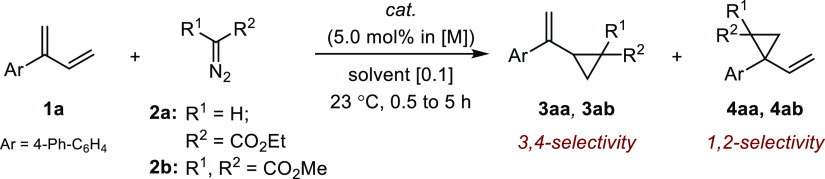
Abstract
A Pd-catalyzed 3,4-regioselective cyclopropanation of 2-substituted 1,3-dienes by decomposition of diazo esters is reported. The vinylcyclopropanes generated are isolated in practical chemical yields with high levels of regioselectivity but low diastereoselectivity. The system operates under mild reaction conditions, is scalable, and tolerates various sensitive functional groups. A series of original postcatalytic derivatizations is presented to highlight the synthetic potential of the catalytic method.
Keywords: palladium catalysis, conjugated dienes, selective catalysis, cyclopropanation, vinylcyclopropanes
Vinylcyclopropanes (VCPs) are highly prevalent structural motifs in natural and synthetic bioactive molecules.1−5 Owing to their propensity to ring-open in the presence of transition-metal catalysts, their reactivity has been widely studied and they are now frequently used as platforms for further transformations.6−12 Retrosynthetically, the transition-metal-catalyzed cyclopropanation of dienes based on diazoalkane decomposition ranks among the most direct routes for their preparation.13,14 In practice, while this is certainly true for symmetrical substrates where both alkenes are equivalent, the situation is more contrasted for unsymmetrical 1,3-dienes. Indeed, the highly enantio- and diastereoselective Cu-catalyzed cyclopropanation of 2,5-dimethyl-2,4-hexadiene (DMHD) for the production of pyrethroids was brought to industrial scale by Aratani and his group at Sumimoto Co., in the 1980s (Figure 1A).2 Today, it still constitutes one of the most significant achievements of selective homogeneous catalysis. In comparison, until recently, the cyclopropanation of 1,3-dienes featuring two distinct alkenes was considered of poor synthetic utility because of the low levels of regio- and diastereoselectivity obtained with the transition-metal catalysts typically used for simple olefins.15−17 Two recent studies have begun to address these shortcomings. Our group has shown that, using readily available diazo esters, the chiral Cu-bisoxazoline system catalyzes the cyclopropanation of 2-substituted 1,3-dienes with excellent levels of regio- and enantioselectivity but modest trans/cis selectivity (from 1:1 to 2:1) (Figure 1B). Because the ester stereocenter (C5) is controlled by the chiral ligand, the lack of diastereocontrol at C2 was circumvented by engaging the VCP mixtures in a subsequent Rh-catalyzed stereoconvergent intermolecular (5 + 2) cycloaddition with a variety of alkynes. Overall, this sequential approach yielded 7-membered rings with high levels of enantiopurity.18 Concurrently to this study, Uyeda and co-workers reported a unique dinuclear Ni catalyst for the cyclopropanation of branched dienes using silylated diazoalkanes. Cyclopropanation occurred exclusively at the most substituted double bond (rr(1,2/3,4) > 20:1), and the corresponding racemic VCPs were isolated with moderate to excellent levels of diastereoselectivity (Figure 1C). Quite notably, an unusual diradical mechanism distinct from the classical (2 + 1) cycloaddition involving M = CR2 intermediates was established.19 Following these advances, we sought to identify a complementary catalytic system for the regioselective cyclopropanation of the terminal alkene in 2-substituted 1,3-dienes (Figure 1D). We report herein the results of our investigations in this direction.
Figure 1.
(A) Cu-catalyzed enantioselective cyclopropanation of DMHD. (B) Cu-catalyzed 1,2-regioselective and enantioselective cyclopropanation of branched dienes. (C) Ni-catalyzed 1,2-regioselective, and diastereoselective cyclopropanation of 2-substituted 1,3-dienes. (D) Pd-catalyzed 3,4-regioselective cyclopropanation of 2-substituted 1,3-dienes.
