; 95% CI, 1.1 to 1.8) (Fig. 3C). Nonskin cancers were found in almost every major organ system in the body, with some less common cancers (kidney, thyroid, parotid, and embryonal) occurring in the thymectomy group. Controls with postoperative cancers were also older at the time of surgery than corresponding patients who had undergone thymectomy (70 years vs. 56 years of age) (Table S6), which suggests that cancers in patients who had undergone thymectomy may have been less age-driven than those in controls.
Figure 3. Characteristics of Post-Thymectomy Cancer (Chart-Review Cohort).
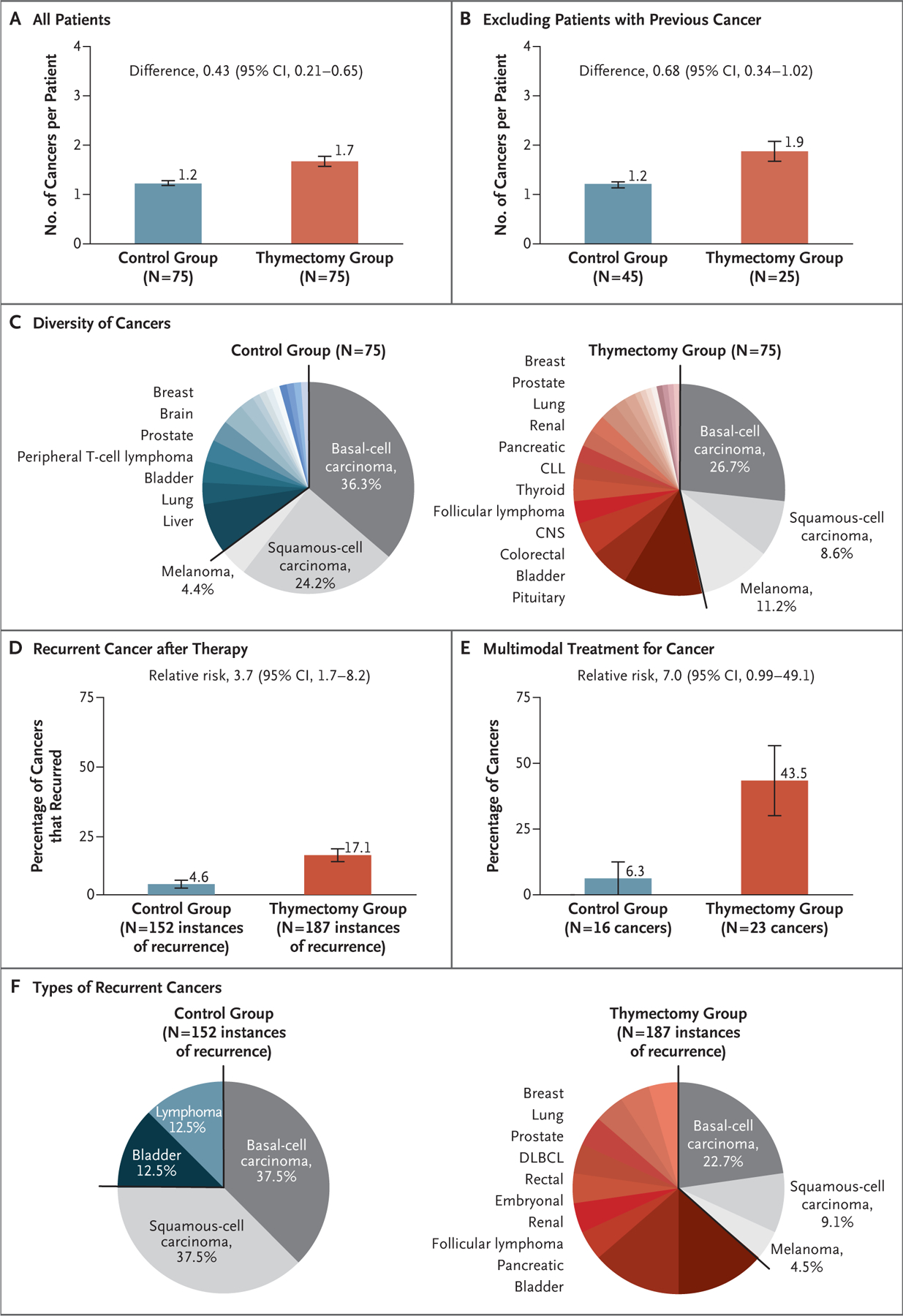
Among the patients with at least one cancer after surgery, the number of cancers per patient was larger, on average, among those who had undergone thymectomy than among controls, regardless of the preoperative history of cancer. Detailed medical record reviews were performed for 75 patients who had undergone thymectomy and 75 controls randomly chosen from the subgroup who had postoperative cancer (chart-review cohort). Panel A shows data for all patients in the cohort, and Panel B shows data for the cohort minus patients with a preoperative history of cancer. Panel C shows the types of cancer that developed after surgery; 64.9% of the cancers in the control group were skin cancers, whereas only 46.5% of the cancers in the thymectomy group were skin cancers. The remainder of the cancers in the thymectomy group represented most major tissue types in the body; those found in more than 1% of the patients in the group are listed next to the pie chart. The total numbers of types of postoperative cancer in the chart-review cohort were 116 in the thymectomy group and 91 in the control group. CLL denotes chronic lymphocytic leukemia, and CNS central nervous system. Panel D shows cancer recurrence after therapy, which was more common in the thymectomy group than in the control group. Panel E shows the percentages of postoperative cancers that would be treated with an intensive regimen involving multimodal therapy (i.e., surgery, chemotherapy, radiation, and monoclonal antibodies) according to National Comprehensive Cancer Network guidelines. Panel F shows the types of cancers that recurred in each group; nonskin cancers that recurred in the thymectomy group are listed next to the pie chart. DLBCL denotes diffuse large B-cell lymphoma.
