Abstract
Designing efficient, economical heterogeneous catalysts for the Knoevenagel condensation reaction is highly significant owing to the importance of reaction products in industries as well as pharmaceutics. Herein, we have designed and synthesized biguanidine-functionalized basic magnetically retrievable cobalt ferrite nanoparticles (CFNPs) for the synthesis of Knoevenagel condensation products using benzaldehydes and active methylene compounds (malononitrile/ethyl cyanoacetate/cyanoacetamide). Several advanced techniques, such as Fourier transform infrared (FT-IR), thermogravimetric analysis (TGA), powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and vibration sample magnetometry (VSM), were utilized to precisely characterize the catalyst. The robust features of the current approach involve outstanding catalytic performance, solvent-free reaction conditions, ease of catalyst retrievability, easy workup procedure, large substrate tolerance, high turnover frequency (TOF) values (up to 486.88 h–1), values of green chemistry metrics such as E-factor (0.15), reaction mass efficiency (RME) value (87.07%), carbon efficiency (93.4%), and atom economy (AE) value (88.10%) close to their ideal values, and recyclability up to eight runs without a considerable reduction in activity, boosting the appeal of this approach from a commercial and ecological point of view.
Keywords: green approach, magnetic nanocatalyst, reusable, Knoevenagel condensation, solvent-free
1. Introduction
The Knoevenagel condensation, a well-known reaction involving aldehydes and activated methylene group-containing molecules, has gained immense significance and widespread adoption as a highly influential and beneficial approach in organic chemistry for the synthesis of C–C bond formation to generate substituted electrophilic alkenes.1,2 The alkenes thus formed act as versatile intermediates in various organic synthesis applications. These applications encompass a wide range of fields, including the production of fine chemicals, formation of heterocyclic compounds, carbohydrates, and synthesis of drug intermediates, natural products, fluorescent dyes, and functional polymers.3−8
Moreover, the applicability of Knoevenagel condensation in various industries such as polymers and pharmaceutical synthesis of various products and intermediates has been well documented.4 This type of condensation reaction turned out to be a key step for producing numerous clinical drugs.9 Some of the commercially available drugs involving the Knoevenagel condensation reaction are shown in Figure 1. Besides their enzyme inhibiting properties, the substituted alkene compounds exhibit a vast array of biological studies such as anticancer, antioxidant, antimalarial, antihypertensive, and antiviral properties.6,10 Furthermore, the Knoevenagel condensation is extensively employed in industries for various synthetic organic methods as this type of condensation reaction is devoid of any waste and byproducts.11
Figure 1.
Some of the commercially available drugs derived using the Knoevenagel condensation reaction.
Benzylidenemalononitrile (BMN) derivatives formed by the Knoevenagel condensation involving benzaldehydes and malononitrile also have gained the attention of many research groups owing to their exceptional properties such as anticancer, antifungal, antibacterial, and anticorrosive.12 The benzylidenemalononitrile derivatives are also used for enhancing cellular resistance to oxidative stress and prostaglandin production, for designing photoconductive cells, and for the activation/inhibition of certain types of enzymes.13−16
Taking into consideration the desirability and applicability of these derivatives, there is an urgent need for a simple and employable synthetic procedure for Knoevenagel condensation which is cost-effective as well as energy-saving.
This condensation reaction is frequently carried out using a basic catalyst, as the basic sites present over the catalyst attract protons from the adsorbed active methylene molecules such as malononitrile in order to generate carbanion intermediates. In order to catalyze this Knoevenagel condensation reaction, several homogeneous and heterogeneous (several different types of) catalysts such as zeolites, ionic liquids, Lewis acids, basic catalysts (urea, thiourea, and piperidine), ammonium salts, amino acids, aliphatic amines, metal oxides, metal–organic frameworks (MOFs), carbon materials, graphene-supported Pd and Ni nanoparticles, and covalent organic frameworks (COFs) have been employed.1,3,5,6,9,11 However, the applicability of these catalysts is limited owing to various environmental issues, such as post-treatment of the generated waste liquid, severe reaction conditions, elevated temperature, use of additives, costly metals, insufficient yields, extensive reaction period, harmful solvents, corrosion of equipment, and tedious workup procedure.5,11 Also, some of these reported procedures suffer from various other serious problems including the formation of unwanted side products and the production of waste due to self-condensation, addition, and polymerization reactions.6
Thus, keeping in mind these restrictions, the aspects of green chemistry, and process sustainability, efforts have been made to develop efficient and reusable heterogeneous catalysts, which can efficiently catalyze this condensation reaction.
Transition metal oxides with the following formula MFe2O4, where M = nickel, copper, cobalt, zinc, and manganese, have gained tremendous attention owing to their exceptional physical as well as chemical properties. Among these, cobalt ferrite nanocomposites are a steady magnetic material, which is well-thought-out as the most prominent support due to its substantial magnetic properties such as higher saturation magnetization (Ms) value, relatively high permeability, high intrinsic coercivity, high Curie temperature, biocompatibility, nontoxicity, and chemical stability.17−20 Several chemical procedures have been developed to synthesize CoFe2O4 nanoparticles including electrospinning, Co precipitation, sol–gel, sonochemical, combustion, and hydrothermal–solvothermal–hydrothermal methods.18−20
These properties have led to their widespread adoption in a wide range of fields, including but not limited to sensors, ferrofluid technology, nanobiotechnology, microwave absorbers, anticancer agent, MRI,21 biosensors, reduced-toxicity drug delivery, magnetic energy storage, photocatalysis, environmental remediation, catalysis, antimicrobials, and more.19,20,22−25
Building on our current protocol to establish synthetic routes that are sustainable for a variety of organic transformations, we have been utilizing a range of advanced materials;26−29 we have designed this solid biguanidine-functionalized base catalyst (CFNP) as a Lewis basic catalyst having abundant N atoms due to the presence of two imine-like functions over the biguanidine group. The Knoevenagel condensation reaction was studied and used to evaluate the more effective material’s catalytic activity. This reaction was carried out under solvent-less conditions, lesser reaction time (4–10 min), and high yields.
2. Experimental Section
2.1. Catalyst Preparation
2.1.1. Experimental Procedure for the Synthesis of CoFe2O4
One-pot solvothermal synthesis (Scheme 1)30 was used to produce magnetic nanoparticles of cobalt ferrite (CoFe2O4). To make a uniform solution, we added CoCl2.6H2O (148 mg, 0.625 mmol) and FeCl3.6H2O (337 mg, 1.25 mmol) to 10 mL of ethane-1,2-diol and agitated the mixture constantly at 50 °C. After obtaining a homogenous reaction mixture, 900 mg of NaOAc and 500 mg of PEG-6000 were added and agitated for an additional 30 min. The resulting liquid was transported to an autoclave and heated to 160 °C for 16 h. The resulting black-colored material was separated magnetically and rinsed repeatedly in double-deionized water. Nanoparticles of cobalt ferrite (CoFe2O4) were produced and dried at 60 °C for 6 h.
Scheme 1. Pictorial Representation of the Synthesis of the CFNP Nanocatalyst.