The renewed interest in Ni and Pd catalysis for the development of cyclopropanation reactions prompted us to initiate our investigations with the evaluation of group X transition-metal precatalysts using branched diene 1a as a model substrate and ethyl diazo acetate 2a (Table 1).20−26 Whereas no reaction was observed with several nickel sources, most of the Pd(II) precatalysts yielded diethyl fumarate and diethyl maleate (entries 1–3 and 5–7). Cyclopropanation occurred to a marginal extent using Pd(OAc)2 but with excellent 3,4-regioselectivity (12% conv., rr3/4 > 20:1; entry 4). The use of Pd2(dba)3 (>98% purity based on Ananikov’s method)27 led to a noticeable increase in catalytic activity, a similarly high level of regiocontrol, but essentially no diastereocontrol (entry 8). While catalytic inhibition was observed when P- or N-based ligands were employed, 64% conv. in 3a (rr3/4 > 20:1) was achieved in tetrahydrofuran (THF) after a brief solvent survey (entries 9–12). The commercially available [(NHC)Pd(0)] complexes C2 and C3 displayed significant reactivity and selectivity but did not outcompete Pd2(dba)3 (entries 13 and 14). In the absence of Lewis acid, no polymerization of ethyl diazo acetate was observed with C2 and C3.28,29 Finally, we found that the optimal results were obtained with C4, an underused though readily available Pd(0) precursor (69% conv., rr3/4 > 20:1, trans/cis 1:1; entry 14).30 Under these conditions, the less reactive diazomalonate 2b enabled the regioselective cyclopropanation of 1a into VCP 3b in 47% conversion (rr3/4 > 20:1), a result that could not be improved at higher temperature (entries 19 and 20).
Table 1. Reaction Optimizationa.
| entry | catalyst | 2 | solvent | conv. (%)b | rr (3,4/1,2)b | trans/cisb |
|---|---|---|---|---|---|---|
| 1 | Ni(cod)2 | 2a | toluene | nrc | ||
| 2 | Ni(cod)(DQ) | 2a | toluene | nr | ||
| 3 | NiCl2(PPh3)2 | 2a | toluene | nr | ||
| 4 | Pd(OAc)2 | 2a | toluene | 12 | >20:1 | 1.4:1.0 |
| 5 | PdCl2(cod) | 2a | toluene | nr | ||
| 6 | (CPhos)Pd(G3) | 2a | toluene | nr | ||
| 7 | C1 | 2a | toluene | nr | ||
| 8 | Pd2(dba)3 | 2a | toluene | 37 | >20:1 | 1.3:1.0 |
| 9 | Pd2(dba)3/L1 | 2a | toluene | 9 | >20:1 | 2.0:1.0 |
| 10 | Pd2(dba)3/PCy3d | 2a | toluene | <5 | nde | nd |
| 11 | Pd2(dba)3 | 2a | CH2Cl2 | 31 | >20:1 | 1.5:1.0 |
