Abstract
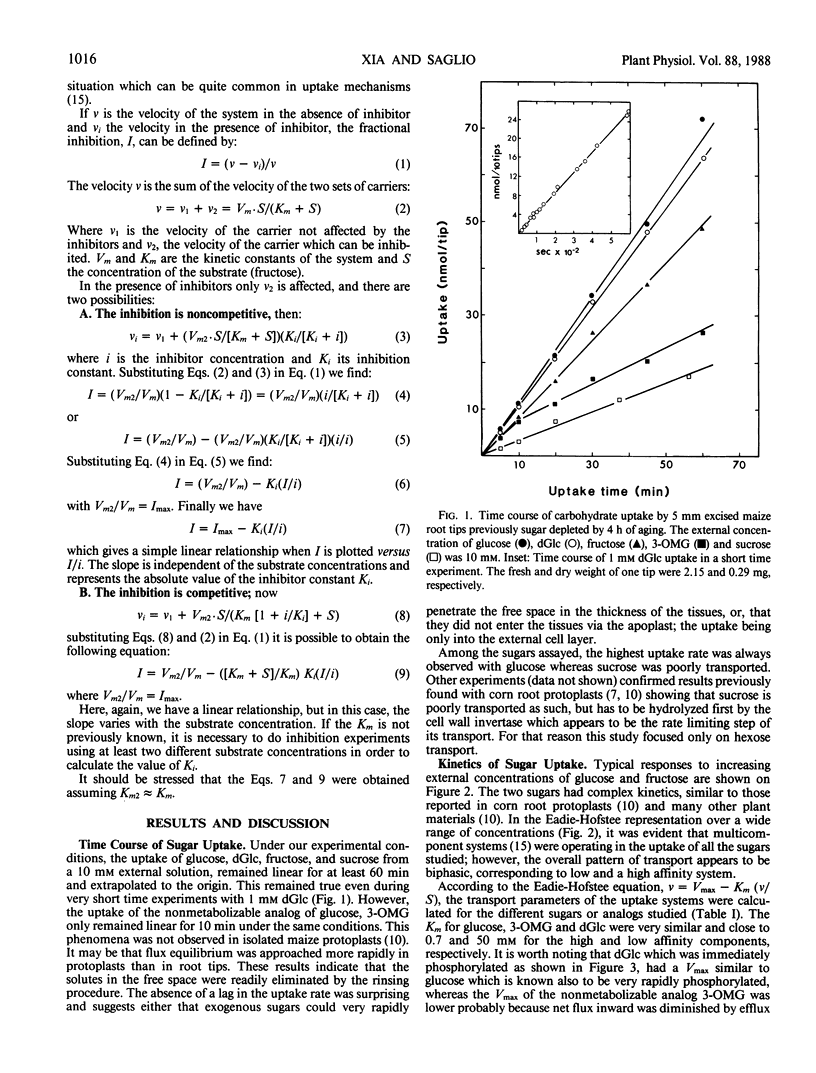
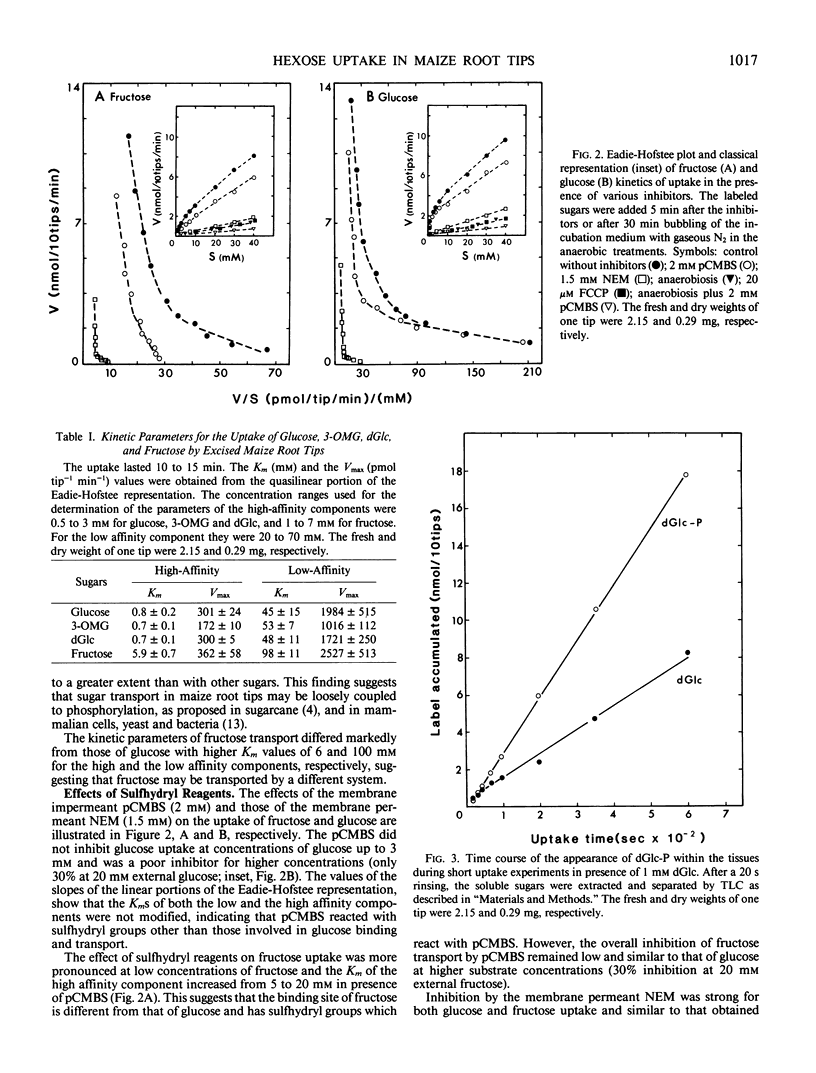
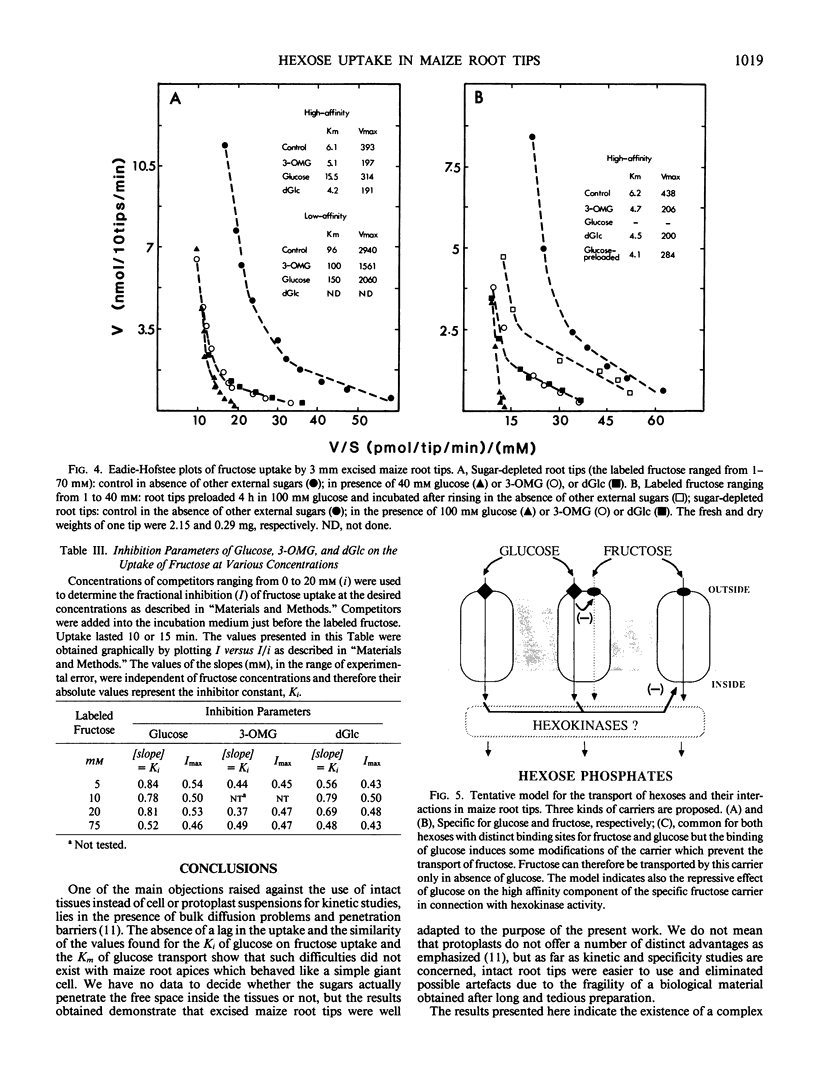
Sugar-depleted excised maize (Zea mays L.) root tips were used to study the kinetics and the specificity of hexose uptake. It was found that difficulties induced by bulk diffusion and penetration barriers did not exist with root tips. Several lines of evidence indicate the existence of a complex set of uptake systems for hexoses showing an overall biphasic dependence on external sugar concentrations. The results suggest that the high and the low affinity components might be located on the same carrier. One uptake system was specific for fructose, but the high affinity component was repressed by high concentrations of external glucose. A second system was specific for glucose and its analogs (2-deoxy-d-glucose and 3-O-methyl-d-glucose), and a third one, more complex, had a high affinity for glucose and its analogs but could transport fructose when glucose was not present in the external solution. A simple method is proposed to determine the inhibitor constants in competition experiments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bisson L. F., Fraenkel D. G. Expression of kinase-dependent glucose uptake in Saccharomyces cerevisiae. J Bacteriol. 1984 Sep;159(3):1013–1017. doi: 10.1128/jb.159.3.1013-1017.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Transport of 6-deoxyglucose in Saccharomyces cerevisiae. J Bacteriol. 