Abstract
Background
Inflammatory, immune, and nutritional status are key factors in obstructive colorectal cancer (OCRC). This study aims to investigate the value of modified Naples prognostic score (M-NPS) in evaluating OCRC prognosis.
Methods
A total of 196 OCRC patients were retrospectively analyzed to construct M-NPS based on serum albumin (ALB), total cholesterol (CHOL), neutrophil:lymphocyte ratio (NLR), and lymphocyte:monocyte ratio (LMR), and then they were divided into three groups. The Kaplan–Meier (KM) method and Cox proportional hazard regression analysis were performed for overall survival (OS) and disease-free survival (DFS) of OCRC patients.
Results
Patients with high M-NPS had worse OS and DFS (P = 0.0001, P = 0.0011). Multivariate COX analysis showed that M-NPS was an independent prognostic factor for OCRC patients. Patients in the M-NPS 2 group had significantly worse OS (hazard ratio [HR] = 4.930 (95% confidence interval [95% CI], 2.217–10.964), P < 0.001) and DFS (HR = 3.508 (95% CI, 1.691–7.277), P < 0.001) than those in the 0 group.
Conclusion
M-NPS was an independent prognostic factor for OCRC patients; it might provide a potential reference for immunonutritional intervention in patients with obstruction.
Keywords: Obstructive colorectal cancer, Modified Naples prognostic score, Prognostic factors, Inflammatory, Nutritional status
Background
Colorectal cancer (CRC) is the third most prevalent cancer in the world, with acute colonic obstruction present in 8–29% of CRC patients [1–3]. Patients with obstructive CRC (OCRC) are often associated with poor nutritional status, prognosis and quality of survival. In addition to surgery as a routine emergency management, the increase in self-expanding metal stent (SEMS) implantation offers a new option to relieve obstructive symptoms [1, 2]. However, the overall prognosis of OCRC patients is still not promising. The construction of new prognostic biomarkers could better assist clinicians in making rational treatment decisions.
The inflammatory, immune and nutritional status of patients are considered to be closely related to the prognosis of CRC [4–7]. Inflammation can significantly increase the risk of cancer development, whereas OCRC usually has more serious local and systemic inflammatory reactions [8, 9]. The CRC tumor microenvironment has complex immune responses, and immunotherapy has become an important pillar of CRC treatment in recent years; moreover, different immune scoring systems for assessing CRC prognosis are constantly being developed [6]. In addition, the relationship between nutritional status and prognosis of CRC receives increased attention, especially in OCRC patients, where nutritional status significantly affects postoperative recovery, prognosis, and quality of survival [7, 10]. Therefore, many inflammatory immune markers have been studied to evaluate the prognosis of CRC. Neutrophil:lymphocyte ratio (NLR), platelet:lymphocyte ratio (PLR), lymphocyte:monocyte ratio (LMR), systemic immune–inflammatory index (SII), prognostic nutrition index (PNI), and Naples prognostic score (NPS) were found to be independent prognostic factors of CRC [7, 11–16]. However, few biomarkers used to evaluate the prognosis of OCRC patients require further exploration.
NPS is a prognostic score constructed by Galizia Gennaro et al. based on serum albumin (ALB), total cholesterol (CHOL), NLR, and LMR [15]. The value of NPS in predicting the prognosis of CRC, metastatic CRC (mCRC), gastric cancer, duodenal ampullary cancer, and non-small cell lung cancer [15, 17–20] has been confirmed by relevant studies, but no relevant exploration in OCRC has been conducted. In this study, we established a modified NPS (M-NPS) based on a cohort of patients with OCRC and explored its value in assessing the prognosis of OCRC.
Methods
Patient cohort
This study finally included 196 OCRC patients, who received treatment at Wuhan Union Hospital, Wuhan Central Hospital, and Jingzhou Central Hospital from December 2014 to May 2018 (Fig. 1). All patients with CRC complicated by colorectal obstruction confirmed by computerized tomography (CT), magnetic resonance imaging (MRI), and endoscopy were eligible. The exclusion criteria were as follows: personal history of prior, synchronous, or metachronous malignancy; inflammatory/hematologic disease and disease affecting immune or nutritional status. The patients were generally followed up every three months by the outpatient service with CT in the first two years and then annually for the next three to five years when no evidence of recurrence was observed. For the patients who did not visit our hospital as scheduled, telephone interviews were conducted to obtain treatment information and progression status. The end of follow up was in September 2021. Median follow-up time was 39 months. The present study has been approved by the ethics committee of Wuhan Union Hospital (No.2018-S377) and conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Fig. 1.
Flow diagram of study population selected
Procedures of treatment
All the enrolled patients received resection of colorectal cancer, and some patients received preoperative SEMS implantation according to their admission conditions and indications. 41 patients were treated with SEMS implantation and underwent surgery within two weeks after SEMS implantation. The interval between bridging is approximately 7–14 days. 104 patients underwent laparoscopic resection of colorectal cancer, and 92 patients underwent open resection of colorectal cancer. Five of the enrolled patients had distant metastases (liver metastases), all of which were single, and radical resection of the metastatic lesion was performed.