2.1.2. Experimental Procedure for the Synthesis of CoFe2O4@SiO2
The Stöber sol–gel process was then used to cover the cobalt ferrite nanoparticles (CoFe2O4) with silica.28−31 To achieve this, 200 mg of CoFe2O4 nanoparticles (0.852 mmol) was sonicated in 200 mL of a solution made up of 160 mL of EtOH and 40 mL of double-deionized water. Then, 1.5 mL of 25% NH3 solution and 1 mL of tetraethyl orthosilicate were added dropwise. The resultant dispersed solution was then constantly stirred at 60 °C for 6 h. The final silica-coated nanoparticles were magnetically separated using an external magnet, washed numerous times with ethanol, and then dried under vacuum.
2.1.3. Experimental Procedure for the Synthesis of (CFNA) CoFe2O4@SiO2@NH2
By using our prior method,29 which included adding 1 mL of (3-aminopropyl)triethoxysilane (APTES) to 0.5 g of CoFe2O4@SiO2 nanoparticles dispersed in 100 mL of EtOH, we were able to introduce amine groups onto the surface of silica-coated cobalt ferrite nanoparticles. The final mixture was agitated at 80 °C for 6 h. After that, the synthesized CFNA nanocomposites were collected using an extrinsic magnet, washed with Et2O to get rid of the silylating agent that had not been reacted, and dried till vacuum.
2.1.4. Synthesis of CFNPs
Biguanidine was added to the surface of the nanocomposite (CoFe2O4@SiO2@NH2) in order to enhance the catalytic sites (NH sites). For this surface modification, 10 mL of EtOH was mixed with 0.5 g of CFNA nanoparticles, 125 mg of dicyandiamide, and 0.3 mL of Et3N and refluxed for 6 h. The end product, CoFe2O4@SiO2@NH2@BG (CFNP) nanoparticles (Scheme 1), was collected by using an external magnet, washed repeatedly with EtOH, and then dried in an oven for 24 h at 100 °C.
2.2. General Procedure for Knoevenagel Condensation Using CFNP as a Catalyst
All the benzaldehydes (1, 4, 7) used during the experiment were purchased from Fluka, Sigma-Aldrich, Merck, Spectrochem, and malononitrile (2), ethyl cyanoacetate (4), and cyanoacetamide (8) were bought from Spectrochem and utilized without undergoing any further purification before being used.
Benzaldehyde (1.0 mmol), an active methylene compound (malononitrile/ethyl cyanoacetate/cyanoacetamide), and 10 mg of the nanocatalyst (CFNP) were placed in a 25 mL oven-dried round-bottom flask. The reaction mixture was stirred constantly at 60 °C. The completion of the condensation process was carefully monitored using TLC with 20% ethyl acetate in hexane as the mobile phase. Ethanol was then added when the reaction was complete, and the catalyst was separated using an external magnet and washed in ethanol. The solvent was evaporated using a rotatory evaporator, and the resultant solid was recrystallized using ethanol.
3. Results and Discussion
3.1. Catalyst Characterization
To validate the structural attributes of the synthesized nanocatalyst, multiple characterization techniques were used, which are discussed below.
3.1.1. Fourier Transform Infrared (FT-IR) Spectroscopy
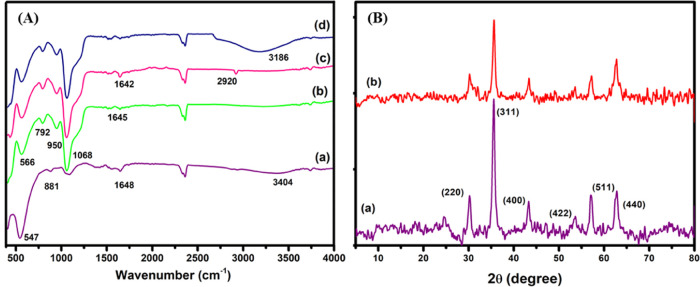
FT-IR spectra of CoFe2O4, CoFe2O4 @SiO2, CFNA, and CFNPs were obtained at room temperature using a Perkin Elmer Spectrum 2000 utilizing the KBr pellet method (400–4000 cm–1), as shown in Figure 2A. Absorption bands at 881 and 547 cm–1 in the FT-IR spectra of CoFe2O4 nanoparticles provide evidence for the existence of inverted spinal cobalt ferrite structures.32 Peaks at 881 and 547 cm–1 correspond to the stretching vibration of the tetrahedral site (Fe3+, O2–) and octahedral site (Co2+, O2–) in the spinel structure, respectively. Figure 2A(a) shows the stretching and bending vibrations of the surface OH groups present at 3404 and 1648 cm–1, respectively.30 In addition, the presence of silica over the surface of cobalt ferrite nanoparticles is confirmed by the bands seen at 792, 950, and 1068 cm–1 in Figure 2A(b),33 which could be attributed to the Si–O–Si symmetric, Si–O symmetric, and Si–O–Si asymmetric stretching modes, respectively. Two additional bands, at 2920 (CH2) and 1642 (NH2) cm–1, occur in the spectrum of APTES-functionalized CoFe2O4@SiO2 nanoparticles, indicating the surface functionalization of CoFe2O4@SiO2 with NH2 groups (Figure 2A(c)).34 Furthermore, Figure 2A(d) represents the FT-IR spectra of CFNPs, which are reported earlier.35 Thus, the findings showed that CFNA nanoparticles were successfully functionalized with the required biguanidine moiety.
Figure 2.
(A) FT-IR spectra of (a) CoFe2O4, (b) silica-coated CoFe2O4 (CoFe2O4@SiO2), (c) CoFe2O4@SiO2@NH2, and (d) CFNP. (B) Powder XRD analysis of (a) CoFe2O4 and (b) CFNPs.
3.1.2. XRD Analysis
X-ray diffraction (XRD) was analyzed by the structural integrity and crystalline phase structure of the synthesized CoFe2O4 and CFNPs. XRD patterns were acquired at room temperature in the 2θ range of 5–80° (Figure 2(B)) using the parameters 0.15406 nm, 40 kV, 40 mA, and scanning rate = 2°/min.
Nanoparticles of inverse cubic spinel cobalt ferrite exhibit characteristic diffraction peaks at 30.24, 35.62, 43.33, 53.60, 57.27, and 62.79°, which correspond to the (220), (311), (400), (422), (511), and (440) planes, respectively, with the space group fd-3m36 (JCPDS card no. 22-1086, Figure 2B(a)).28,30Figure 2B(a) shows that the XRD structure of the sample is consistent with that reported in the previous literature37 for CoCl2.6H2O (JCPDS file (no. 01-0173)), with the exception of a slight shift in the peaks caused by the presence of bimetallic nature (Co and Fe). Additionally, the Bragg diffraction peaks for CFNP were similar and matched with characteristics peaks of cobalt ferrite nanoparticles, confirming that the spinel structure of CoFe2O4 nanoparticles remains even after chemical transformations (Figure 2B(b)). The XRD pattern showed intense peaks, which is consistent with increased CoFe2O4 crystallization.