Breast cancers among patients who had undergone thymectomy had hormonal status and mutation rates (Tables S7 and S8) similar to those among controls but had a higher mean histologic grade30 (Fig. S8A). Gastrointestinal, genitourinary, and hematologic cancers in patients who had undergone thymectomy were more frequently widespread in the body than those in controls (percentage of cancers with distant tissue or nodal metastasis, 52.2% vs. 18.8%; relative risk, 2.8; 95% CI, 0.9 to 8.3) (Fig. S8B). After treatment, cancer recurrence was more common among patients who had undergone thymectomy than among controls (percentage of cancers that recurred, 17.1% vs. 4.6%; relative risk, 3.7; 95% CI, 1.7 to 8.2) (Fig. 3D), and postoperative cancers in the thymectomy group were more likely to receive multimodal treatment than those in the control group (43.5% vs. 6.3% of cancers; relative risk, 7.0; 95% CI, 0.99 to 49.1) (Fig. 3E). Details of the cancers that recurred are shown in Figure 3F. Mortality from cancer was higher in the thymectomy group than in the control group (2.3% vs. 1.0%; relative risk, 2.3; 95% CI, 1.02 to 5.1) and higher in the thymectomy group than in an age-, year-, and state-adjusted general U.S. population (2.3% vs. 1.5%) (analysis based on 951 patients in the thymectomy group and 786 in the control group). Thymectomy was associated with more frequent, varied, and aggressive cancers that had a higher incidence of recurrence and resulted in increased mortality from cancer.
RISK OF AUTOIMMUNE DISEASE
T-cell–mediated immunity is critical for health, so we assessed whether thymectomy is associated with uncontrolled inflammation or autoimmunity. Although the risk of autoimmune disease was not higher in the thymectomy group than in the control group as a whole 5 years after surgery, an analysis in which patients with preoperative infection, cancer, or autoimmune disease were excluded revealed a higher risk of postoperative autoimmune disease in the thymectomy group (12.3% vs. 7.9%; relative risk, 1.5; 95% CI, 1.02 to 2.2) (Fig. 4). Because this difference dissipated when risk was evaluated over a 20-year period, thymectomy appeared to have transiently and modestly increased the risk of autoimmune disease. An analysis of data censored on the basis of each patient’s last MGB system interaction showed similar results.