Data collection
The following clinicopathological features were collected from the patient’s medical records: age, sex, body mass index (BMI), American Society of Anesthesiologists Physical Status Classification (ASA class), tumor location, tumor size, tumor differentiation, TNM stage [21], vascular tumor thrombus, nerve invasion, SEMS implantation, chemotherapy, radiotherapy, lymph node ratio (LNR), laboratory examination included ALB, CHOL, whole blood count (neutrophils, lymphocytes, and monocytes), and CEA. Blood samples were taken within two weeks prior to radical surgery or stent placement, and M-NPS was calculated based on the results of this blood sample.
Construction of M-NPS
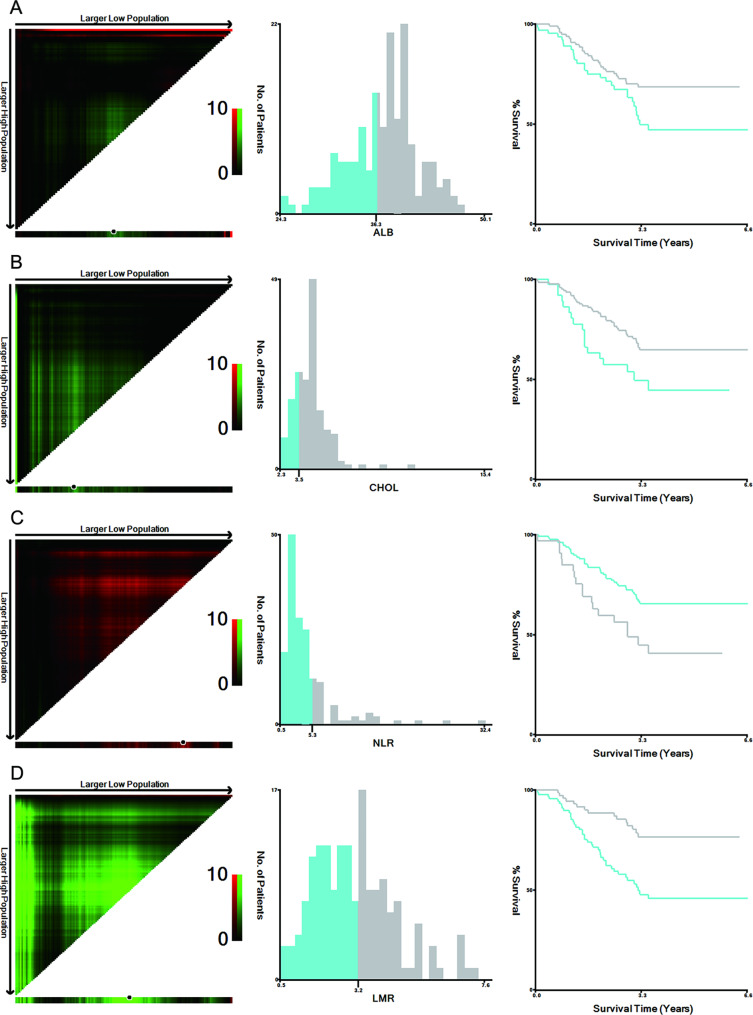
NPS was composed of ALB, CHOL, NLR, and LMR. Since conventional NPS did not demonstrate significant evaluation significance in this study cohort, based on the particularity of patients with obstruction, x-Tiles 3.6.1 (Yale University, New Haven, CT, USA) was used for data analysis to obtain the optimal cut-points of the four indicators (Fig. 2) [22]. The modified prognostic score composition is as follows: the score of ALB concentration > 36.3 g/L was 0, ≤ 36.3 g/L was 1; CHOL concentration > 3.48 mmol/L was 0, ≤ 3.48 mmol/L was 1; NLR ≤ 5.33 was 0, > 5.33 was 1; LMR > 3.19 was 0, and ≤ 3.19 was 1. The scores of the four indicators were M-NPS, and the patients with M-NPS of 0, 1 and 2, and 3 and 4 were assigned to groups 0, 1, and 2, respectively, for analysis.
Fig. 2.
Optimal cut-points of ALB, CHOL, NLR, and LMR were determined by X-tile analysis. The plot shows the χ2 log-rank values produced when dividing the cohort with optimal cut-points, producing high and low population. X-tile plots are shown in the left panels, red coloration of cut-points indicates an inverse correlation with survival, whereas green coloration represents direct associations. The optimal cut-point highlighted by the black circle in the left panels is shown on a histogram (middle panels), and Kaplan–Meier plots are presented in right panels. In terms of overall survival, the optimal cut-points of ALB (A), CHOL (B), NLR (C) and LMR (D) were 36.3, 3.48, 5.33, and 3.19, respectively
Statistical analysis
The main result of this study is the overall survival (OS), and the secondary result is disease-free survival (DFS). OS was defined as the time from the diagnosis of CRC with obstruction to the date of death, follow-up, or end of follow-up, whichever came first. DFS was calculated as the interval between the diagnosis of CRC with obstruction and the first documentation of disease recurrence, death, or end of follow-up, whichever came first. Continuous variables were expressed as the mean ± standard deviation or median and interquartile ranges (IQR). Mann–Whitney U or Kruskal–Wallis Chi-Squared was used to compare the differences of variables between groups. The categorical variables were expressed as frequency and percentage, and the differences between groups were compared using Pearson Chi-square test, Fischer precision test, or Spearman’s rank correlation test. Some variables were dichotomized using normal or median values, and X-tile software was used to analyze the four basic indicators of M-NPS for classification conversion. Kaplan–Meier (KM) method and log-rank test were applied to compare the survival difference between groups. The receiver operating characteristic (ROC) curve of 12-month, 24-month, and 36-month was established, and the area under curve (AUC) was analyzed to compare the predictive ability of the prognosis scoring system. Cox proportional hazard regression was used to calculate hazard ratios (HRs), and the corresponding 95% confidence intervals (95%CIs). Variables with P < 0.05 in univariate analysis were included in the multivariate analysis. Statistical analyses were performed using IBM SPSS Statistics 26.0 (IBM Corp., Armonk, NY, USA) and R 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). All analyses were two-sided, and P values < 0.05 were considered statistically significant.