| 12 | Pd2(dba)3 | 2a | THF | 64 | >20:1 | 1.2:1.0 |
| 13 | C2 | 2a | THF | 38 | >20:1 | 2.0:1.0 |
| 14 | C3 | 2a | THF | 41 | >20:1 | 1.2:1.0 |
| 15 | C4 | 2a | THF | 69 | >20:1 | 1.0:1.0 |
| 16 | C4/L1 | 2a | THF | nr | ||
| 17 | C4/PCy3d | 2a | THF | 18 | >20:1 | 1.0:1.0 |
| 18 | C3 | 2b | THF | nr | ||
| 19 | C4 | 2b | THF | 47 | >20:1 | nag |
| 20f | C4 | 2b | THF | 23 | >20:1 | na |
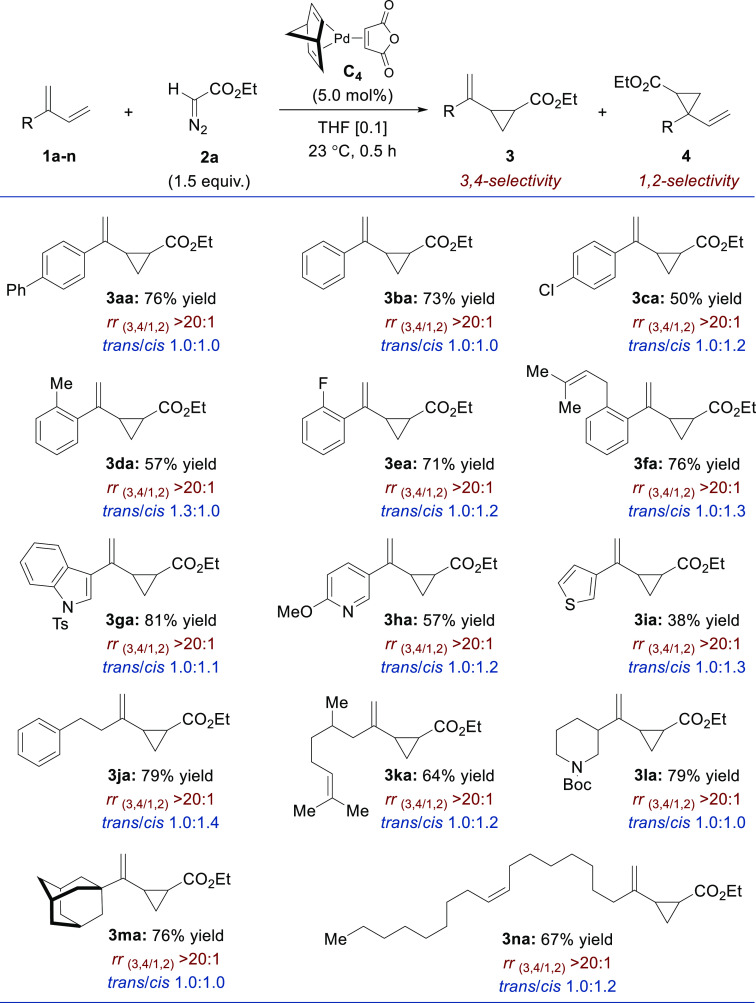
The generality of the optimized protocol was subsequently evaluated with a representative selection of 2-substituted 1,3-dienes 1a–n using ethyl diazo acetate 2a (Figure 2). Substrates containing an electron-poor, an electron-neutral, an electron-rich aromatic ring, or a heteroaromatic substituent delivered the vinylcyclopropanes in usually high yield, low cis/trans ratio but with consistently excellent 3,4-regioselectivity. Only the 3-thiophene derivative 3ia was isolated in low yield (38% yield). Ortho-substitution was well-tolerated (3da–3fa). Primary, secondary, and even sterically demanding tertiary aliphatic derivatives were cyclopropanated with similar catalytic efficiency (3ja–3na). Among the diverse functional groups that were accommodated, the perfect chemo- and regioselectivity observed for substrates featuring a 1,2-(Z)-disubstituted alkenes (3na), as well as trisubstituted alkenes (3fa, 3ka), is particularly noticeable.
Figure 2.
Scope of the Pd-catalyzed 3,4-regioselective cyclopropanation of 2-substituted 1,3-dienes (0.5 mmol scale). Regio- and diastereoselectivity determined by 1H NMR. Yield after purification.
The optimized reaction conditions using C4 were next applied to other classes of 1,3-dienes (Figure 3). We found that terminal diene 1o underwent cyclopropanation with a very high level of 1,2-regioselectivity, affording 3oa in 86% yield (trans/cis 1:1). In contrast, symmetrical dienes such as 1p and 1q were less reactive, delivering 3pa and 3qa/3qa′ in 17% and 39% yields, respectively. The robustness of the cyclopropanation protocol was confirmed by successfully conducting the model reaction between 1a and 2a on a gram scale (Figure 4). Gratifyingly, the combined yield for this experiment was slightly improved, and, more importantly, we showed that both diastereoisomers could be separated by standard chromatographic purification affording 530 mg of trans-3aa and 560 mg of cis-3aa.