Figure 4. Effect of Thymectomy on the Risk of Autoimmune Disease.
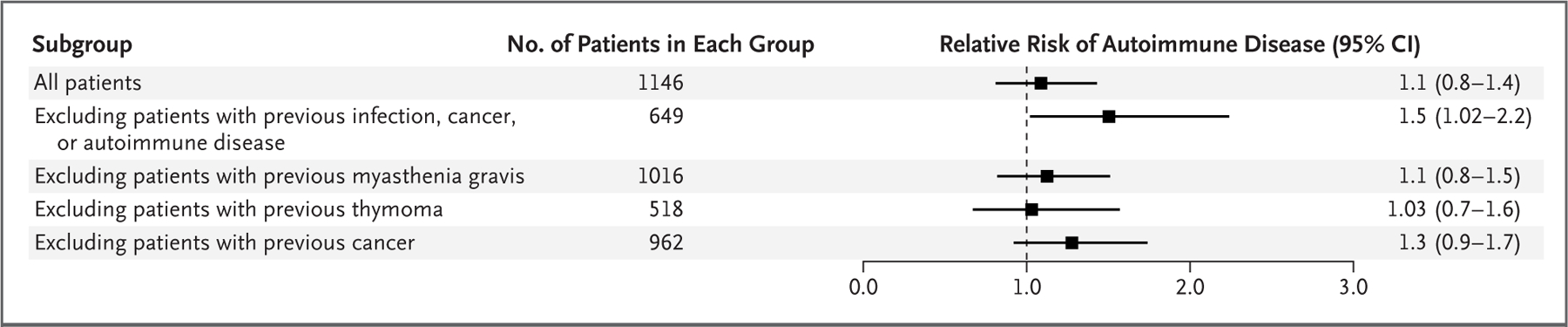
The risk of autoimmune disease in the thymectomy group was compared with that among age-, race-, and sex-matched controls in the first 5 years after surgery. The relative risk of autoimmune disease was increased among patients who had undergone thymectomy who did not have a history of preoperative infections, cancers, or autoimmune disease.
Similar to the way in which we analyzed cancers, we analyzed the medical records of 75 patients in the thymectomy group and 75 controls who were randomly chosen from the cohort with a postoperative autoimmune disease (Table S9). As in the analysis of cancer, controls with a postoperative autoimmune disease were older at the time of surgery (65 years vs. 50 years), which suggests that autoimmune disease among the patients who had undergone thymectomy may have been less age driven than that among controls. Among the patients with an autoimmune disease, those who had undergone thymectomy had more autoimmune diseases per patient than controls (1.7 vs. 1.1; difference, 0.60; 95% CI, 0.31 to 0.89) (Fig. S9A). When patients with a preoperative history of autoimmune disease (e.g., myasthenia gravis) were excluded, the number of postoperative autoimmune diseases per patient was still higher in the thymectomy group than in the control group (1.7 vs. 1.2; difference, 0.54; 95% CI, 0.24 to 0.83) (Fig. S9B). The types of postoperative autoimmune disease found in the patients are shown in Figure S9C.
ADULT T-CELL PRODUCTION
Study staff obtained consent for the collection of the plasma and peripheral-blood T cells from 22 patients who had undergone thymectomy and 20 controls in total; the blood sample from 1 control was of insufficient quality to use for study, which left 19 controls in these analyses. The patients in the thymectomy group did not differ from those in the control group with respect to age, race, or sex.