Results
Patient characteristics and correlation between M-NPS and patients’ clinicopathological characteristics
A total of 196 OCRC patients were included in the final statistical analysis (Fig. 1). The median age of the patients was 65 years, with 102 males and 94 females. Median OS and DFS were 39 and 36 months, respectively (Table 1). According to M-NPS, patients were divided into three groups: 57 cases in group 0, 110 cases in group 1, and 29 cases in group 2. M-NPS showed significant inter-group differences in age (P = 0.005), sex (P = 0.010), ASA class (P = 0.011), tumor size (P = 0.047) and four M-NPS factors (P < 0.001), whereas no significant inter-group differences were found in other variables (Table 1).
Table 1.
Relationships between Modified Naples prognostic score and clinicopathological characteristics
| Modified Naples prognostic score | |||||
|---|---|---|---|---|---|
| Variables | Total | Group 0 | Group 1 | Group 2 | P* |
| Age(yr) | 65(55–71) | 63 (54–67) | 65(55–71) | 71(64–79) | 0.005 |
| Sex | 0.010 | ||||
| Male | 102(52.0) | 21(36.8) | 61(55.5) | 20(69.0) | |
| Female | 94(48.0) | 36(63.2) | 49(44.5) | 9(31.0) | |
| BMI (mean ± SD) | 22.1 ± 2.8 | 21.9 ± 2.7 | 22.2 ± 2.7 | 22.0 ± 3.2 | 0.768 |
| ASA class | 0.011 | ||||
| 1 | 5(2.5) | 2(3.5) | 3(2.7) | 0(0.0) | |
| 2 | 126(64.3) | 42(73.7) | 68(61.8) | 16(55.2) | |
| 3 | 35(17.9) | 10(17.5) | 19(17.3) | 6(20.7) | |
| 4 | 30(15.3) | 3(5.3) | 20(18.2) | 7(24.1) | |
| 1/2 | 131(66.8) | 44(77.2) | 71(64.5) | 16(55.2) | 0.090 |
| 3/4 | 65(33.2) | 13(22.8) | 39(35.5) | 13(44.8) | |
| Location | 0.214 | ||||
| Right colon | 63(32.1) | 19(33.3) | 38(34.5) | 6(20.7) | |
| Left colon | 78(39.8) | 19(33.3) | 42(38.2) | 17(58.6) | |
| Rectum | 55(28.1) | 19(33.3) | 30(27.3) | 6(20.7) | |
| Size (cm) | 0.047 | ||||
| d<5 | 97(49.5) | 36(63.2) | 49(44.5) | 12(41.4) | |
| d ≥ 5 | 99(50.5) | 21(36.8) | 61(55.5) | 17(58.6) | |
| Differentiation | 0.487 | ||||
| Low | 28(14.3) | 5(8.8) | 18(16.4) | 5(17.2) | |
| Medium | 136(69.4) | 43(75.4) | 74(67.3) | 19(65.5) | |
| High | 32(16.3) | 9(15.8) | 18(16.4) | 5(17.2) | |
| TNM stage | |||||
| I | 5(2.5) | 1(1.8) | 4(3.6) | 0(0.0) | 0.745 |
| II | 85(43.4) | 26(45.6) | 46(41.8) | 13(44.8) | |
| III | 94(48.0) | 27(47.4) | 53(48.2) | 14(48.3) | |
| IV | 12(6.1) | 3(5.3) | 7(6.4) | 2(6.9) | |
| I/II | 90(45.9) | 27(47.4) | 50(44.5) | 13(44.8) | 0.965 |
| III/IV | 106(54.1) | 30(52.6) | 60(55.5) | 16(55.2) | |
| Tumor | |||||
| T1 | 6(3.1) | 0(0.0) | 6(5.5) | 0(0.0) | 0.237 |
| T2 | 23(11.7) | 5(8.8) | 11(10.0) | 7(24.1) | |
| T3 | 89(45.4) | 30(52.6) | 45(40.9) | 14(48.3) | |
| T4 | 78(39.8) | 22(38.6) | 48(43.6) | 8(27.6) | |
| T1/2 | 29(14.8) | 5(8.8) | 17(15.5) | 7(24.1) | 0.158 |
| T3/4 | 167(85.2) | 52(91.2) | 93(84.5) | 22(75.9) | |
| Node | 0.675 | ||||
