Abstract
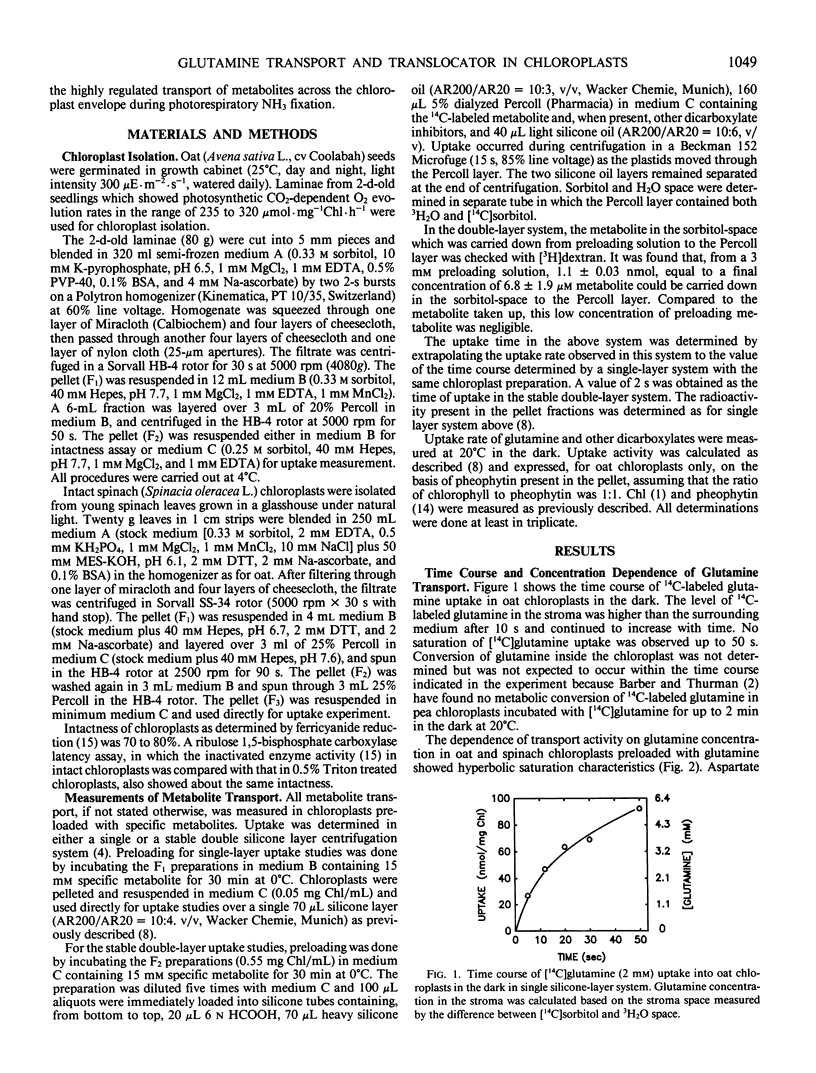
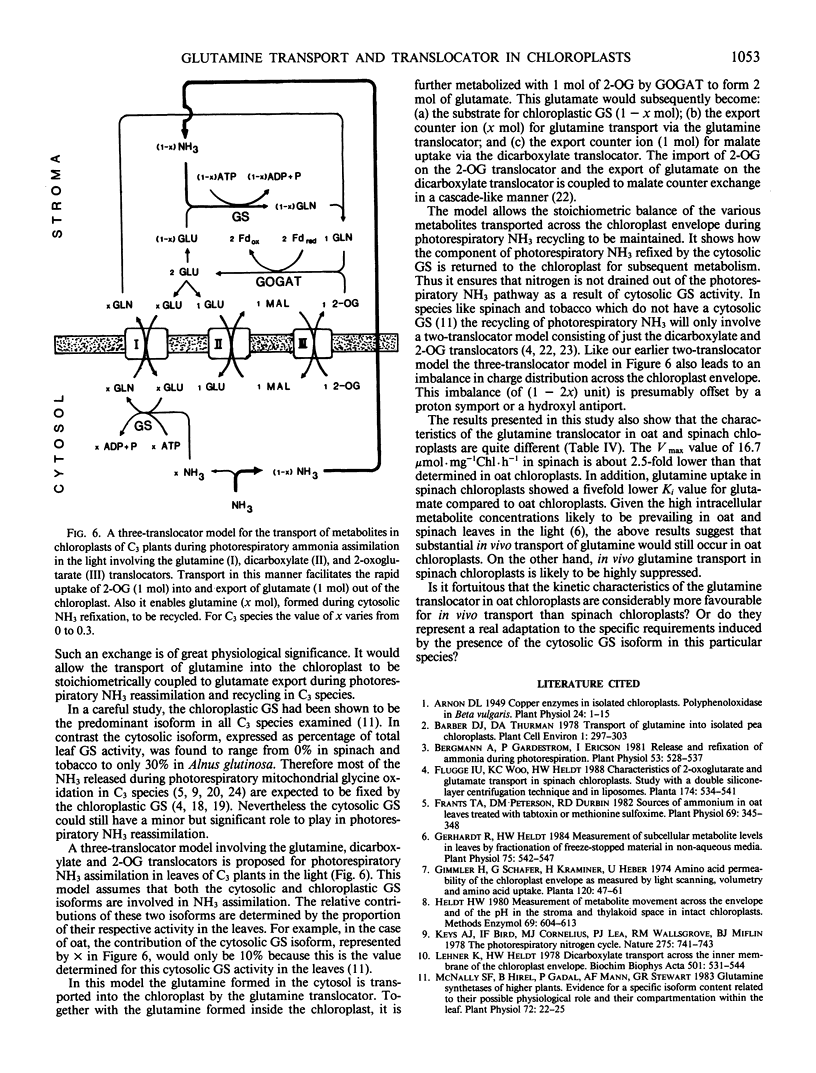
The transport of l-[14C]glutamine in oat (Avena sativa L.) and spinach (Spinacia oleracea L.) chloroplasts was studied by a conventional single-layer and a newly developed stable double-layer silicone oil filtering system. [14C]Glutamine was actively transported into oat chloroplasts against a concentration gradient. Metabolite uptake was greatly affected by the endogenous dicarboxylate pools, which could be easily changed by preloading the chloroplast with specific exogenous substrate. Glutamine uptake was decreased by 44 to 75% in oat chloroplasts preloaded with malate, 2-oxoglutarate (2-OG), and aspartate, but increased by 52% in chloroplasts preloaded with l-glutamate. On the other hand, the uptake of the other four dicarboxylates was decreased by 47 to 79% in chloroplasts preloaded with glutamine. In glutamine-preloaded chloroplasts the uptake of glutamine was inhibited only by l-glutamate. The observed inhibition by l-glutamate was competitive with an apparent Ki value of 32.1 millimolar in oat and 6.7 millimolar in spinach chloroplasts. This study indicates that there are two components involved in glutamine transport in chloroplasts. The major component was mediated via a specific glutamine translocator. It was specific for glutamine and did not transport other dicarboxylates except l-glutamate. A K0.5 value of 1.25 millimolar and Vmax of 45.5 micromoles per milligram of chlorophyll per hour were determined for the glutamine translocator in oat chloroplasts. The respective values were 1.0 millimolar and 16.7 micromoles per milligram of chlorophyll per hour in spinach chloroplasts. A three translocator model, involving the glutamine, dicarboxylate, and 2-OG translocators, is proposed for the reassimilation of photorespiratory NH3 in chloroplasts of C3 species. In this three-translocator model the additional transport of glutamine into the chloroplast is coupled to the export of glutamate via the glutamine translocator. This is an extension of the two-translocator model, involving the dicarboxylate and 2-OG translocators, proposed for spinach chloroplasts, (KC Woo, UI Flügge, HW Heldt 1987 Plant Physiol 84: 624-632).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz T. A., Peterson D. M., Durbin R. D. Sources of ammonium in oat leaves treated with tabtoxin or methionine sulfoximine. Plant Physiol. 1982 Feb;69(2):345–348. doi: 10.1104/pp.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardt R., Heldt H. W. Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in nonaqueous media. Plant Physiol. 1984 Jul;75(3):542–547. doi: 10.1104/pp.75.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner K., Heldt H. W. Dicarboxylate transport across the inner membrane of the chloroplast envelope. Biochim Biophys Acta. 1978 Mar 13;501(3):531–544. doi: 10.1016/0005-2728(78)90119-6. [DOI] [PubMed] [Google Scholar]