New T-cell production can be measured by quantifying sjTRECs, the by-products of VDJ recombination in each newly developing T cell in the thymus. In our cohort (demographic characteristics are shown in Tables S10 and S11), the mean sjTREC counts in CD4+ and CD8+ lymphocytes were higher among controls than among patients who had undergone thymectomy (CD4+: 1451 vs. 526 per microgram of DNA [difference, 925; 95% CI, 82 to 1768; P = 0.009]; CD8+: 1466 vs. 447 per microgram of DNA [difference, 1019; 95% CI, 330 to 1707; P<0.001]) (Fig. 5A and 5B). This result indicates that the thymus continued to contribute to new T-cell production in adulthood and that premature cessation of thymopoiesis might have affected T-cell–mediated immunity.
Figure 5. T-Cell Production and Inflammatory Responses.
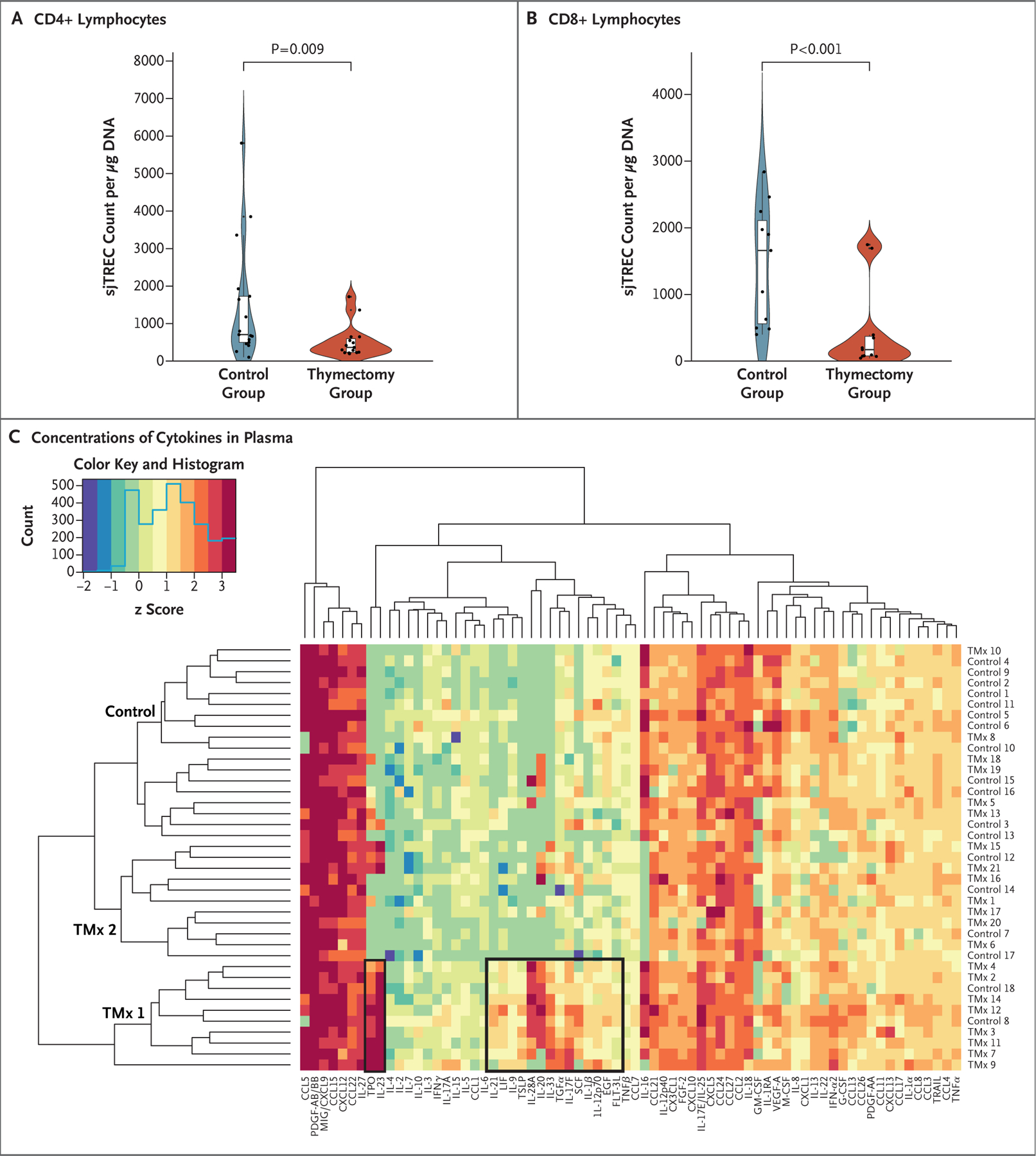
Panels A and B show an analysis of signal joint T-cell receptor excision circles (sjTRECs) in CD4+ (Panel A) and CD8+ (Panel B) lymphocytes, with results expressed as the sjTREC count per microgram of DNA. CD4+ and CD8+ lymphocytes isolated from patients in the control group had larger mean sjTREC counts than those isolated from patients in the thymectomy group (CD4+: 1451 vs. 526 per microgram of DNA; difference, 925 [95% CI, 82 to 1768]; P = 0.009; and CD8+: 1466 vs. 447 per microgram of DNA; difference, 1019 [95% CI, 330 to 1707]). The CD4+ experiment included 15 patients in the thymectomy group and 17 in the control group; the CD8+ experiment included 11 patients in each group. In the violin plots, dots indicate individual samples and the width of the shaded area indicates the probability density. In each box-and-whisker plot within a violin plot, the horizontal line indicates the median, the top and bottom of the box indicate the interquartile range, and the whiskers indicate 1.5 times the interquartile range. Panel C shows a heat map of cytokines measured in blood plasma obtained from 21 patients who had undergone thymectomy (labeled TMx) and 19 controls. A panel of 71 cytokines was used, 15 of which differed significantly between the groups (P<0.05 with Bonferroni correction). One control (of 19) was excluded from this analysis because