| N0 | 96(49.0) | 27(47.4) | 55(50.0) | 14(48.3) | |
| N1 | 68(34.7) | 18(31.6) | 40(36.4) | 10(34.5) | |
| N2 | 32(16.3) | 12(21.1) | 15(13.6) | 5(17.2) | |
| N0a | 96(49.0) | 27(47.4) | 55(50.0) | 14(48.3) | 0.946 |
| N1/2 | 100(51.0) | 30(52.6) | 55(50.0) | 15(51.7) | |
| Metastasis | 1.000 | ||||
| M0 | 191(97.4) | 56(98.2) | 107(97.3) | 28(96.6) | |
| M1 | 5(2.6) | 1(1.8) | 3(2.7) | 1(3.4) | |
| Vascular Tumor Thrombus | 0.195 | ||||
| No | 150(76.5) | 39(68.4) | 89(80.9) | 22(75.9) | |
| Yes | 46(23.5) | 18(31.6) | 21(19.1) | 7(24.1) | |
| Nerve Invasion | 0.453 | ||||
| No | 137(69.9) | 40(70.2) | 74(67.3) | 23(79.3) | |
| Yes | 59(30.1) | 17(29.8) | 36(32.7) | 6(20.7) | |
| LNR (%) | 2.6(0.0-14.4) | 3.3(0.0-17.6) | 0.0(0.0-14.2) | 3.1(0.0-12.5) | 0.786 |
| SEMS implantation | 0.068 | ||||
| No | 155(79.1) | 51(89.5) | 83(75.5) | 21(72.4) | |
| Yes | 41(20.9) | 6(10.5) | 27(24.5) | 8(27.6) | |
| Chemotherapy | 0.303 | ||||
| No | 110(56.1) | 28(49.1) | 67(60.9) | 15(51.7) | |
| Yes | 86(43.9) | 29(50.9) | 43(39.1) | 14(48.3) | |
| Radiotherapy | 0.353 | ||||
| No | 190(96.9) | 56(98.2) | 107(97.3) | 27(93.1) | |
| Yes | 6(3.1) | 1(1.8) | 3(2.7) | 2(6.9) | |
| CEA (ng/ml) | 5.7(2.7–16.4) | 5.1(2.4–12.4) | 6.7(2.9–20.6) | 5.5(3.5–17.6) | 0.364 |
| NPS factors | |||||
| ALB (g/L) | 38.1(34.7–40.3) | 39.8(38.3–42.6) | 37.8(34.2–40.3) | 33.6(31.3–35.9) | < 0.001 |
| CHOL (mmol/L) | 4.3(3.6–4.8) | 4.4(4.3–4.8) | 4.3(3.6-5.0) | 3.0(2.6–3.3) | < 0.001 |
| NLR | 3.1(2.0-5.2) | 2.0(1.6–2.5) | 3.6(2.4–5.3) | 6.2(4.3–13.6) | < 0.001 |
| LMR | 2.9(2.0–4.0) | 4.4(3.6-5.0) | 2.6(2.0-3.3) | 1.8(1.3–2.2) | < 0.001 |
ASA class adopted binary classification (1/2 vs. 3/4)
TNM stage adopted binary classification (I/II vs. III/IV).
Tumor stage adopted binary classification (T1/2 vs. T3/4)
Node stage adopted binary classification (N0 vs. N1/2)
Right colon: the proximal two-thirds of the transverse colon, ascending colon and caecum
left colon: the distal third of the transverse colon, splenic flexure, descending colon, sigmoid colon
*P was calculated by the Kruskal–Wallis tests and the analysis of variance (ANOVA) for continuous variables and the Chi-square test and the Spearman’s rank correlation test for categorical variables
Prognosis (OS and DFS) of patients based on M-NPS
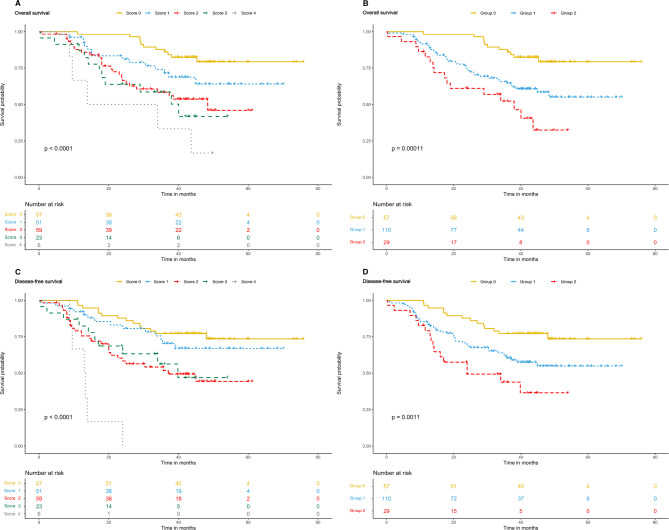
The correlation between MNPS (0–4 scores and 0–2 groups) and OS/DFS were shown in Fig. 3. The KM method showed that MNPS (0–4 scores) were significantly associated with OS and DFS in OCRC patients (P < 0.0001, P < 0.0001). In the M-NPS group, the mean OS values of group 0,1, and 2 were 45.4, 32.2, and 27.2 months, respectively, and the mean DFS values were 42.7, 30.1, and 23.8 months. The OS and DFS in group 0 were significantly higher than those in groups 1 and 2 (P = 0.0001, P = 0.0011).
Fig. 3.