- McNally S. F., Hirel B., Gadal P., Mann A. F., Stewart G. R. Glutamine Synthetases of Higher Plants : Evidence for a Specific Isoform Content Related to Their Possible Physiological Role and Their Compartmentation within the Leaf. Plant Physiol. 1983 May;72(1):22–25. doi: 10.1104/pp.72.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville S. C., Ogren W. L. An Arabidopsis thaliana mutant defective in chloroplast dicarboxylate transport. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1290–1294. doi: 10.1073/pnas.80.5.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallsgrove R. M., Lea P. J., Miflin B. J. Distribution of the Enzymes of Nitrogen Assimilation within the Pea Leaf Cell. Plant Physiol. 1979 Feb;63(2):232–236. doi: 10.1104/pp.63.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Boyle F. A., Flugge I. U., Heldt H. W. N-ammonia assimilation, 2-oxoglutarate transport, and glutamate export in spinach chloroplasts in the presence of dicarboxylates in the light. Plant Physiol. 1987 Nov;85(3):621–625. doi: 10.1104/pp.85.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C. Effect of inhibitors on ammonia-, 2-oxoglutarate-, and oxaloacetate-dependent o(2) evolution in illuminated chloroplasts. Plant Physiol. 1983 Jan;71(1):112–117. doi: 10.1104/pp.71.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Flügge U. I., Heldt H. W. A Two-Translocator Model for the Transport of 2-Oxoglutarate and Glutamate in Chloroplasts during Ammonia Assimilation in the Light. Plant Physiol. 1987 Jul;84(3):624–632. doi: 10.1104/pp.84.3.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Morot-Gaudry J. F., Summons R. E., Osmond C. B. Evidence for the Glutamine Synthetase/Glutamate Synthase Pathway during the Photorespiratory Nitrogen Cycle in Spinach Leaves. Plant Physiol. 1982 Nov;70(5):1514–1517. doi: 10.1104/pp.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo K. C., Osmond C. B. Stimulation of ammonia and 2-oxoglutarate-dependent o(2) evolution in isolated chloroplasts by dicarboxylates and the role of the chloroplast in photorespiratory nitrogen recycling. Plant Physiol. 1982 Mar;69(3):591–596. doi: 10.1104/pp.69.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]