anomalous, out-of-range readings were obtained with repeated testing. Bonferroni correction was used for all statistical testing. The figure is based on log10-transformed cytokine concentrations in order to adjust for differences in scales of expression. Unsupervised clustering showed that the patients who had undergone thymectomy had similar cytokine-expression profiles; these patients dominated two clusters, labeled TMx 1 and TMx 2, that differed from the control cluster in their strong expression of type 2 and type 17 helper T-cell cytokines and acute-phase reactants (especially in cluster TMx 1; some patients in cluster TMx 2 shared this phenotype). The z score is highlighted in the top left with a histogram showing the frequency of cytokine expression at different z-score values. EGF denotes epidermal growth factor, FGF fibroblast growth factor, FLT-3L FMS-like tyrosine kinase 3 ligand, G-CSF granulocyte colony-stimulating factor, GM-CSF granulocyte–macrophage colony-stimulating factor, IFN interferon, IL interleukin, LIF leukemia inhibitory factor, M-CSF macrophage colony-stimulating factor, MIG monokine induced by interferon-γ, PDGF platelet-derived growth factor, SCF stem-cell factor, TNF tumor necrosis factor, TPO thrombopoietin, TRAIL tumor necrosis factor–related apoptosis-inducing ligand, TSLP thymic stromal lymphopoietin, and VEGF vascular endothelial growth factor.
T-cell clonality (i.e., the degree of sameness of the TCR repertoire) has been implicated in autoimmunity and the risk of cancer.31–33 To investigate the effect of thymectomy on the complexity of the TCR repertoire, CD4+ and CD8+ lymphocytes were purified in blood samples obtained from patients who had undergone thymectomy and from controls and were analyzed by TCR sequencing. Among the patients in the thymectomy group who had postoperative cancer (characteristics of the patients are shown in Tables S12 through S15), TCR clonality was more evident and the richness of the TCR repertoire was decreased in CD4+ and CD8+ lymphocyte populations (Fig. S10). These data suggest that the increased risk of cancer seen in patients who had undergone thymectomy may have been related to decreased TCR complexity.