Kaplan–Meier survival analysis according to Modified Naples Prognostic Score and group. (A) Significant differences in the overall survival among patients were found with five scores (P < 0.0001). (B) Significant differences in the overall survival among patients were found with three score groups (P = 0.0001). (C) Significant differences in the disease-free survival among patients were found with five scores (P < 0.0001). (D) Significant differences in the disease-free survival among patients were found with three score groups (P = 0.0011)
Univariate and multivariate analysis of OCRC prognosis
The univariate and multivariate COX proportional hazard regression analysis of OS is shown in Table 2. The Univariate analysis showed that the M-NPS group was significantly correlated with OS (P = 0.003, P < 0.001). Further multivariate analysis confirmed that the M-NPS group was an independent prognostic factor of OS. Group 0 had significantly better prognosis than group 1 (HR = 2.635 (95%CI, 1.330–5.221), P = 0.006) and group 2 (HR = 4.930 (95%CI, 2.217–10.964), P < 0.001). Other independent prognostic factors for OS included TNM stage (P = 0.009) and nerve invasion (P = 0.016).
Table 2.
Univariate and multivariate analyses of Prognostic factor for overall survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P* | HR (95% CI) | P* |
| Age | 1.022 (1.000-1.044) | 0.048 | 1.008 (0.984–1.033) | 0.516 |
| Sex | ||||
| Male | 1 | |||
| Female | 0.916 (0.567–1.479) | 0.720 | ||
| BMI | 0.971 (0.890–1.058) | 0.499 | ||
| ASA class | ||||
| 1/2 | 1 | 1 | ||
| 3/4 | 1.810 (1.115–2.938) | 0.016 | 1.713 (0.950–3.092) | 0.074 |
| Location | ||||
| Right colon | 1 | |||
| Left colon | 0.756 (0.429–1.332) | 0.333 | ||
| Rectum | 0.852 (0.467–1.556) | 0.602 | ||
| Size | ||||
| d<5 | 1 | |||
| d ≥ 5 | 0.986 (0.610–1.592) | 0.954 | ||
| Differentiation | ||||
| Low | 1 | |||
| Medium | 0.838 (0.410–1.716) | 0.630 | ||
| High | 1.000 (0.427–2.342) | 0.999 | ||
| TNM stage | ||||
| TNM I/II | 1 | 1 | ||
| TNM III/IV | 2.093 (1.255–3.490) | 0.005 | 4.320 (1.446–12.903) | 0.009 |
| Tumor | ||||
| T1/2 | 1 | |||
| T3/4 | 1.980 (0.856–4.582) | 0.110 | ||
| Node | ||||
| N0 | 1 | 1 | ||
| N1/2 | 1.753 (1.071–2.868) | 0.026 | 0.488 (0.169–1.408) | 0.185 |
| Metastasis | ||||
| 0 | 1 | |||
| 1 | 1.882 (0.591–5.992) | 0.285 | ||
| Vascular Tumor Thrombus | ||||
| No | 1 | |||
| Yes | 1.503 (0.875–2.582) | 0.140 | ||
| Nerve Invasion | ||||
| No | 1 | 1 | ||
| Yes | 1.856 (1.126–3.059) | 0.015 | 1.890 (1.124–3.178) | 0.016 |
| LNR (%) | 2.096 (0.680–6.464) | 0.198 | ||
| SEMS implantation | ||||
| No | 1 | |||
| Yes | 1.230 (0.681–2.222) | 0.492 | ||
| Chemotherapy | ||||
| No | 1 | |||
| Yes | 0.961 (0.591–1.562) | 0.872 | ||
| Radiotherapy | ||||
| No | 1 | |||
| Yes | 0.519 (0.072–3.742) | 0.515 | ||
| CEA | 1.000 (1.000–1.000) | 0.132 | ||
| M-NPS Group | ||||
| 0 | 1 | 1 | ||
| 1 | 2.730 (1.398–5.332) | 0.003 | 2.635 (1.330–5.221) | 0.006 |
| 2 | 4.893 (2.259–10.601) | < 0.001 | 4.930 (2.217–10.964) | < 0.001 |
* (OS) Multivariate analysis adjusted for age, ASA class, TNM stage, node and nerve Invasion. We use a stepwise regression approach for multivariate analysis
At the same time, univariate and multivariate COX proportional hazard regression analysis was conducted for DFS (Table 3). The Univariate analysis showed that M-NPS group was significantly correlated with DFS (P = 0.015, P < 0.001). Further multivariate analysis confirmed that the M-NPS group was an independent prognostic factor of DFS. Group 0 had significantly better prognosis than group 1 (HR = 2.031 (95%CI, 1.104–3.737), P = 0.023) and group 2 (HR = 3.508 (95%CI, 1.691–7.277), P < 0.001).
Table 3.