1983 Sep;155(3):995–1000. doi: 10.1128/jb.155.3.995-1000.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J. E. Sugar transport in immature internodal tissue of sugarcane: I. Mechanism and kinetics of accumulation. Plant Physiol. 1972 Jan;49(1):82–86. doi: 10.1104/pp.49.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore T., Lo T. C. Hexose transport in L6 rat myoblasts. I. Rate-limiting step, kinetic properties, and evidence for two systems. J Cell Physiol. 1986 Apr;127(1):95–105. doi: 10.1002/jcp.1041270113. [DOI] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Lin W., Sadler N. L., Franceschi V. R. Pathway of Phloem unloading of sucrose in corn roots. Plant Physiol. 1983 Jun;72(2):362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J. M., Cirillo V. P. Glucose transport in a kinaseless Saccharomyces cerevisiae mutant. J Bacteriol. 1987 Jul;169(7):2932–2937. doi: 10.1128/jb.169.7.2932-2937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Schmitt M. R., Hitz W. D., Giaquinta R. T. Sugar transport in isolated corn root protoplasts. Plant Physiol. 1984 Dec;76(4):894–897. doi: 10.1104/pp.76.4.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Schmitt M. R., Hitz W. D., Giaquinta R. T. Sugar transport into protoplasts isolated from developing soybean cotyledons : I. Protoplast isolation and general characteristics of sugar transport. Plant Physiol. 1984 Aug;75(4):936–940. doi: 10.1104/pp.75.4.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D., Devadatham M. S. Sugar transport in Coprinus cinereus. Biochim Biophys Acta. 1979 Feb 2;550(3):515–526. doi: 10.1016/0005-2736(79)90153-6. [DOI] [PubMed] [Google Scholar]