3.1.3. SEM and TEM Analysis
In order to derive information about the shape and morphology of the synthesized nanoparticles (CoFe2O4, CoFe2O4@SiO2, and CFNP), scanning electron microscopy (SEM) images were collected through using a Carl Zeiss, India (Jeol Japan Mode: JSM 6610LV), and transmission electron microscopy (TEM) images of the synthesized nanoparticles were acquired using an FEI TECHNAI (model number G2 T20) operated at 200 kV by casting their dispersed ethanolic solution over carbon-coated copper grids. The morphology and their texture illustration were attained using scanning electron microscopy and transmission electron microscopy analysis (Figure 3). The SEM pictures collected for CoFe2O4 nanoparticles substantiated the formation of the monodispersed spherical shape without agglomeration despite the dipolar interaction between the particles.38 (Figure 3a). The SEM analysis of silica-coated nanoparticles (CoFe2O4@SiO2) and after surface modification by the biguanidine group (CFNP) depicted the intactness of their spherical shape (Figure 3b–c). Furthermore, the TEM analysis confirmed that the spherical shape of CoFe2O4 nanoparticles is monodisperse and the size is about 200 nm (Figure 3d). TEM imaging (Figure 3e) confirms the existence of a uniform silica covering of roughly 30 nm around the CoFe2O4 core, which prevents agglomeration. The TEM picture of CFNPs further supported the preservation of the same spherical shape and the expanded size of these nanoparticles (Figure 3f). Figure S1b (Supporting information) represents the SAED pattern of CoFe2O4 nanoparticles, which confirmed the polycrystalline nature of the synthesized nanoparticles due to the existence of diffraction rings with white spots.39
Figure 3.
SEM images of (a) CoFe2O4, (b) CoFe2O4@SiO2, and (c) CFNP and TEM images of (d) CoFe2O4, (e) CoFe2O4@SiO2, and (f) CFNP.
Furthermore, to understand the elemental composition of the CFNP nanocatalyst, the SEM-coupled EDX spectrum was collected, and it confirms the presence of expected elements such as cobalt (Co, 1.45%), iron (Fe, 9.40%), silicon (Si, 6.72%), carbon (C, 18.54%), nitrogen (N, 25.27%), and oxygen (O, 38.61%), indicating the successful conjugation of biguanidine with CoFe2O4@SiO2@NH2 nanoparticles (Figure 4).
Figure 4.
SEM-coupled EDX spectrum of CFNP.
3.1.4. VSM Analysis
The magnetic properties of the synthesized CoFe2O4, CoFe2O4@SiO2, CoFe2O4@SiO2@NH2, and CFNPs were evaluated using a vibration sample magnetometer (VSM), model number EV-9, Microsense at room temperature (Figure 5). As it can be seen clearly from Figure 5, there is the absence of the hysteresis phenomenon, remanent magnetization, and coercivity, implying the super paramagnetic behavior of bare as well as surface-modified nanoparticles.40 The values of saturation magnetization (Ms) for CoFe2O4, silica-coated CoFe2O4, CFNA, and CFNP were determined to be 63, 53, 48, and 34 emu/g, respectively. The monolayer covering of APTES on the surface caused a very slight drop in the Ms value of CoFe2O4 after APTES functionalization.39 This difference in Ms values of CoFe2O4 and CFNP showed that a great number of biguanidine moieties were bonded to Co2Fe2O4 nanoparticles.32 The addition of nonmagnetic silica and other functional groups may reduce the surface moments for distinct particles, which contributes to the overall drop in magnetism. Despite the lower Ms value for the CFNP in comparison to bare CoFe2O4, it is still sufficient to perform magnetic separation more quickly from the crude solution by introducing an extrinsic magnetic field. The Ms value for the recovered catalyst was 26 emu/g at rt, which is more than adequate for the efficient and rapid recovery utilizing an external magnet (Figure 5e).
Figure 5.

VSM analysis of (a) CoFe2O4, (b) CoFe2O4@SiO2, (c) CoFe2O4@SiO2@NH2, (d) CFNP, and (e) reused CFNP after eight consecutive cycles.
3.1.5. Thermogravimetric Analysis (TGA)
Thermogravimetric analysis26 (TGA) of the sample was carried out on a Shimadzu TG/DTA simultaneous measuring instrument (Model: DTG-60) from ambient temperature (rt) to 800 °C at a steady 10 °C per min heating rate and 100 mL per min nitrogen gas flow to determine the thermal stability of CFNPs. The results indicated that the synthesized CFNPs were stable up to 300 °C in a N2 environment (Figure 6). Evaporation of the entrapped water and organic solvents causes a minor loss in weight between 230 and 300 °C, but the significant weight loss above 400 °C is due to the disintegration of organic layers. A total weight loss of up to 75% shows that a large portion of the catalyst was composed of organic layers. After 400 °C, the CFNPs start decomposing at 400 °C, which is completed at 650 °C. A further increase in the temperature did not show any decomposition of CFNPs.
Figure 6.
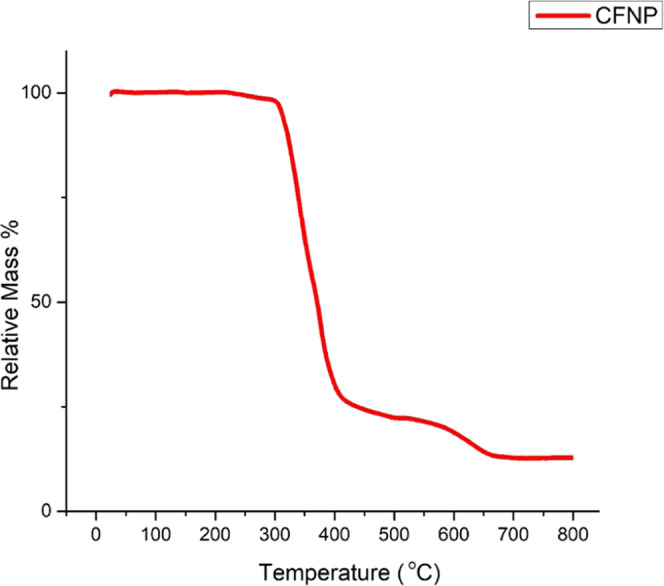
TGA analysis of CFNPs.
3.1.6. Basicity of Catalysis
The quantification of basic sites over the present CFNA and CFNP nanocatalysts was carried out using the conductometric titration method as reported elsewhere.27 The experimental results showed that the number of basic sites as calculated using conductometric titration was found to be 1.4 mmol/g for CFNA and 3.05 mmol/g for CFNP.
3.1.7. X-ray Crystallographic Structure of Compound 6b
Data were collected using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) on an XtaLAB Synergy, Dualflex, HyPix3000 diffractometer running at 50 kV and 30 mA. The crystal was analyzed at 293(2) K while the data were being recorded. Using Olex2,41 the structure was analyzed and refined using the SHELXL42 refinement package involving least squares minimization. For crystal growth, the Knoevenagel adduct 6b was added in a 50 mL beaker, dissolved in 10 mL of DCM, and kept for few days. The structure analysis of one of the synthesized compound 6b is done by using X-ray single-crystal diffraction (CCDC-2210747, Figure 7). The detailed crystallographic studies of compound 6b43 C12H10ClNO2 (M = 235.66 g/mol) indicated that the compound has a monoclinic structure in the crystal lattice, space group P21/n, and the Z value is 4.