Univariate and multivariate analyses of prognostic factor for disease-free survival
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P* | HR (95% CI) | P* |
| Age | 1.012 (0.992–1.032) | 0.236 | ||
| Sex | ||||
| Male | 1 | |||
| Female | 1.145 (0.721–1.820) | 0.565 | ||
| BMI | 1.021 (0.938–1.110) | 0.636 | ||
| ASA class | ||||
| 1/2 | 1 | 1 | ||
| 3/4 | 1.742 (1.092–2.780) | 0.020 | 1.763 (1.090–2.851) | 0.021 |
| Location | ||||
| Right colon | 1 | |||
| Left colon | 0.768 (0.446–1.323) | 0.341 | ||
| Rectum | 0.859 (0.480–1.539) | 0.610 | ||
| Size | ||||
| d<5 | 1 | |||
| d ≥ 5 | 0.923 (0.581–1.465) | 0.733 | ||
| Differentiation | ||||
| Low | 1 | |||
| Medium | 0.983 (0.483–2.001) | 0.961 | ||
| High | 1.256 (0.543–2.905) | 0.594 | ||
| TNM stage | ||||
| TNM I/II | 1 | 1 | ||
| TNM III/IV | 1.655 (1.029–2.664) | 0.038 | 1.902 (1.165–3.104) | 0.010 |
| Tumor | ||||
| T1/2 | 1 | |||
| T3/4 | 1.633 (0.783–3.407) | 0.191 | ||
| Node | ||||
| N0 | 1 | |||
| N1/2 | 1.298 (0.815–2.066) | 0.272 | ||
| Metastasis | ||||
| 0 | 1 | |||
| 1 | 1.851 (0.582–5.886) | 0.297 | ||
| Vascular Tumor Thrombus | ||||
| No | 1 | |||
| Yes | 1.243 (0.721–2.145) | 0.434 | ||
| Nerve Invasion | ||||
| No | 1 | |||
| Yes | 1.573 (0.964–2.567) | 0.070 | ||
| LNR (%) | 1.360 (0.422–4.388) | 0.607 | ||
| SEMS implantation | ||||
| No | 1 | |||
| Yes | 1.163 (0.657–2.057) | 0.604 | ||
| Chemotherapy | ||||
| No | 1 | |||
| Yes | 0.937 (0.586–1.496) | 0.784 | ||
| Radiotherapy | ||||
| No | 1 | |||
| Yes | 1.133 (0.278–4.624) | 0.862 | ||
| CEA | 1.000 (1.000–1.000) | 0.193 | ||
| M-NPS Group | ||||
| 0 | 1 | 1 | ||
| 1 | 2.128 (1.160–3.905) | 0.015 | 2.031 (1.104–3.737) | 0.023 |
| 2 | 3.691 (1.791–7.608) | < 0.001 | 3.508 (1.691–7.277) | < 0.001 |
* (DFS) Multivariate analysis adjusted for ASA class and TNM stage
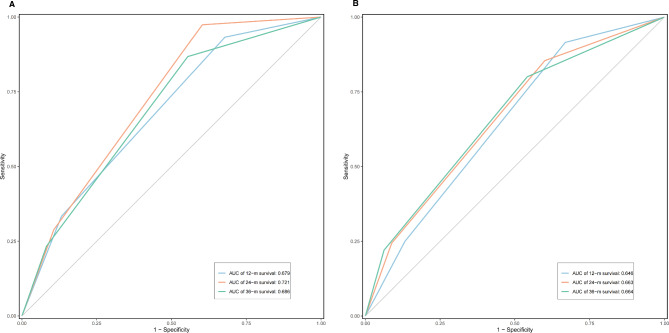
Subsequently, the ROC curve was applied to compare the prediction ability of OS and DFS in the M-NPS group at 12, 24, and 36 months (Fig. 4). The results showed that the M-NPS group could effectively predict OS (AUC = 0.679, 0.721, 0.686) and DFS (AUC = 0.646, 0.663, 0.664) in OCRC patients within 36 months.
Fig. 4.
Time-dependent receiver operating characteristic (ROC) to assess Modified Naples Prognostic Score. Time-dependent ROC to assess the accuracy of Modified Naples Prognostic Score group in predicting overall survival (A) and disease-free survival (B) at 12, 24, and 36 months. (A) The area under the curve (AUC) values for 12-, 24-, and 36-month overall survival were 0.679, 0.721, and 0.686, respectively. (B) AUC values for 12-, 24-, and 36-month disease-free survival were 0.646, 0.663, and 0.664, respectively
Discussion
Although previous studies have explored the value of inflammatory or immunonutritional status indicators in evaluating the prognosis of OCRC [23–25], no study has combined these indicators to achieve a more effective prognosis assessment of patients with obstruction. A comprehensive score combined with these status indicators could provide a more comprehensive, effective, and intuitive reference basis for prognosis evaluation of OCRC patients, considering the complex inflammatory, immune, and nutritional status of OCRC patients. In this study, M-NPS composed of inflammatory, nutritional, and immune status-related indicators was constructed and validated as an independent prognostic factor for OS and DFS in OCRC. In addition, M-NPS demonstrated good efficiency in predicting survival at 12, 24, and 36 months.
As an important component of tumor microenvironment, inflammation plays a complex role in the occurrence and development of cancer; it is considered the seventh hallmark of cancer [4, 8]. In OCRC, due to colorectal obstruction, a large number of bacteria in the intestinal tract proliferated, resulting in dysbacteriosis. Patients usually have more serious local and systemic inflammatory reactions, and even septic shock [9, 26]. Inflammation caused by obstruction leads to aggregation of neutrophils and monocytes, further promoting the development of cancer through the production and release of a large number of inflammatory mediators, transformation factors, cytokines, and chemokines [4, 8]. On the contrary, the local immune response caused by inflammation leads to the increase in monocytes and lymphocytes, which can play as a certain anticancer activity. However, a degree of immunosuppression may also be present in OCRC patients; thus, tumor–host immunity and inflammatory response exhibit a complicated interaction [6, 27, 28]. CRC patients present a heterogeneous immune landscape according to microsatellite status and other factors. Most patients have microsatellite-stable (MSS) tumors with poor immune cell infiltration, and the difference in immune response has a more profound impact on the prognosis of patients with obstruction [29]. The association between MSI status and OCRC deserves further study in the future. Therefore, the changes in immune and inflammatory indicators, such as NLR and LMR, can undoubtedly provide a powerful reference for assessing the prognosis of OCRC, and some studies have also investigated the association between the biomarkers and prognosis of OCRC [23–25].
