Abstract
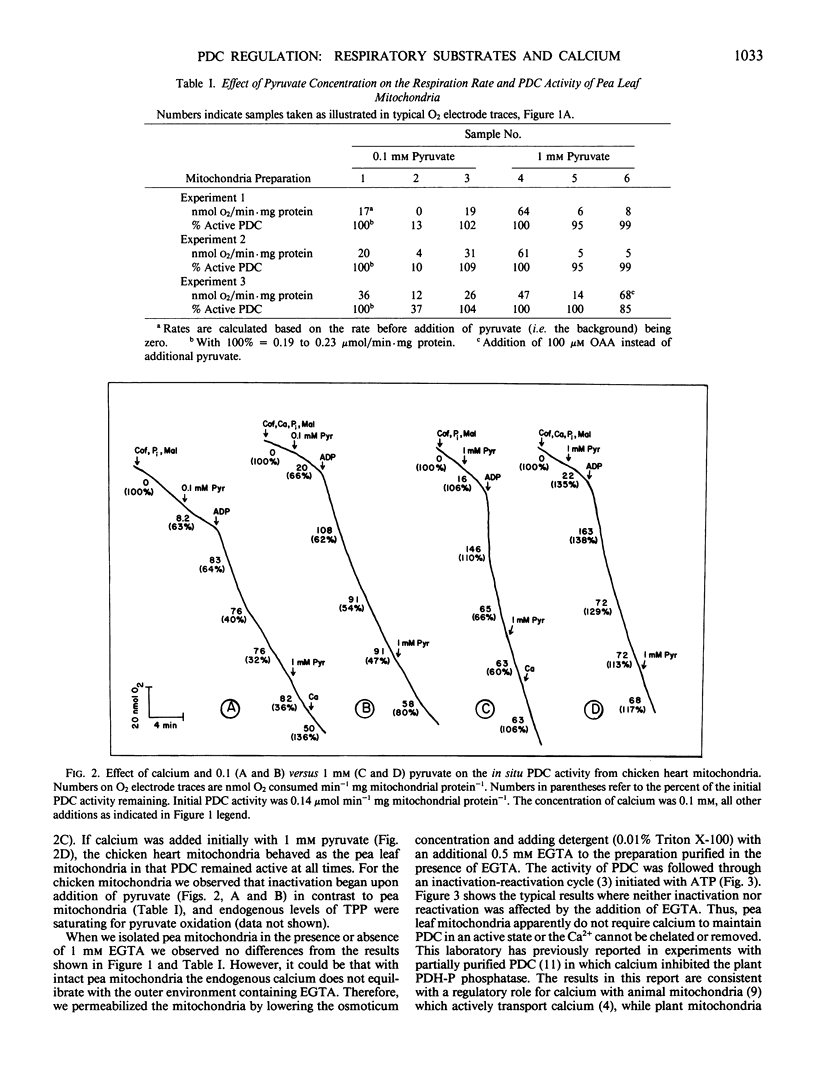
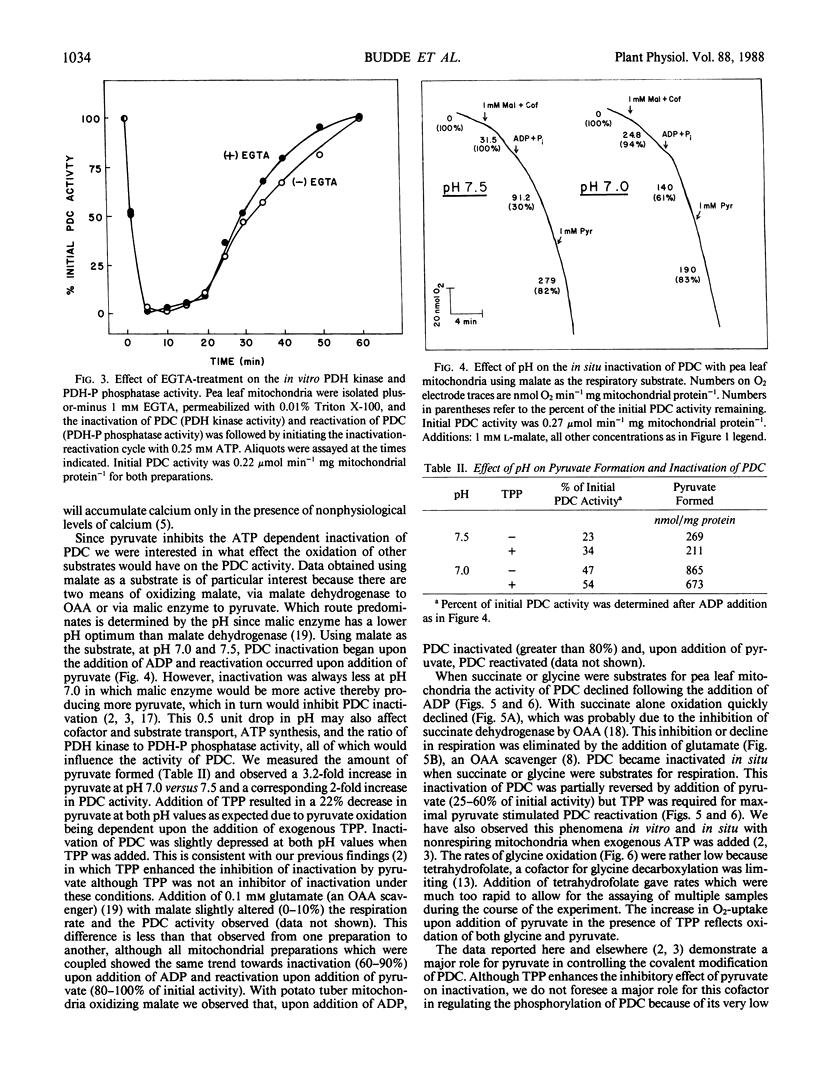
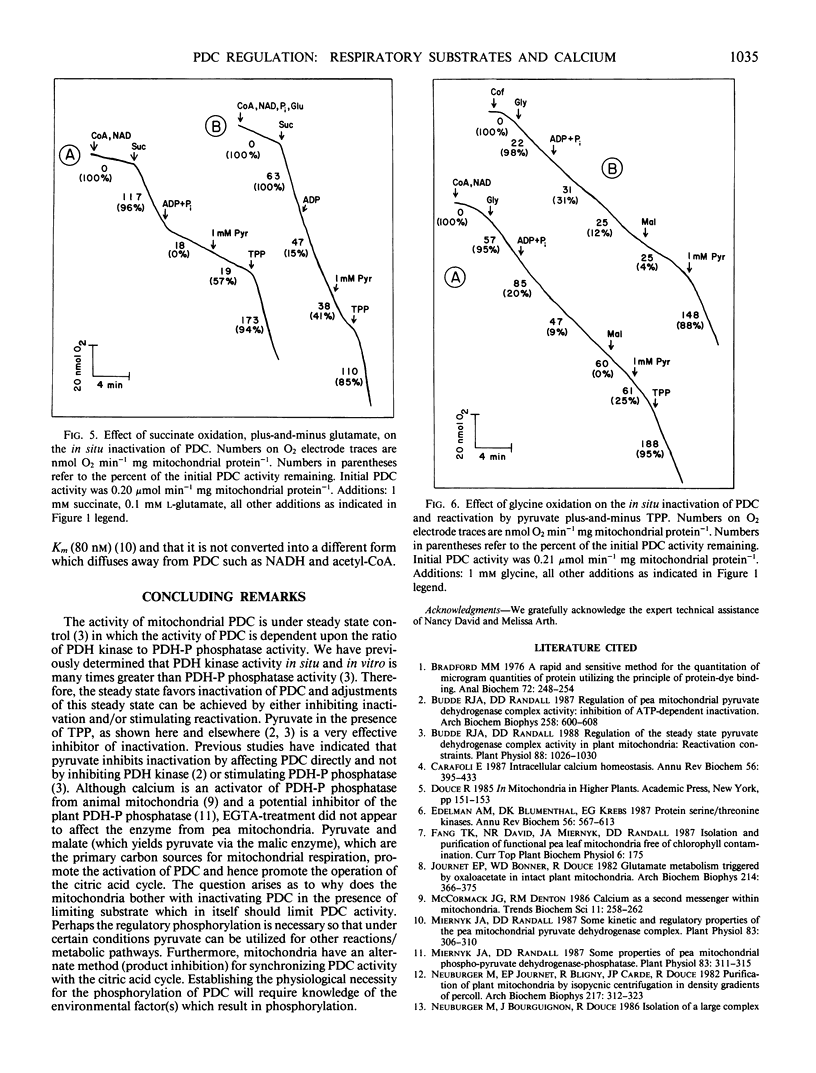
The activity of the pyruvate dehydrogenase complex (PDC), as controlled by reversible phosphorylation, was studied in situ with mitochondria oxidizing dfifferent substrates. PDCs from both plant and animal tissues were inactivated when pyruvate became limiting. The PDC did not inactivate in the presence of saturating levels of pyruvate. Calcium stimulated reactivation of PDC in chicken heart but not pea (Pisum sativum L.) leaf mitochondria. With pea leaf mitochondria oxidizing malate, inactivation of PDC was pH dependent corresponding to the production of pyruvate via malic enzyme. When pea leaf mitochondria oxidized succinate or glycine, PDC was inactivated. This inactivation was reversed by the addition of pyruvate. Reactivation by pyruvate was enhanced by the addition of thiamine pyrophosphate, as previously observed with nonrespiring mitochondria. These results indicate a major role for pyruvate in regulating the covalent modification of the PDC.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Randall D. D. Regulation of pea mitochondrial pyruvate dehydrogenase complex activity: inhibition of ATP-dependent inactivation. Arch Biochem Biophys. 1987 Nov 1;258(2):600–606. doi: 10.1016/0003-9861(87)90382-1. [DOI] [PubMed] [Google Scholar]