Figure 3.
Pd-catalyzed cyclopropanation of differently substituted 1,3-dienes (0.5–1.0 mmol scale). Regio- and diastereoselectivity determined by 1H NMR. aNot determined.
Figure 4.
Large-scale experiment. Regio- and diastereoselectivity determined by 1H NMR.
The synthetic utility of the VCP obtained was demonstrated through a series of comparative postcatalytic derivatizations using trans-3aa and cis-3aa or the corresponding primary alcohols trans-5aa and cis-5aa prepared following a standard reduction procedure (see the Supporting Information for details). We initiated our investigations by evaluating two protocols recently reported by the Marek group for the cyclopropanation and epoxidation of densely substituted alkenyl cyclopropyl carbynols.31 Our interest stemmed from the fact that—to the best of our knowledge—the substitution pattern of the VCP generated by the Pd-catalyzed cyclopropanation of 2-substituted 1,3-dienes had not been explored in previous studies.32−34 While we did not observe product formation using diiodomethane for the Zn-mediated Simmons–Smith–Furukawa cyclopropanation of either trans-5aa or cis-5aa, reactivity was restored with the use of chloroiodomethane (Figure 5A).35 Biscyclopropyl carbinol trans-6aa was obtained in a 53% yield after purification by column chromatography starting from trans-5aa. In contrast, when cis-5aa was subjected to similar reaction conditions, cyclopropanation of the C=C bond was accompanied by competitive O–H insertion, a feature that is commonly observed with transition metals.36,37 The biscyclopropyl carbinol cis-6aa and the biscyclopropyl methyl ether cis-7aa thus generated could be separated by column chromatography and isolated in 28% and 48% yields, respectively. While no reaction occurred when attempting to perform a V-catalyzed cyclopropanation of trans-5aa using tBuOOH as an oxidant, alkenyl cyclopropyl carbynol cis-5aa afforded 8aa as a single diastereoisomer (Figure 5B).38−40 Unexpectedly, benzoylation of 8aa using 3,5-dinitrobenzoyl chloride led to the diastereoselective formation of the 3-oxabicyclo[3.1.0]hexane derivative 9aa, generated through intramolecular SN2 ring-opening of the epoxide by the pendant alcohol functionality and subsequent benzoylation. The relative stereochemistry of the three contiguous stereocenters in 8aa and 9aa was assigned by growing crystals of suitable quality for X-ray analysis of the latter. Finally, we found that Cu-catalyzed protoboration of both trans-3aa and cis-3aa led to the formation of the same ring-opened polyfunctional allyl boronate ester (E)-10aa in good yields and with excellent level of stereocontrol (E/Z > 20:1) (Figure 5C).41−44 Quite notably, the use of morpholine trifluoroacetic acid salt appeared crucial to avoid deborylation, a phenomenon we observed using various alcohols as proton sources. It is worth noting that the stereoconvergent nature of this unprecedented Cu-catalyzed protoboration alleviates the lack of stereocontrol of the Pd-catalyzed 3,4-regioselective cyclopropanation of branched dienes and obviates the need to separate the cis and trans VCPs.
Figure 5.
(A) Simmons–Smith–Furukawa cyclopropanations, (B) V-catalyzed epoxidations, and (C) Cu-catalyzed borylative ring-opening reactions.
In conclusion, we have developed a 3,4-regioselective Pd-catalyzed cyclopropanation of 2-substituted 1,3-dienes using readily available diazo esters. The vinylcyclopropanes generated are isolated in practical chemical yields with exquisite regioselectivity and low diastereoselectivity. The method operates under mild reaction conditions, is compatible with a wide number of potentially sensitive functional groups, and can be performed on a gram scale, thus allowing separation of the cis and trans isomers. A series of postcatalytic derivatizations served to highlight some of the intrinsic reactivity differences between these diastereoisomeric structures. Among these, an original stereoconvergent Cu-catalyzed ring-opening protoboration mitigated the modest level of stereocontrol of the catalytic cyclopropanation. Current efforts in our laboratory are directed toward understanding the origin of the high levels of regioselectivity obtained in the Pd-catalyzed 3,4-cyclopropanation of branched 1,3-dienes.