The association between nutritional indicators and OCRC prognosis deserves attention. The nutritional status of CRC patients, especially those complicated with obstruction, has received increased attention in the first-line clinical practice, and nutritional therapy plays a key role in comprehensive treatment [30, 31]. The nutritional status of patients is generally worse due to the presence of digestive tract obstruction symptoms, and their surgical tolerance, postoperative or post-treatment complication risk, recovery ability, and anti-infection ability are lower than those of patients without obstruction [9, 32]. Therefore, we could better assess the status and prognosis of OCRC patients and make more appropriate therapeutic interventions by focusing on pre-treatment nutritional status. Serum ALB content is a crucial indicator in the clinical front-line of gastrointestinal surgery, which does not only effectively present the nutritional status of patients with obstruction, but also serves as a marker of systemic inflammation; it has been widely included in various scoring systems [7, 16]. Previous study has shown that low CHOL is associated with poor prognosis of CRC [33], and the inclusion of CHOL could also help to evaluate the nutritional status of OCRC patients more effectively.
The conventional NPS contains inflammatory factors such as neutrophils, lymphocytes, monocytes, and nutritional status indicators such as cholesterol and albumin, and has been validated in the prognostic evaluation of CRC [15, 20]. However, OCRC patients would have worse systemic nutritional status and inflammation compared with conventional CRC, and conventional NPS did not have good suitability in OCRC cohort. Therefore, based on the characteristics of OCRC patients with severe inflammatory response and poor immunonutritional status, x-tile software was used to re-intercept the cut-points of NPS factors suitable for this special cohort, and a prognostic scoring system M-NPS suitable for evaluating the inflammatory immunonutritional status of OCRC patients was constructed. OCRC patients had significantly worse prognosis as M-NPS increased, with patients in the M-NPS group 2 having significantly worse OS and DFS than patients in group 0. M-NPS was an important prognostic factor for OCRC patients independent of tumor stage; it can effectively predict the survival of OCRC patients within 36 months. A major advantage of this M-NPS is that it covers clinically focused indicators of inflammation, immunity, and nutritional status in patients with obstruction. The application of scoring can provide reference for OCRC patients to obtain optimal treatment decisions; it could help appropriate patients to obtain more active immunonutritional intervention.
This study has some limitations, mainly in the retrospective analysis and limited sample size. Whether to receive transitional SEMS treatment prior to surgery was not designed separately. Thus, the predictive value of M-NPS should be further evaluated by prospective studies with a large sample size.
Conclusion
According to the characteristics of OCRC patients, such as severe inflammation and poor nutrition, this study combined the inflammatory, immune, and nutritional indicators to construct a prognostic score, M-NPS, and verified that it is an independent prognostic factor for OS and DFS.
Acknowledgements
Not applicable.
Abbreviations
- ASA class
American Society of Anesthesiologists Physical Status Classification
- ALB
Albumin
- AUC
Area under the curve
- BMI
Body Mass Index
- CHOL
Total cholesterol
- CRC
Colorectal cancer
- CT
Computerized tomography
- DFS
Disease-free survival
- HRs
Hazard ratios
- IQR
Interquartile ranges
- KM
Kaplan-Meier
- LMR
Lymphocyte:monocyte ratio
- LNR
Lymph node ratio
- mCRC
Metastatic CRC
- M-NPS
Modified Naples prognostic score
- MRI
Magnetic resonance imaging
- NLR
Neutrophil:lymphocyte ratio
- NPS
Naples prognostic score
- OCRC
Obstructive colorectal cancer
- OS
Overall survival
- PLR
Platelet:lymphocyte ratio
- PNI
Prognostic nutrition index
- ROC
Receiver operating characteristic
- SEMS
Self-expanding metal stent
- SII
Systemic immune–inflammatory index
- 95%CIs
95% confidence Intervals
Authors’ contributions
JNG, SHD and ZXJ lead the study. FWM, YFX, LQ, JGS, JY and JY collected the data. JNG performed the data analysis, implemented the methodology. JNG prepared the original draft. SHD and ZXJ helped to perfect the figures. HLL, KL and KW reviewed the manuscript. YHC and KLC reviewed and edited the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No.82170678) and Hubei Province Key Research and Development Program of China (Science and Technology Innovation Special Project No.2021BAA044), and Wuhan Strong Magnetic Field Interdisciplinary Fund (No. WHMF202113).
Data Availability
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee and the Institutional Review Committee of Wuhan Union Hospital (No.2018-S377) and was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junnan Gu, Shenghe Deng and Zhenxing Jiang contributed equally to this work.
Contributor Information
Yinghao Cao, Email: d201981630@hust.edu.cn.
Kailin Cai, Email: caikailin@hust.edu.cn.