- Naftalin R. J., Smith P. M. A model for accelerated uptake and accumulation of sugars arising from phosphorylation at the inner surface of the cell membrane. Biochim Biophys Acta. 1987 Feb 12;897(1):93–111. doi: 10.1016/0005-2736(87)90318-x. [DOI] [PubMed] [Google Scholar]
- Saglio P. H., Drew M. C., Pradet A. Metabolic Acclimation to Anoxia Induced by Low (2-4 kPa Partial Pressure) Oxygen Pretreatment (Hypoxia) in Root Tips of Zea mays. Plant Physiol. 1988 Jan;86(1):61–66. doi: 10.1104/pp.86.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H. Effect of path or sink anoxia on sugar translocation in roots of maize seedlings. Plant Physiol. 1985 Feb;77(2):285–290. doi: 10.1104/pp.77.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Pradet A. Soluble Sugars, Respiration, and Energy Charge during Aging of Excised Maize Root Tips. Plant Physiol. 1980 Sep;66(3):516–519. doi: 10.1104/pp.66.3.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio P. H., Raymond P., Pradet A. Metabolic Activity and Energy Charge of Excised Maize Root Tips under Anoxia: CONTROL BY SOLUBLE SUGARS. Plant Physiol. 1980 Dec;66(6):1053–1057. doi: 10.1104/pp.66.6.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. F., Copeland L. Hexokinase II of Pea Seeds. Plant Physiol. 1981 Nov;68(5):1123–1127. doi: 10.1104/pp.68.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]