INFLAMMATORY RESPONSES
Blood plasma cytokine concentrations were assessed by enzyme-linked immunosorbent assay in 21 patients in the thymectomy group and 19 patients in the control group (Fig. 5C). Unsupervised clustering of cytokine profiles grouped the patients into three clusters, two dominated by patients who had undergone thymectomy and one largely made up of controls (Fig. 5C). Thymectomy clusters 1 and 2 had a shared proinflammatory pattern. This pattern included interleukin-33, thymic stromal lymphopoietin, and interleukin-23, as well as acute-phase reactants (thrombopoietin and granulocyte colony-stimulating factor). In addition, both interleukin-10 and interleukin-1Ra, which are known suppressors of inflammation,34,35 were substantially down-regulated in patients who had undergone thymectomy (Table S16). The control group did not show a consistent proinflammatory profile. Taken together, these data suggest that thymectomy is associated with a shared cytokine signature of immune dysregulation.
DISCUSSION
In this study, we found that thymectomy in adulthood was associated with an increased risk of death from any cause and an increased risk of cancer. These observations held true even in separate analyses in which patients with a preoperative history of potentially confounding conditions such as cancer, autoimmune disease, infection, myasthenia gravis, or thymoma were excluded. In addition, in the subgroup of patients without a history of confounding conditions, an association between thymectomy and postoperative autoimmune disease was noted. Patients across age groups who had undergone thymectomy were more likely to die from any cause and more likely to die from cancer than controls and the U.S. general population. Among patients with postoperative cancer, thymectomy was associated with more aggressive, recurrent disease. In addition, thymectomy was associated with autoimmune disease in conjunction with a proinflammatory modification of plasma cytokine levels, including substantially elevated levels of type 2 helper T cell–promoting factors (interleukin-33 and thymic stromal lymphopoietin) and type 17 helper T cell–promoting factors (interleukin-23) that have been experimentally associated with cancer and autoimmune disease.36–39 Thymectomy was associated with reduced production of newly formed T cells, as reflected by persistently depressed sjTREC counts extending to a mean follow-up of 14.2 postoperative years (range, 8 to 26). This finding is consistent with that of another study in which decreased sjTREC counts were found during shorter follow-up (500 days) of patients who had undergone thymectomy in adulthood for myasthenia gravis.40 Finally, patients who had undergone thymectomy and had postoperative cancer were found to have more oligoclonal, less diverse TCR repertoires, which could conceivably contribute to the development of cancer and autoimmune disease.31–33
Together, these findings support a role for the thymus contributing to new T-cell production in adulthood and to the maintenance of adult human health. The disruption of homeostasis caused by thymectomy is sufficient to adversely affect critical health outcomes, which argues strongly that the adult thymus remains functionally important.
This study is retrospective and observational, and therefore the data cannot be used to identify causation. However, they provide evidence of an association between thymectomy and adverse outcomes in patients. These results strongly suggest that when possible, preservation of the thymus should be a clinical priority.
Supplementary Material
Acknowledgments
We thank Jag Singh, M.D., Ph.D., Lauren Maranian, N.P., Laura Aseltine, N.P., and all the providers who gave permission for our study staff to obtain consent from their patients for their participation in this study; John Higgins, M.D., for his support, expertise, and facilitation of access to the Mass General Brigham Research Patient Data Registry; and the Harvard Stem Cell Institute–Center for Regenerative Medicine Flow Cytometry Core Facility at Massachusetts General Hospital for technical assistance.