Figure 7.
Single-crystal XRD of compound 6b (CCDC- 2210747). The displacement ellipsoid contour is drawn at the 50% probability level.
From crystal structure, it is clear that for the given organic molecule 6b and its derivatives, the configuration is E, by using IUPAC convention. Important bond length and bond angle can be seen in Tables S8 and S9, respectively, as mentioned in the Supporting Information.
3.2. Catalytic Potential Analysis of the Biguanidine-Functionalized Cobalt Ferrite Nanocatalyst for the Knoevenagel Condensation
The catalytic potential in the Knoevenagel condensation with benzaldehydes
and malononitrile was investigated using the synthesized CFNP nanocatalyst.
The reaction conditions were optimized by taking 4-bromobenzaldehyde
and malononitrile as test substrates under solvent-free conditions.
To accomplish the optimal reaction conditions, effects of the quantity
of the catalyst, solvent, temperature, and reaction completion time
were studied and optimized (Table 1).
Table 1. Optimization of Reaction Conditionsa.
| entry | catalyst amountb | solvent | temp (°C) | timec (min) | yield (%)d |
|---|---|---|---|---|---|
| 1. | 0 | 60 | 30 | NR | |
| 2. | 5 | 60 | 10 | 70 | |
| 3. | 7 | 60 | 10 | 90 | |
| 4. | 10 | 60 | 5 | 99 | |
| 5. | 10 | EtOH | 60 | 10 | 95 |
| 6. | 10 | DCM | 60 | 10 | 70 |
| 7. | 10 | CH3CN | 60 | 10 | 78 |
| 7. | 10 | THF | 60 | 10 | 85 |
| 8. | 10 | H2O | 60 | 10 | 90 |
| 9. | 10 | toluene | 60 | 10 | 80 |
| 10. | 15 | 60 | 5 | 99 | |
| 11 | 10 | rt | 10 | 72 | |
| 12. | 10 | 80 | 5 | 98 | |
| 13. | 10e | 40 | 5 | 88 | |
| 14. | 10f | 60 | 8 | 88 |
Reaction Conditions: 4-bromobenzaldehyde (1.0 mmol) and malononitrile (1.2 mmol).
catalyst (mg).
time (min).
(%) isolated yield.
catalyst27 (ASMNPs),.
catalyst (CFNA).
Primarily, the impact of the quantity of the catalyst on the product yield was investigated. The catalytic amount selected for the reaction varies from 0 mg to 15 mg (Table 1, entry 1–4 and 10) at 60 °C, and it was observed that the best results were obtained when 10 mg of CFNP was utilized to perform the reaction (Table 1, entry 4). Using the lower amount of the catalyst, i.e., 5 mg and 7 mg, the yield of the product was found to be less with 70 and 90% yield, respectively (Table 1, entry 2–3), whereas no noticeable change in the isolated yield of the product even after increasing the quantity of the catalyst is observed. When performing the reaction at 60 °C and varying the quantity of the catalyst CFNP from 0 to 15 mg (Table 1, entries 1–4 and 10), it was found that 10 mg of CFNP produced the finest results (Table 1, entry 4). Using a lower quantity of the catalyst, i.e., 5 mg and 7 mg, resulted in a lesser product yield of 70 and 90%, respectively (Table 1, entries 2–3), while increasing the catalyst amount had no noticeable effect on the isolated yield. Therefore, using a catalyst amount of 10 mg yielded the optimum outcomes. Furthermore, on varying the solvents such as EtOH, DCM, CH3CN, THF, H2O, and toluene, we did not observe any significant increase in the overall yield of the product (Figure 8, entry 5–9 and Table 1).
Figure 8.
Solvents effect on the yield of the Knoevenagel condensation product [SF corresponds to solvent-free conditions].
Subsequently, the impact of temperature on the reaction’s progression was also studied. The yield of the product at rt was found to be 72% only, whereas the increase of temperature from 40 to 60 °C leads to an increase in the isolated yield of the product. One possible explanation for the higher yield is that increasing the temperature also increases the reactant solubility, which ultimately improves the catalytic performance. In addition, neither the pace of the reaction nor the isolated yield of the product was significantly changed by an increase in temperature from 60 to 80 °C (entry 12, Table 1). Along with the present catalyst CFNP, the model reaction was also carried out using our previously reported magnetic basic nanocatalyst (ASMNPs),27 which is (Fe3O4@SiO2@NH2), and a noteworthy yield (88%) of the model reaction product was found at 40 °C within 5 min. However, the current magnetic nanocatalyst (CFNP) has a great number of catalytic active sites compared to ASMNPs; the reaction proceeded more efficiently and smoothly. Additionally, we used the catalyst CFNAf (CoFe2O4@SiO2@NH2) and CFNP to compare the model reaction’s notable yield, which we found to be 88 and 99%, respectively. Considering this, CFNPs show superior catalytic activity compared to CFNA and ASMNPs.
3.2.1. Green Chemistry Parameters
For the synthesis of the Knoevenagel condensation product (3a), the green chemistry metrics for the condensation process between 4-bromobenzaldehyde and malononitrile were also analyzed. Table 2 shows some of the variables considered when analyzing the cost effectiveness of green organic synthesis under ideal circumstances. The radar chart (Figure 9) showed the harmonious relationship between reaction mass efficiency, carbon efficiency, atom economy, and the E-factor, which shows that this approach is environmentally friendly. The supplementary file explains every calculation used to determine green chemistry parameters.
Table 2. Calculation of Green Chemistry Metrics.
| yield | atom economy (AE) | reaction mass efficiency (RME) | E-factor | carbon efficiency (CE) |
|---|---|---|---|---|
| 99% | 88.10% | 87.07% | 0.15 | 93.4% |
Figure 9.
Green chemistry metrics for the synthetic ″3a″ from a model Knoevenagel condensation process shown on a radar chart plot.
3.3. Knoevenagel Condensation of Aldehydes with an Active Methylene Molecule (Malononitrile, Ethyl Cyanoacetate, or Cyanoacetamide) and the Catalytic Role of CFNPs
After optimizing the reaction conditions for the aforesaid Knoevenagel condensation, the capability of the synthesized nanocatalyst (CFNP) toward the synthesis of the condensation product (benzylidene) was extended and investigated by taking a diverse range of aromatic aldehydes/benzaldehydes and malononitrile (Schemes 2 and 3). Briefly, this condensation reaction was achieved by heating aldehyde (1.0 mmol), malononitrile (1.2 mmol), and the catalyst (10 mg) in an oil bath to 60 °C for a suitable amount of time. Using malononitrile and several aromatic aldehydes containing either electron-withdrawing or electron-donating groups, we were able to generate a wide variety of useful Knoevenagel condensation products in good to great yield, as illustrated in Scheme 2. In addition, the outcomes demonstrated that the electronic environment of the benzylidene utilized affects how quickly benzylidene converts and how much is produced. Electron-withdrawing benzaldehyde derivatives, such as Br, Cl, NO2, CHO, F, and COOH (entry 1a, 1b, 1h, 1l, 1d. 1k), showed superior conversion and better yield as compared to benzaldehydes having electron-donating groups including OMe or CH3 (entry 1c, 1i).