Experimental Section
General Cyclopropanation Procedure
In a N2-filled glovebox, a 15% solution of ethyl diazo acetate in toluene (2a or 2b, 0.75 mmol, 1.50 equiv) was added at once to a Schlenk flask containing the appropriate diene 1a–n (0.5 mmol, 1.00 equiv) and precatalyst C4, (5 mol %) in THF (5 mL, 0.1 M) at room temperature. The reaction mixture was stirred at 25 °C for 30 min. The Schlenk was taken out of the glovebox, and the reaction was quenched by dilution using THF (5 mL). The crude mixture was concentrated under reduced pressure and adsorbed in silica using CH2Cl2. Purification by flash chromatography using the appropriate eluent yielded the desired vinylcyclopropanes (VCPs).
Acknowledgments
Financial support was provided by the University of Geneva and the Swiss National Foundation (grants 200020_175489 and 200021_188490). Stéphane Rosset (University of Geneva) was acknowledged for measuring HRMS. Marion Pupier (University of Geneva) was acknowledged for assistance with NMR analyses. Dr. Sylvain Taillemaud (University of Geneva) was acknowledged for fruitful scientific discussions.
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsorginorgau.3c00024.
Experimental procedures and characterization of all new compounds and X-ray data for compound 9aa (PDF)
Author Contributions
CRediT: Agonist Kastrati formal analysis (lead), methodology (lead), writing-review & editing (supporting); Vincent Jaquier formal analysis (lead), methodology (lead), writing-review & editing (supporting); Michele Garbo data curation (lead), methodology (lead), writing-review & editing (supporting); Celine Besnard data curation (lead), formal analysis (lead); Clément Mazet conceptualization (lead), formal analysis (supporting), funding acquisition (lead), investigation (supporting), methodology (supporting), project administration (lead), resources (lead), supervision (lead), writing-original draft (lead), writing-review & editing (lead).
The authors declare no competing financial interest.
Supplementary Material
References
- Aratani T.; Yoneyoshi Y.; Nagase T. Asymmetric synthesis of chrysanthemic acid. An application of copper carbenoid reaction. Tetrahedron Lett. 1975, 16, 1707–1710. 10.1016/S0040-4039(00)72239-8. [DOI] [Google Scholar]
- Aratani T. Catalytic asymmetric synthesis of cyclopropanecarboxylic acids: an application of chiral copper carbenoid reaction. Pure Appl. Chem. 1985, 57, 1839–1844. 10.1351/pac198557121839. [DOI] [Google Scholar]
- Doyle M. P.; Protopopova M. N. New Aspects of Catalytic Asymmetric Cyclopropanation. Tetrahedron 1998, 54, 7919–7946. 10.1016/S0040-4020(98)00222-1. [DOI] [Google Scholar]
- Suenobu K.; Itagaki M.; Nakamura E. Reaction Pathway and Stereoselectivity of Asymmetric Synthesis of Chrysanthemate with the Aratani C1-Symmetric Salicylaldimine–Copper Catalyst. J. Am. Chem. Soc. 2004, 126, 7271–7280. 10.1021/ja031524c. [DOI] [PubMed] [Google Scholar]
- Talele T. T. The “cyclopropyl fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem. 2016, 59, 8712–8756. 10.1021/acs.jmedchem.6b00472. [DOI] [PubMed] [Google Scholar]