Supported by the Tracey and Craig A. Huff Harvard Stem Cell Institute Research Support Fund, the Gerald and Darlene Jordan Professorship of Medicine, and a grant (U19AI149676, to Dr. Scadden) from the National Institutes of Health. Dr. Kooshesh received support from the American Society of Hematology. Dr. Gustafsson received support from the Swedish Research Council and the John S. Macdougall Jr. and Olive R. Macdougall Fund.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Kameron A. Kooshesh, Centers for Regenerative Medicine, Massachusetts General Hospital, the Harvard Stem Cell Institute, Department of Stem Cell and Regenerative Biology, Harvard University, Harvard Medical School, Boston
Brody H. Foy, Systems Biology, Harvard Medical School, Boston
David B. Sykes, Centers for Regenerative Medicine, Harvard Medical School, Boston
Karin Gustafsson, Centers for Regenerative Medicine, Massachusetts General Hospital, the Harvard Stem Cell Institute, Department of Stem Cell and Regenerative Biology, Harvard University, Harvard Medical School, Boston
David T. Scadden, Centers for Regenerative Medicine, Massachusetts General Hospital, the Harvard Stem Cell Institute, Department of Stem Cell and Regenerative Biology, Harvard University, Harvard Medical School, Boston
References
- 1.van Gent R, Schadenberg AWL, Otto SA, et al. Long-term restoration of the human T-cell compartment after thymectomy during infancy: a role for thymic regeneration? Blood 2011;118:627–34. [DOI] [PubMed] [Google Scholar]
- 2.Brearley S, Gentle TA, Baynham MI, Roberts KD, Abrams LD, Thompson RA. Immunodeficiency following neonatal thymectomy in man. Clin Exp Immunol 1987; 70:322–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Wells WJ, Parkman R, Smogorzewska E, Barr M. Neonatal thymectomy: does it affect immune function? J Thorac Cardiovasc Surg 1998;115:1041–6. [DOI] [PubMed] [Google Scholar]
- 4.Kurobe H, Tominaga T, Sugano M, et al. Complete but not partial thymectomy in early infancy reduces T-cell-mediated immune response: three-year tracing study after pediatric cardiac surgery. J Thorac Cardiovasc Surg 2013;145(3):656–662, 662.e1–662.e2. [DOI] [PubMed] [Google Scholar]
- 5.Prelog M, Wilk C, Keller M, et al. Diminished response to tick-borne encephalitis vaccination in thymectomized children. Vaccine 2008;26:595–600. [DOI] [PubMed] [Google Scholar]
- 6.Sauce D, Larsen M, Fastenackels S, et al. Evidence of premature immune aging in patients thymectomized during early childhood. J Clin Invest 2009;119:3070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elder RW, George RP, McCabe NM, et al. Immunologic aging in adults with congenital heart disease: does infant sternotomy matter? Pediatr Cardiol 2015;36:1411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murray JM, Kaufmann GR, Hodgkin PD, et al. Naive T cells are maintained by thymic output in early ages but by proliferation without phenotypic change after age twenty. Immunol Cell Biol 2003;81:487–95. [DOI] [PubMed] [Google Scholar]
- 9.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MRM, Boyd R, Sempowski GD. Thymic involution and immune reconstitution. Trends Immunol 2009;30:366–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williams KM, Hakim FT, Gress RE. T cell immune reconstitution following lymphodepletion. Semin Immunol 2007;19: 318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weng N-P. Aging of the immune system: how much can the adaptive immune system adapt? Immunity 2006;24:495–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas R, Wang W, Su D-M. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing 2020;17:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmer S, Albergante L, Blackburn CC, Newman TJ. Thymic involution and rising disease incidence with age. Proc Natl Acad Sci U S A 2018;115:1883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadasz Z, Haj T, Kessel A, Toubi E. Age-related autoimmunity. BMC Med 2013;11: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naylor K, Li G, Vallejo AN, et al. The influence of age on T cell generation and TCR diversity. J Immunol 2005;174:7446–52. [DOI] [PubMed] [Google Scholar]