Scheme 2. Substrate Scope for the Knoevenagel Condensation Involving Aldehydes and Malononitrile using CFNP as a Catalyst.
Reaction Conditions: Benzaldehyde (1 equiv, 1.0 mmol) and malononitrile (79.27 mg, 0.66 mL, 1.2 equiv, 1.2 mmol), catalyst (10 mg), and temp (60 °C) under neat reaction conditions.
Scheme 3. Substrate Scope for the Knoevenagel Condensation Involving Aldehydes and Ethyl Cyanoacetate using CFNP as a Catalyst.
Reaction Conditions: Benzaldehyde (1 equiv, 1.0 mmol) and ethyl cyanoacetate (135.7 mg, 1.2 equiv, 1.2 mmol), catalyst (10 mg), and temp (60 °C) under neat reaction conditions
Additionally, broadening of the use of the Knoevenagel condensation reaction between aldehydes with ethyl cyanoacetate and cyanoacetamide acting as an active methylene compound was also carried out under similar reaction conditions (Schemes 3 and 4). When compared to Scheme 2, it was seen that the reaction proceeds efficiently, which produced an excellent amount of the product.
Scheme 4. Substrate Scope for the Knoevenagel Condensation between Aldehydes and Cyanoacetamide using CFNP as a Catalyst.
Reaction Conditions: Benzaldehyde (1 equiv, 1.0 mmol) and cyanoacetamide (100.89 mg, 1.2 equiv, 1.2 mmol), catalyst (10 mg), and temp (60 °C) under neat reaction conditions.
Additionally, from an industrial perspective, gram-scale synthesis of 2-(4-nitrobenzylidene)malononitrile (3h) was performed. To accomplish this, 1.51 g of p-nitrobenzaldehyde (10 mmol), 0.6 mL of malononitrile (12 mmol), and 100 mg of the catalyst CFNP were heated in an oil bath with continuous stirring. After completion of the reaction for compound 3 h, it was found that the reaction proceeded smoothly within 35 min with an excellent yield of 1.49 g (99%). In addition, the current catalytic system was found to be suitable for the conversion involving moderate reaction conditions and an easier workup procedure.
3.4. Probable Reaction Mechanism
A plausible mechanism for the Knoevenagel reaction involving the reactants benzaldehyde and malononitrile using CFNPs as a nanocatalyst for the formation of benzylidene by the condensation method is illustrated in Figure 10. In the beginning, the hydrogen bonds on the catalyst surface were responsible for absorbing the substrates (A). Then, the imine group of the biguanidine moiety (CFNP) abstracts the active α-H of malononitrile and the carbanion formed, which attack the carbonyl carbon of the benzaldehyde to generate an oxyanion (B). After that, the oxyanion thus obtained reacts with the proton of amino cation (C) to form the corresponding Knoevenagel condensation product (benzylidene malononitrile) by removal of a water molecule (D), and the catalyst (CFNPs) is concurrently generated back. Hence, the basic sites of the nanocatalyst played a very vital role in attaining superior performance of the present Knoevenagel condensation reaction.
Figure 10.

Plausible reaction mechanism for the Knoevenagel condensation reaction involving aldehydes and malononitrile using a basic CFNP nanocatalyst.44
3.5. Analysis of the Recyclability of the Magnetic Biguanidine-Functionalized Cobalt Ferrite (CFNPs)
Several criteria of green chemistry, including excellent recovery and reusability of the catalyst, are extremely critical and desired features that point in the direction of its commercial implementation. In this regard, 4-bromobenzaldehyde and malononitrile were used as model substrates at the appropriate condensation conditions for the assay of the operational stability and recyclability of the CFNP nanocatalyst. The catalyst was removed magnetically from the reaction mixture when the condensation reaction was complete, washed with ethanol (4–5 times), and then dried at 60 °C under vacuum. After recovering the catalyst, an identical model reaction with benzaldehyde and malononitrile was repeated. The recycling results indicated no appreciable loss in the catalytic activity of the nanocatalyst up to eight consecutive cycles (Figure 11), revealing its exceptional long-term stability and reusability. The CFNP nanocatalyst’s activity may have dropped somewhat because the pores were blocked by reactants or products. To further examine the amine group leaching into the reaction mixture, the CFNP was removed from the reaction medium after 3 min using an extrinsic magnet. Now, the residual reaction mixture was reacted under similar circumstances, and no further progress in the reaction was observed even after 40 min, which confirmed the heterogeneous nature as well as stability of the CFNP nanocatalyst. The obtained outcomes were further supported by the FT-IR, powder XRD, and SEM analyses of the recovered catalyst (obtained after the 8th run in the Supporting Information (SI)) and VSM analyses of the recovered catalyst (Figure 5e). The basic nanocatalyst’s structure and shape remained unaltered even after eight runs, proving its stability and durability, when the findings of fresh and recovered catalysts were compared.
Figure 11.
Recyclability chart of the synthesized CFNP catalyst.
3.6. Comparison of the CFNP-Catalyzed Knoevenagel Condensation to Form Benzylidenemalononitrile Derivatives with the Reported Precedents
Numerous homogeneous and heterogeneous catalysts have been identified for the Knoevenagel condensation reaction involving aldehydes and malononitrile, according to a thorough literature review (Table 3). Our magnetic solid heterogeneous CFNP nanocatalyst outperformed previous reports in ambient reaction conditions, catalyst loading, solvent-free conditions, time, product yield, no need for column chromatography, wide functional group tolerance, catalyst magnetic retrievability, and recyclability. The current magnetic nanocatalyst performs well because it has several basic catalytic sites that support condensation product formation. Thus, the current catalytic system offers a clean and green method without a reaction solvent, simple workup, recyclability, and stability of the biguanidine-functionalized nanocatalyst by applying an external magnetic force.