- Rubin M.; Rubina M.; Gevorgyan V. Transition Metal Chemistry of Cyclopropenes and Cyclopropanes. Chem. Rev. 2007, 107, 3117–3179. 10.1021/cr050988l. [DOI] [PubMed] [Google Scholar]
- Jiao L.; Yu Z.-X. Vinylcyclopropane Derivatives in Transition-Metal-Catalyzed Cycloadditions for the Synthesis of Carbocyclic Compounds. J. Org. Chem. 2013, 78, 6842–6848. 10.1021/jo400609w. [DOI] [PubMed] [Google Scholar]
- Souillart L.; Cramer N. Catalytic C-C Bond Activations via Oxidative Addition to Transition Metals. Chem. Rev. 2015, 115, 9410–9464. 10.1021/acs.chemrev.5b00138. [DOI] [PubMed] [Google Scholar]
- Fumagalli G.; Stanton S.; Bower J. F. Recent Methodologies That Exploit C-C Single-Bond Cleavage of Strained Ring Systems by Transition Metal Complexes. Chem. Rev. 2017, 117, 9404–9432. 10.1021/acs.chemrev.6b00599. [DOI] [PubMed] [Google Scholar]
- Meazza M.; Guo H.; Rios R. Synthetic applications of vinyl cyclopropane opening. Org. Biomol. Chem. 2017, 15, 2479–2490. 10.1039/C6OB02647H. [DOI] [PubMed] [Google Scholar]
- Pirenne V.; Muriel B.; Waser J. Catalytic Enantioselective Ring-Opening Reactions of Cyclopropanes. Chem. Rev. 2021, 121, 227–263. 10.1021/acs.chemrev.0c00109. [DOI] [PubMed] [Google Scholar]
- Wang J.; Blaszczyk S. A.; Li X.; Tang W. Transition Metal-Catalyzed Selective Carbon-Carbon Bond Cleavage of Vinylcyclopropanes in Cycloaddition Reactions. Chem. Rev. 2021, 121, 110–139. 10.1021/acs.chemrev.0c00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel H.; Marcoux J.-F.; Molinaro C.; Charette A. B. Stereoselective Cyclopropanation Reactions. Chem. Rev. 2003, 103, 977–1050. 10.1021/cr010007e. [DOI] [PubMed] [Google Scholar]
- Davies H. M. L.; Manning J. R. Catalytic C-H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. 10.1038/nature06485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. P.; Dorow R. L.; Tamblyn W. H.; Buhro W. E. Regioselectivities of Cyclopropanation Reactions with Ethyl Diazoacetate. Tetrahedron Lett. 1982, 23, 2161–2264. 10.1016/S0040-4039(00)87316-5. [DOI] [Google Scholar]
- Doyle M. P.; Dorow R. L.; Buhro W. E.; Griffin J. H.; Tamblyn W. H.; Trudell M. L. Stereoselectivity of Catalytic Cyclopropanation Reactions. Catalyst Dependence in Reactions of Ethyl Diazoacetate with Alkenes. Organometallics 1984, 3, 44–52. 10.1021/om00079a010. [DOI] [Google Scholar]
- Murelli R. P.; Catalán S.; Gannon M. P.; Snapper M. L. Ruthenium-Catalyzed Tandem Enyne-Cross Metathesis-Cyclopropanation: Three-Component Access to Vinyl Cyclopropanes. Tetrahedron Lett. 2008, 49, 5714–5717. 10.1016/j.tetlet.2008.07.119. [DOI] [Google Scholar]
- Garbo M.; Besnard C.; Guenée L.; Mazet C. Access to Optically Active 7-Membered Rings by a 2-Step Synthetic Sequence: Cu Catalyzed Stereoselective Cyclopropanation of Branched 1,3-Dienes/Rh-Catalyzed Stereoconvergent [5 + 2] Cycloaddition. ACS Catal. 2020, 10, 9604–9611. 10.1021/acscatal.0c02956. [DOI] [Google Scholar]