- 16.Douek DC, McFarland RD, Keiser PH, et al. Changes in thymic function with age and during the treatment of HIV infection. Nature 1998;396:690–5. [DOI] [PubMed] [Google Scholar]
- 17.Xu JQ, Murphy SL, Kochanek KD, Arias E. Deaths: final data for 2019. National vital statistics reports Vol. 70. No. 8. Hyatsville, MD: National Center for Health Statistics, 2021. [Google Scholar]
- 18.National Center for Health Statistics. Death rates by 10-year age groups: United States and each state, 2007 Hyattsville, MD: Centers for Disease Control and Prevention, September 2010. (https://www.cdc.gov/nchs/data/dvs/MortFinal2007_Worktable23r.pdf). [Google Scholar]
- 19.Levin N, Abramsky O, Lossos A, et al. Extrathymic malignancies in patients with myasthenia gravis. J Neurol Sci 2005;237: 39–43. [DOI] [PubMed] [Google Scholar]
- 20.Verwijst J, Westerberg E, Punga AR. Cancer in myasthenia gravis subtypes in relation to immunosuppressive treatment and acetylcholine receptor antibodies: a Swedish nationwide register study. Eur J Neurol 2021;28:1706–15. [DOI] [PubMed] [Google Scholar]
- 21.Pan CC, Chen PC, Wang LS, Chi KH, Chiang H. Thymoma is associated with an increased risk of second malignancy. Cancer 2001;92:2406–11. [DOI] [PubMed] [Google Scholar]
- 22.Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010;5:Suppl 4:S260–S265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamata T, Yoshida S, Wada H, et al. Extrathymic malignancies associated with thymoma: a forty-year experience at a single institution. Interact Cardiovasc Thorac Surg 2017;24:576–81. [DOI] [PubMed] [Google Scholar]
- 24.Evoli A, Punzi C, Marsili F, et al. Extrathymic malignancies in patients with thymoma. Ann Oncol 2004;15:692–3. [DOI] [PubMed] [Google Scholar]
- 25.Age and cancer risk Bethesda, MD: National Cancer Institute, March 2021. (https://www.cancer.gov/about-cancer/causes-prevention/risk/age). [Google Scholar]
- 26.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol 2015;151:1081–6. [DOI] [PubMed] [Google Scholar]
- 27.Guy GP Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002–2006 and 2007–2011. Am J Prev Med 2015;48:183–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brin L, Zubair AS, Brewer JD. Optimal management of skin cancer in immunosuppressed patients. Am J Clin Dermatol 2014; 15:339–56. [DOI] [PubMed] [Google Scholar]
- 29.Dahlke E, Murray CA, Kitchen J, Chan A-W. Systematic review of melanoma incidence and prognosis in solid organ transplant recipients. Transplant Res 2014;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakha EA, El-Sayed ME, Lee AHS, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol 2008;26:3153–8. [DOI] [PubMed] [Google Scholar]
- 31.Britanova OV, Putintseva EV, Shugay M, et al. Age-related decrease in TCR repertoire diversity measured with deep and normalized sequence profiling. J Immunol 2014; 192:2689–98. [DOI] [PubMed] [Google Scholar]
- 32.Mittelbrunn M, Kroemer G. Hallmarks of T cell aging. Nat Immunol 2021;22:687–98. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Nguyen T, Achour A, et al. Longitudinal analysis reveals age-related changes in the T cell receptor repertoire of human T cell subsets. J Clin Invest 2022; 132(17):e158122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 2009;27:519–50. [DOI] [PubMed] [Google Scholar]
- 36.Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science 2013; 339:286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nevala WK, Vachon CM, Leontovich AA, Scott CG, Thompson MA, Markovic SN. Evidence of systemic Th2-driven chronic inflammation in patients with metastatic melanoma. Clin Cancer Res 2009;15:1931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokumaru Y, Le L, Oshi M, et al. Association of Th2 high tumors with aggressive features of breast cancer. J Clin Oncol 2020; 38(15)Suppl:e12584. abstract ( 10.1200/JCO.2020.38.15_suppl.e12584). [DOI] [Google Scholar]
- 39.Marwaha AK, Leung NJ, McMurchy AN, Levings MK. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front Immunol 2012;3:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sempowski G, Thomasch J, Gooding M, et al. Effect of thymectomy on human peripheral blood T cell pools in myasthenia gravis. J Immunol 2001;166:2808–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