- Budde R. J., Randall D. D. Regulation of steady state pyruvate dehydrogenase complex activity in plant mitochondria : reactivation constraints. Plant Physiol. 1988 Dec;88(4):1026–1030. doi: 10.1104/pp.88.4.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Journet E. P., Bonner W. D., Douce R. Glutamate metabolism triggered by oxaloacetate in intact plant mitochondria. Arch Biochem Biophys. 1982 Mar;214(1):366–375. doi: 10.1016/0003-9861(82)90041-8. [DOI] [PubMed] [Google Scholar]
- Miernyk J. A., Randall D. D. Some kinetic and regulatory properties of the pea mitochondrial pyruvate dehydrogenase complex. Plant Physiol. 1987 Feb;83(2):306–310. doi: 10.1104/pp.83.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernyk J. A., Randall D. D. Some properties of pea mitochondrial phospho-pyruvate dehydrogenase-phosphatase. Plant Physiol. 1987 Feb;83(2):311–315. doi: 10.1104/pp.83.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuburger M., Journet E. P., Bligny R., Carde J. P., Douce R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch Biochem Biophys. 1982 Aug;217(1):312–323. doi: 10.1016/0003-9861(82)90507-0. [DOI] [PubMed] [Google Scholar]
- Randle P. J. Mitochondrial 2-oxoacid dehydrogenase complexes of animal tissues. Philos Trans R Soc Lond B Biol Sci. 1983 Jul 5;302(1108):47–57. doi: 10.1098/rstb.1983.0037. [DOI] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed L. J. Regulation of mammalian pyruvate dehydrogenase complex by a phosphorylation-dephosphorylation cycle. Curr Top Cell Regul. 1981;18:95–106. doi: 10.1016/b978-0-12-152818-8.50012-8. [DOI] [PubMed] [Google Scholar]
- Rubin P. M., Randall D. D. Regulation of plant pyruvate dehydrogenase complex by phosphorylation. Plant Physiol. 1977 Jul;60(1):34–39. doi: 10.1104/pp.60.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T. P., Oestreicher G., Hogue P. Regulation of Succinate Dehyrogenase in Higher Plants: I. Some General Characteristics of the Membrane-bound Enzyme. Plant Physiol. 1973 Dec;52(6):616–621. doi: 10.1104/pp.52.6.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P. P., Ferguson-Miller S. M., Suelter C. H. Isolation of highly coupled heart mitochondria in high yield using a bacterial collagenase. Methods Enzymol. 1986;125:16–27. doi: 10.1016/s0076-6879(86)25004-1. [DOI] [PubMed] [Google Scholar]