- Maity A. K.; Kalb A. E.; Zeller M.; Uyeda C. A Dinickel Catalyzed Cyclopropanation without the Formation of a Metal Carbene Intermediate. Angew. Chem., Int. Ed. 2021, 60, 1897–1902. 10.1002/anie.202011602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomilov Y. V.; Bordakov V. G.; Dolgii I. E.; Nefedov O. M. Reaction of diazoalkanes with unsaturated compounds. Cyclopropanation of olefins by diazomethane in presence of palladium compounds. Bull. Acad. Sci. USSR, Div. Chem. Sci. 1984, 3, 533–538. 10.1007/BF00995691. [DOI] [Google Scholar]
- Denmark S. E.; Stavenger R. A.; Faucher A.-M.; Edwards J. P. Cyclopropanation with Diazomethane and Bis(oxazoline)palladium(II) Complexes. J. Org. Chem. 1997, 62, 3375–3389. 10.1021/jo970044z. [DOI] [PubMed] [Google Scholar]
- Chen S.-F.; Ma J.; Wang J.-B. Palladium-catalyzed cyclopropanation of electron-deficient olefins with aryldiazocarbonyl compounds. Tetrahedron Lett. 2008, 49, 6781–6783. 10.1016/j.tetlet.2008.09.024. [DOI] [Google Scholar]
- Martín C.; Molina F.; Alvarez E.; Belderrain T. R. Stable N-Heterocyclic Carbene (NHC)–Palladium(0) Complexes as Active Catalysts for Olefin Cyclopropanation Reactions with Ethyl Diazoacetate. Chem. - Eur. J. 2011, 17, 14885–14895. 10.1002/chem.201102900. [DOI] [PubMed] [Google Scholar]
- Zhou Y.-Y.; Uyeda C. Reductive Cyclopropanations Catalyzed by Dinuclear Nickel Complexes. Angew. Chem., Int. Ed. 2016, 55, 3171–3175. 10.1002/anie.201511271. [DOI] [PubMed] [Google Scholar]
- Sakurai S.; Inagaki T.; Kodama T.; Yamanaka M.; Tobisu M. Palladium-Catalyzed Siloxycyclopropanation of Alkenes Using Acylsilanes. J. Am. Chem. Soc. 2022, 144, 1099–1105. 10.1021/jacs.1c11497. [DOI] [PubMed] [Google Scholar]
- Inagaki T.; Sakurai S.; Yamanaka M.; Tobisu M. Palladium-Catalyzed Silylacylation of Allenes Using Acylsilanes. Angew. Chem., Int. Ed. 2022, 61, e202202387 10.1002/anie.202202387. [DOI] [PubMed] [Google Scholar]
- Zalesskiy S. S.; Ananikov V. P. Pd2(dba)3 as a Precursor of Soluble Metal Complexes and Nanoparticles: Determination of Palladium Active Species for Catalysis and Synthesis. Organometallics 2012, 31, 2302–2309. 10.1021/om201217r. [DOI] [Google Scholar]
- Ihara E.; Ishiguro Y.; Yoshida N.; Hiraren T.; Itoh T.; Inoue K. (N-Heterocyclic Carbene) Pd/Borate Initiating Systems for Polymerization of Ethyl Diazoacetate. Macromolecules 2009, 42, 8608–8610. 10.1021/ma901857s. [DOI] [Google Scholar]
- Zhukhovitskiy A. V.; Kobylianskii I. J.; Thomas A. A.; Evans A. M.; Delaney C. P.; Flanders N. C.; Denmark S. E.; Dichtel W. R.; Toste F. D. A Dinuclear Mechanism Implicated in Controlled Carbene Polymerization. J. Am. Chem. Soc. 2019, 141, 6473–6478. 10.1021/jacs.9b01532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K.; Ueda F.; Hirai K.; Ishii Y. New mixed olefin complexes of palladium (0). A stabilization of the Pd(0) state with a combination of electron-donating and electron-withdrawing olefins. Chem. Lett. 1977, 6, 877–880. 10.1246/cl.1977.877. [DOI] [Google Scholar]