Table 3. Previously Reported Methods and Catalyst for the Knoevenagel Condensation Reaction.
| s. no. | catalyst | solvent | temp. (°C) | time | yield (%) | refs |
|---|---|---|---|---|---|---|
| 1. | NiCu@MW CNT NPs | H2O/MeOH (1:1) | rt | 10–180 min | 96 | (12) |
| 2. | FeNPs/PPD@rGO | toluene | 40 | 3.5 h | 100 | (5) |
| 3. | Ce0.4Bi0.6O1.7 | EtOH | 35 | 6h | 100 | (45) |
| 4. | Fe3O4@CFR-S-PNIPAM@ Pd/CD | H2O | 25 | 24 h | 98 | (46) |
| 5. | GO-NH2(1.8)–Si (0.2) | H2O | 40 | 1 h | 99 | (47) |
| 6. | PS–NH2 HMOPBs | EtOH | rt | 2 h | 98 | (48) |
| 7. | Cu-based MOF | toluene | 100 | 10 h | 99 | (49) |
| 8. | Ga4B2O9 | N2/DMSO | 40 | 0.5 h | 98 | (9) |
| 9. | Fe3O4/SiO2/PPI-G3 | N2/EtOH | rt | 0.5 h | 93 | (50) |
| 10. | [CaZn] NUC-21 | 60 | 1 h | 97 | (51) | |
| 11. | UiO-67-diamine | DMF | 25 | 2 h | 99 | (52) |
| 12. | (NUC-42) | H2O | 60 | 6 h | 99 | (53) |
| 13. | CFNP | solvent-free | 60 | 5–20 min | 99 | P.W. |
4. Conclusions
In conclusion, a novel biguanidine-functionalized basic magnetic cobalt ferrite nanocatalyst (CFNP) for the Knoevenagel condensation reaction involving aldehydes and an active methylene compound (malononitrile/ethyl cyanoacetate/cyanoacetamide) has been designed, characterized, and examined for its performance. The current approach’s notable features are its superiority over the conventional magnetic core-based nanocatalyst reported previously, which include ambient reaction conditions, the heterogeneous nature of the catalyst, a shorter reaction period (4–30 min), low catalyst loading, a broad substrate scope, high conversion, high TOF values (52.46–486.88 h–1), and high yield of condensation products. The produced nanocatalyst also showed recyclability up to eight successive runs without any notable reduction in its activity, and its removal from the reaction mixture is as easy as using magnetic attraction. In addition, the proposed protocol is green and sustainable to easily synthesize the Knoevenagel condensation products, with use of nonhazardous chemicals, cost effectiveness, solvent-free settings, and a straightforward workup process. We believe that the developed nanocatalyst can find a wide variety of industrial applications for significant organic transformation reactions under green and sustainable conditions due to the presence of a large number of NH groups, which is enabled by the immobilization of a wide variety of chemical and metal moieties.
Acknowledgments
A.M. thanks the Department of Science and Technology (DST INSPIRE) for providing a Senior Research Fellowship. P.Y. expresses gratitude to the UGC for the Senior Research Fellowship. S.K.A. acknowledges the financial support received from the University of Delhi, Delhi 110007, India.
Data Availability Statement
The data supporting the findings of this study are available in the published article and its accompanying Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.3c00002.
Materials and reagents, instrumentation, green chemistry parameters, TOF calculations, EDX and SAED spectrum of CoFe2O4, SEM, powder XRD, and FT-IR of the reused catalyst after eight runs, FTIR, 1H, and 13C NMR shift value, HRMS/elemental analysis, 1H and 13C NMR spectra of the synthesized compounds, and single-crystal XRD data for compound 6b and references (PDF).
Author Contributions
$ A.M. and P.Y. contributed equally in this manuscript. CRediT: Anupam Mishra data curation (equal), formal analysis (equal), investigation (equal), methodology (equal), project administration (equal), resources (equal), software (equal), writing-review & editing (equal); Priyanka Yadav writing-original draft (equal); Satish Kumar Awasthi conceptualization (equal), supervision (equal), visualization (equal).
The authors declare no competing financial interest.
Supplementary Material
References
- Taher A.; Lumbiny B. J.; Lee I.-M. A facile microwave-assisted Knoevenagel condensation of various aldehydes an d ketones using amine-functionalized metal organic frameworks. Inorg. Chem. Commun. 2020, 119, 108092 10.1016/j.inoche.2020.108092. [DOI] [Google Scholar]
- Varnaseri N.; Rouhani F.; Ramazani A.; Morsali A. Size and function influence study on enhanced catalytic performance of a cooperative MOF for mild, green and fast C–C bond formation. Dalton Trans. 2020, 49, 3234–3242. 10.1039/D0DT00433B. [DOI] [PubMed] [Google Scholar]
- Joharian M.; Morsali A.; Tehrani A. A.; Carlucci L.; Proserpio D. M. Water-stable fluorinated metal–organic frameworks (F-MOFs) with hydrophobic properties as efficient and highly active heterogeneous catalysts in aqueous solution. Green Chem. 2018, 20, 5336–5345. 10.1039/C8GC02367K. [DOI] [Google Scholar]
- Li C.; Zhong D.; Huang X.; Shen G.; Li Q.; Du J.; Li Q.; Wang S.; Li J.; Dou J. Two organic–inorganic hybrid polyoxovanadates as reusable catalysts for Knoevenagel condensation. New J. Chem. 2019, 43, 5813–5819. 10.1039/C8NJ06460A. [DOI] [Google Scholar]
- Patel D.; Vithalani R.; Modi C. K. Highly efficient FeNP-embedded hybrid bifunctional reduced graphene oxide for Knoevenagel condensation with active methylene compounds. New J. Chem. 2020, 44, 2868–2881. 10.1039/C9NJ05821D. [DOI] [Google Scholar]
- Jain K.; Chaudhuri S.; Pal K.; Das K. The Knoevenagel condensation using quinine as an organocatalyst under solvent-free conditions. New J. Chem. 2019, 43, 1299–1304. 10.1039/C8NJ04219E. [DOI] [Google Scholar]
- Zare E.; Rafiee Z. Cellulose stabilized Fe3O4 and carboxylate-imidazole and Co-based MOF growth as an exceptional catalyst for the Knoevenagel reaction. Appl. Organomet. Chem. 2020, 34, e5516 10.1002/aoc.5516. [DOI] [Google Scholar]
- Kalantari F.; Rezayati S.; Ramazani A.; Aghahosseini H.; Ślepokura K.; Lis T. Proline-Cu Complex Based 1, 3, 5-Triazine Coated on Fe3O4 Magnetic Nanoparticles: A Nanocatalyst for the Knoevenagel Condensation of Aldehyde with Malononitrile. ACS Appl. Nano Mater. 2022, 5, 1783–1797. 10.1021/acsanm.1c03169. [DOI] [Google Scholar]
- Yang Y.; Wang D.; Jiang P.; Gao W.; Cong R.; Yang T. Structure-induced Lewis-base Ga4B2O9 and its superior performance in Knoevenagel condensation reaction. Mol. Catal. 2020, 490, 110914 10.1016/j.mcat.2020.110914. [DOI] [Google Scholar]
- Wu J.; Hua W.; Yue Y.; Gao Z. A Highly Efficient Bifunctional Catalyst CoOx/tri-g-C3N4 for One-Pot Aerobic Oxidation–Knoevenagel Condensation Reaction. Catalysts 2020, 10, 712. 10.3390/catal10060712. [DOI] [Google Scholar]
- Miao Z.; Yang F.; Luan Y.; Shu X.; Ramella D. Synthesis of Fe3O4@ P4VP@ ZIF-8 core-shell microspheres and their application in a knoevenagel condensation reaction. J. Solid State Chem. 2017, 256, 27–32. 10.1016/j.jssc.2017.07.032. [DOI] [Google Scholar]
- Zengin N.; Burhan H.; Şavk A.; Göksu H.; Şen F. Synthesis of benzylidenemalononitrile by Knoevenagel condensation through monodisperse carbon nanotube-based NiCu nanohybrids. Sci. Rep. 2020, 10, 12758 10.1038/s41598-020-69764-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turpaev K.; Ermolenko M.; Cresteil T.; Drapier J. C. Benzylidenemalononitrile compounds as activators of cell resistance to oxidative stress and modulators of multiple signaling pathways. A structure–activity relationship study. Biochem. Pharmacol. 2011, 82, 535–547. 10.1016/j.bcp.2011.05.028. [DOI] [PubMed] [Google Scholar]
- Michel F.; Mercklein L.; de Paulet A. C.; Dore J. C.; Gilbert J.; Miquel J. The effect of various acrylonitriles and related compounds on prostaglandin biosynthesis. Prostaglandins 1984, 27, 69–84. 10.1016/0090-6980(84)90221-1. [DOI] [PubMed] [Google Scholar]
- Shahid M.; Misra A. A simple and sensitive intramolecular charge transfer fluorescent probe to detect CN– in aqueous media and living cells. Anal. Methods 2013, 5, 434–437. 10.1039/C2AY25921D. [DOI] [Google Scholar]
- Levitzki A.; Mishani E. Tyrphostins and other tyrosine kinase inhibitors. Annu. Rev. Biochem. 2006, 75, 93–109. 10.1146/annurev.biochem.75.103004.142657. [DOI] [PubMed] [Google Scholar]
- Hedayatnasab Z.; Abnisa F.; Daud W. M. A. W. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017, 123, 174–196. 10.1016/j.matdes.2017.03.036. [DOI] [Google Scholar]
- Gharibshahian M.; Mirzaee O.; Nourbakhsh M. Evaluation of superparamagnetic and biocompatible properties of mesoporous silica coated cobalt ferrite nanoparticles synthesized via microwave modified Pechini method. J. Magn. Magn. Mater. 2017, 425, 48–56. 10.1016/j.jmmm.2016.10.116. [DOI] [Google Scholar]
- Senthil V.; Gajendiran J.; Raj S. G.; Shanmugavel T.; Kumar G. R.; Reddy C. P. Study of structural and magnetic properties of cobalt ferrite (CoFe2O4) nanostructures. Chem. Phys. Lett. 2018, 695, 19–23. 10.1016/j.cplett.2018.01.057. [DOI] [Google Scholar]
- Taghavi Fardood S.; Moradnia F.; Mostafaei M.; Afshari Z.; Faramarzi V.; Ganjkhanlu S. Biosynthesis of MgFe2O4 magnetic nanoparticles and its application in photo-degradation of malachite green dye and kinetic study. Nanochem. Res. 2019, 4, 86–93. [Google Scholar]
- Aguiar A.; Michels L.; da Silva F.; Kern C.; Gomide G.; Ferreira C.; Depeyrot J.; Aquino R.; da Silva G. The use of a laponite dispersion to increase the hydrophilicity of cobalt-ferrite magnetic nanoparticles. Appl. Clay Sci. 2020, 193, 105663 10.1016/j.clay.2020.105663. [DOI] [Google Scholar]
- Sorbiun M.; Shayegan Mehr E.; Ramazani A.; Mashhadi Malekzadeh A. Biosynthesis of metallic nanoparticles using plant extracts and evaluation of their antibacterial properties. Nanochem. Res. 2018, 3, 1–16. 10.22036/NCR.2018.01.001. [DOI] [Google Scholar]
- Kalam A.; Al-Sehemi A. G.; Assiri M.; Du G.; Ahmad T.; Ahmad I.; Pannipara M. Modified solvothermal synthesis of cobalt ferrite (CoFe2O4) magnetic nanoparticles photocatalysts for degradation of methylene blue with H2O2/visible light. Results Phys. 2018, 8, 1046–1053. 10.1016/j.rinp.2018.01.045. [DOI] [Google Scholar]
- Chandekar K. V.; Shkir M.; Alshahrani T.; Ibrahim E. H.; Kilany M.; Ahmad Z.; Manthrammel M. A.; AlFaify S.; Kateb B.; Kaushik A. One-spot fabrication and in-vivo toxicity evaluation of core-shell magnetic nanoparticles. Mater. Sci. Eng. C 2021, 122, 111898 10.1016/j.msec.2021.111898. [DOI] [PubMed] [Google Scholar]
- Zhang H.; Wang J.; Zeng Y.; Wang G.; Han S.; Yang Z.; Li B.; Wang X.; Gao J.; Zheng L.; et al. Leucine-coated cobalt ferrite nanoparticles: Synthesis, characterization and potential biomedical applications for drug delivery. Phys. Lett. A 2020, 384, 126600 10.1016/j.physleta.2020.126600. [DOI] [Google Scholar]
- Mittal R.; Awasthi S. K. Bimetallic Oxide Catalyst for the Dehydrogenative Oxidation Reaction of Alcohols: Practical Application in the Synthesis of Value-Added Chemicals. ACS Sustainable Chem. Eng. 2022, 10, 1702–1713. 10.1021/acssuschemeng.1c07799. [DOI] [Google Scholar]
- Singh P.; Yadav P.; Mishra A.; Awasthi S. K. Green and mechanochemical one-pot multicomponent synthesis of bioactive 2-amino-4 H-benzo [b] pyrans via highly efficient amine-functionalized SiO2@ Fe3O4 nanoparticles. ACS Omega 2020, 5, 4223–4232. 10.1021/acsomega.9b04117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P.; Kakati P.; Singh P.; Awasthi S. K. Application of sulfonic acid fabricated cobalt ferrite nanoparticles as effective magnetic nanocatalyst for green and facile synthesis of benzimidazoles. Appl. Catal., A 2021, 612, 118005 10.1016/j.apcata.2021.118005. [DOI] [Google Scholar]
- Yadav P.; Awasthi S. K. Probing the catalytic activity of highly efficient sulfonic acid fabricated cobalt ferrite magnetic nanoparticles for the clean and scalable synthesis of dihydro, spiro and bis quinazolinones. New J. Chem. 2021, 45, 15928–15941. 10.1039/D1NJ01149A. [DOI] [Google Scholar]
- Sharma R. K.; Yadav S.; Sharma S.; Dutta S.; Sharma A. Expanding the horizon of multicomponent oxidative coupling reaction via the design of a unique, 3D copper isophthalate MOF-based catalyst decorated with mixed spinel CoFe2O4 nanoparticles. ACS Omega 2018, 3, 15100–15111. 10.1021/acsomega.8b02061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezayati S.; Kalantari F.; Ramazani A.; Sajjadifar S.; Aghahosseini H.; Rezaei A. Magnetic Silica-Coated Picolylamine Copper Complex [Fe3O4@ SiO2@ GP/Picolylamine-Cu (II)]-Catalyzed Biginelli Annulation Reaction. Inorg. Chem. 2022, 61, 992–1010. 10.1021/acs.inorgchem.1c03042. [DOI] [PubMed] [Google Scholar]