- Cohen A.; Siddaraju Y.; Marek I. Directed Diastereoselective Cyclopropanation and Epoxidation of Alkenyl Cyclopropyl Carbinol Derivatives. Org. Lett. 2022, 24, 8322–8325. 10.1021/acs.orglett.2c03305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons H. E.; Smith R. D. A New Synthesis of Cyclopropanes from Olefins. J. Am. Chem. Soc. 1958, 80, 5323–5324. 10.1021/ja01552a080. [DOI] [Google Scholar]
- Charette A. B.; Beauchemin A. Simmons-Smith Cyclopropanation Reaction. Org. React. 2001, 58, 1–415. 10.1002/0471264180.or058.01. [DOI] [Google Scholar]
- Nakamura M.; Hirai A.; Nakamura E. Reaction Pathways of the Simmons–Smith Reaction. J. Am. Chem. Soc. 2003, 125, 2341–2350. 10.1021/ja026709i. [DOI] [PubMed] [Google Scholar]
- Denmark S. E.; Edwards J. P. A Comparison of (Chloromethyl)- and (Iodomethyl)zinc Cyclopropanation Reagents. J. Org. Chem. 1991, 56, 6974–6981. 10.1021/jo00025a007. [DOI] [Google Scholar]
- Reactive Intermediate Chemistry; Moss R. A.; Platz M. S.; Jones M. Jr., Eds.; John Wiley & sons, Inc.: Hoboken, NJ, 2004. [Google Scholar]
- Zhu S.-F.; Zhou Q.-L. Transition-Metal-Catalyzed Enantioselective Heteroatom–Hydrogen Bond Insertion Reactions. Acc. Chem. Res. 2012, 45, 1365–1377. 10.1021/ar300051u. [DOI] [PubMed] [Google Scholar]
- Ligtenbarg A. G. J.; Hage R.; Feringa B. L. Catalytic Oxidations by Vanadium Complexes. Coord. Chem. Rev. 2003, 237, 89–101. 10.1016/S0010-8545(02)00308-9. [DOI] [Google Scholar]
- Mihelich E. D.; Daniels K.; Eickhoff D. J. Vanadium-Catalyzed Epoxidations. 2. Highly Stereoselective Epoxidations of Acyclic Homoallylic Alcohols Predicted by a Detailed Transition-State Model. J. Am. Chem. Soc. 1981, 103, 7690–7692. 10.1021/ja00415a067. [DOI] [Google Scholar]
- When the epoxidation was conducted with mCPBA, we observed a dr >10:1 based on 1H NMR analysis of the crude reaction mixture.
- For related (seminal) work on 1,3-dienes, see:; Sasaki Y.; Zhong C.; Sawamura M.; Ito H. Copper(I)-Catalyzed Asymmetric Monoborylation of 1,3-Dienes: Synthesis of Enantioenriched Cyclic Homoallyl- and Allylboronates. J. Am. Chem. Soc. 2010, 132, 1226–1227. 10.1021/ja909640b. [DOI] [PubMed] [Google Scholar]
- Sebelius S.; Olsson V. J.; Szabó K. J. Palladium Pincer Complex Catalyzed Substitution of Vinyl Cyclopropanes, Vinyl Aziridines, and Allyl Acetates with Tetrahydroxydiboron. An Efficient Route to Functionalized Allylboronic Acids and Potassium Trifluoro(allyl)borates. J. Am. Chem. Soc. 2005, 127, 10478–10479. 10.1021/ja052885q. [DOI] [PubMed] [Google Scholar]
- Chen C.; Wang H.; Li T.; Lu D.; Li J.; Zhang X.; Hong X.; Lu Z. Cobalt-Catalyzed Asymmetric Sequential Hydroboration/Isomerization/Hydroboration of 2-Aryl Vinylcyclopropanes. Angew. Chem., Int. Ed. 2022, 61, e202205619 10.1002/anie.202205619. [DOI] [PubMed] [Google Scholar]
- Lu D.; Chen C.; Zheng L.; Ying J.; Lu Z.. Regio- and Stereoselective Cobalt-Catalyzed Hydroboration of Vinylcyclopropanes to Access Homoallylic Boronates Organometallics 2023, 10.1021/acs.organomet.2c00592. [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this study are available in the published article and its Supporting Information.