- Altun S.; Çakıroğlu B.; Özacar M.; Özacar M. A facile and effective immobilization of glucose oxidase on tannic acid modified CoFe2O4 magnetic nanoparticles. Colloids Surf., B 2015, 136, 963–970. 10.1016/j.colsurfb.2015.10.053. [DOI] [PubMed] [Google Scholar]
- Ren C.; Ding X.; Fu H.; Li W.; Wu H.; Yang H. Core–shell superparamagnetic monodisperse nanospheres based on amino-functionalized CoFe2O4@ SiO2 for removal of heavy metals from aqueous solutions. RSC Adv. 2017, 7, 6911–6921. 10.1039/C6RA27728D. [DOI] [Google Scholar]
- Donia A. M.; Atia A. A.; Al-Amrani W. A.; El-Nahas A. M. Effect of structural properties of acid dyes on their adsorption behaviour from aqueous solutions by amine modified silica. J. Hazard. Mater. 2009, 161, 1544–1550. 10.1016/j.jhazmat.2008.05.042. [DOI] [PubMed] [Google Scholar]
- Niknam E.; Moaddeli A.; Khalafi-Nezhad A. Palladium anchored on guanidine-terminated magnetic dendrimer (G3-Gu-Pd): An efficient nano-sized catalyst for phosphorous-free Mizoroki-Heck and copper-free Sonogashira couplings in water. J. Organomet. Chem. 2020, 923, 121369 10.1016/j.jorganchem.2020.121369. [DOI] [Google Scholar]
- Malinowska I.; Ryżyńska Z.; Mrotek E.; Klimczuk T.; Zielińska-Jurek A. Synthesis of CoFe2O4 nanoparticles: the effect of ionic strength, concentration, and precursor type on morphology and magnetic properties. J. Nanomater. 2020, 2020, 1–12. 10.1155/2020/9046219. [DOI] [Google Scholar]
- Datta S.; Mahapatra A.; Sett P.; Ghosh M.; Mallick P.; Chakrabarti P. Magnetic measurements, Raman and infrared spectra of metal–ligand complex derived from CoCl2·6H2O and 2-benzoyl pyridine. Bull. Mater. Sci. 2018, 41, 1–10. 10.1007/s12034-018-1560-z. [DOI] [Google Scholar]
- Agouriane E.; Rabi B.; Essoumhi A.; Razouk A.; Sahlaoui M.; Costa B.; Sajieddine M. Structural and magnetic properties of CuFe2O4 ferrite nanoparticles synthesized by co-precipitation. J. Mater. Environ. Sci 2016, 7, 4116–4120. [Google Scholar]
- Karade V. C.; Sharma A.; Dhavale R. P.; Shingte S. R.; Patil P. S.; Kim J. H.; Zahn D. R. T.; Chougale A. D.; Salvan G.; Patil P. APTES monolayer coverage on self-assembled magnetic nanospheres for controlled release of anticancer drug Nintedanib. Sci. Rep. 2021, 11, 5674 10.1038/s41598-021-84770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W.; Zhang C. Production of medium-chain structured lipids using dual acidic ionic liquids supported on Fe3O4@ SiO2 composites as magnetically recyclable catalysts. LWT 2018, 93, 71–78. 10.1016/j.lwt.2018.03.017. [DOI] [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/S0021889808042726. [DOI] [Google Scholar]
- Sheldrick G. XS. version 2013/1, Georg-August-Universität Göttingen, Germany, 2013; b) GM Sheldrick. Acta Crystallogr., Sect. A 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [Google Scholar]
- Zhang Y.; Liu J.-W. Ethyl 3-(4-chlorophenyl)-2-cyanoacrylate. Acta Crystallogr., Sect. E: Struct. Rep. Online 2006, 62, o5286–o5287. 10.1107/S1600536806044199. [DOI] [Google Scholar]
- Yuan X.; Wang Z.; Zhang Q.; Luo J. An intramolecular relay catalysis strategy for Knoevenagel condensation and 1, 3-dipolar cycloaddition domino reactions. RSC Adv. 2019, 9, 23614–23621. 10.1039/C9RA04081A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga G.; Kukovecz Á.; Kónya Z.; Sipos P.; Pálinkó I. Green and selective toluene oxidation–Knoevenagel-condensation domino reaction over Ce-and Bi-based CeBi mixed oxide mixtures. J. Catal. 2020, 381, 308–315. 10.1016/j.jcat.2019.11.011. [DOI] [Google Scholar]
- Yang Y.; Zhu W.; Shi B.; Lü C. Construction of a thermo-responsive polymer brush decorated Fe3O4@ catechol-formaldehyde resin core–shell nanosphere stabilized carbon dots/PdNP nanohybrid and its application as an efficient catalyst. J. Mater. Chem. A 2020, 8, 4017–4029. 10.1039/C9TA12614G. [DOI] [Google Scholar]
- Qian B.; Wang F.; Li D.; Li Y.; Zhang B.; Zhu J. Preparation of a Pickering emulsion by modification of an amine-functionalized graphene oxide surface with organosilane: efficient catalyst for the Knoevenagel condensation of malononitrile with aldehydes at mild temperature. New J. Chem. 2020, 44, 5995–6002. 10.1039/C9NJ06097A. [DOI] [Google Scholar]
- Zhang L.; Zhang J.; Wei S.; Li S.; Ma X. Amine-functionalized hollow mesoporous nano-bowl with bulky acid-imprinted free space around base sites and DMF-annealed mesoporous channels as an efficient solid base catalyst. Appl. Catal., A 2020, 600, 117560 10.1016/j.apcata.2020.117560. [DOI] [Google Scholar]
- Jin F. An excellently stable heterovalent copper–organic framework based on Cu4I4 and Cu (COO) 2N2 SBUs: The catalytic performance for CO2 cycloaddition reaction and Knoevenagel condensation reaction. Inorg. Chem. Commun. 2020, 116, 107940 10.1016/j.inoche.2020.107940. [DOI] [Google Scholar]
- Kannappan L.; Rajmohan R. Synthesis of structurally enhanced magnetite cored poly (propyleneimine) dendrimer nanohybrid material and evaluation of its functionality in sustainable catalysis of condensation reactions. React. Funct. Polym. 2020, 152, 104579 10.1016/j.reactfunctpolym.2020.104579. [DOI] [Google Scholar]
- Chen H.; Fan L.; Zhang X. Highly Robust 3s–3d {CaZn}–Organic Framework for Excellent Catalytic Performance on Chemical Fixation of CO2 and Knoevenagel Condensation Reaction. ACS Appl. Mater. Interfaces 2020, 12, 54884–54892. 10.1021/acsami.0c18267. [DOI] [PubMed] [Google Scholar]
- Xi F.-G.; Liu H.; Yang N.-N.; Gao E.-Q. Aldehyde-tagged zirconium metal–organic frameworks: a versatile platform for postsynthetic modification. Inorg. Chem. 2016, 55, 4701–4703. 10.1021/acs.inorgchem.6b00598. [DOI] [PubMed] [Google Scholar]
- Chen H.; Zhang Z.; Hu T.; Zhang X. Nanochannel {InZn}–Organic framework with a high catalytic performance on CO2 chemical fixation and Deacetalization–Knoevenagel condensation. Inorg. Chem. 2021, 60, 16429–16438. 10.1021/acs.inorgchem.1c02262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available in the published article and its accompanying Supporting Information.