References
- 1.Kye BH, Lee YS, Cho HM, Kim JG, Oh ST, Lee IK, Kang WK, Ahn CH, Lee SC, Park JK, et al. Comparison of long-term outcomes between emergency surgery and bridge to surgery for malignant obstruction in right-sided Colon cancer: a Multicenter Retrospective Study. Ann Surg Oncol. 2016;23(6):1867–74. doi: 10.1245/s10434-015-5053-7. [DOI] [PubMed] [Google Scholar]
- 2.Park YE, Park Y, Park SJ, Cheon JH, Kim WH, Kim TI. Outcomes of stent insertion and mortality in obstructive stage IV colorectal cancer patients through 10 year duration. Surg Endosc. 2019;33(4):1225–34. doi: 10.1007/s00464-018-6399-2. [DOI] [PubMed] [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 4.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Lucas C, Barnich N, Nguyen HTT. Microbiota, inflammation and colorectal Cancer. Int J Mol Sci 2017, 18(6). [DOI] [PMC free article] [PubMed]
- 6.Malka D, Lièvre A, André T, Taïeb J, Ducreux M, Bibeau F. Immune scores in colorectal cancer: where are we? Eur J Cancer. 2020;140:105–18. doi: 10.1016/j.ejca.2020.08.024. [DOI] [PubMed] [Google Scholar]
- 7.Tokunaga R, Sakamoto Y, Nakagawa S, Miyamoto Y, Yoshida N, Oki E, Watanabe M, Baba H. Prognostic Nutritional Index predicts severe complications, recurrence, and poor prognosis in patients with Colorectal Cancer undergoing primary Tumor Resection. Dis Colon Rectum. 2015;58(11):1048–57. doi: 10.1097/DCR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 8.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–81. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 9.Yeo HL, Lee SW. Colorectal emergencies: review and controversies in the management of large bowel obstruction. J Gastrointest Surg. 2013;17(11):2007–12. doi: 10.1007/s11605-013-2343-x. [DOI] [PubMed] [Google Scholar]
- 10.Reisinger KW, van Vugt JL, Tegels JJ, Snijders C, Hulsewé KW, Hoofwijk AG, Stoot JH, Von Meyenfeldt MF, Beets GL, Derikx JP, et al. Functional compromise reflected by sarcopenia, frailty, and nutritional depletion predicts adverse postoperative outcome after colorectal cancer surgery. Ann Surg. 2015;261(2):345–52. doi: 10.1097/SLA.0000000000000628. [DOI] [PubMed] [Google Scholar]
- 11.Chan JC, Chan DL, Diakos CI, Engel A, Pavlakis N, Gill A, Clarke SJ. The lymphocyte-to-monocyte ratio is a Superior Predictor of overall survival in comparison to established biomarkers of Resectable Colorectal Cancer. Ann Surg. 2017;265(3):539–46. doi: 10.1097/SLA.0000000000001743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen JH, Zhai ET, Yuan YJ, Wu KM, Xu JB, Peng JJ, Chen CQ, He YL, Cai SR. Systemic immune-inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261–72. doi: 10.3748/wjg.v23.i34.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chua W, Charles KA, Baracos VE, Clarke SJ. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer. 2011;104(8):1288–95. doi: 10.1038/bjc.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Y, Chen X, Song Y, Huang X, Chen Q, Lv X, Gao P, Wang Z. The platelet to lymphocyte ratio is a potential inflammatory marker predicting the effects of adjuvant chemotherapy in patients with stage II colorectal cancer. BMC Cancer. 2021;21(1):792. doi: 10.1186/s12885-021-08521-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galizia G, Lieto E, Auricchio A, Cardella F, Mabilia A, Podzemny V, Castellano P, Orditura M, Napolitano V. Naples Prognostic score, based on Nutritional and Inflammatory Status, is an independent predictor of long-term outcome in patients undergoing surgery for Colorectal Cancer. Dis Colon Rectum. 2017;60(12):1273–84. doi: 10.1097/DCR.0000000000000961. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki Y, Okabayashi K, Hasegawa H, Tsuruta M, Shigeta K, Kondo T, Kitagawa Y. Comparison of preoperative inflammation-based Prognostic Scores in patients with Colorectal Cancer. Ann Surg. 2018;267(3):527–31. doi: 10.1097/SLA.0000000000002115. [DOI] [PubMed] [Google Scholar]
- 17.Guo D, Liu J, Li Y, Li C, Liu Q, Ji S, Zhu S. Evaluation of predictive values of Naples Prognostic score in patients with Unresectable Stage III Non-Small Cell Lung Cancer. J Inflamm Res. 2021;14:6129–41. doi: 10.2147/JIR.S341399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin J, Wang H, Peng F, Wang X, Wang M, Zhu F, Xiong G, Qin R. Prognostic significance of preoperative Naples prognostic score on short- and long-term outcomes after pancreatoduodenectomy for ampullary carcinoma. Hepatobiliary Surg Nutr. 2021;10(6):825–38. doi: 10.21037/hbsn-20-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieto E, Auricchio A, Tirino G, Pompella L, Panarese I, Del Sorbo G, Ferraraccio F, De Vita F, Galizia G, Cardella F. Naples Prognostic Score Predicts Tumor Regression Grade in Resectable Gastric Cancer Treated with Preoperative Chemotherapy. Cancers (Basel) 2021, 13(18). [DOI] [PMC free article] [PubMed]
- 20.Miyamoto Y, Hiyoshi Y, Daitoku N, Okadome K, Sakamoto Y, Yamashita K, Kuroda D, Sawayama H, Iwatsuki M, Baba Y, et al. Naples Prognostic score is a useful prognostic marker in patients with metastatic colorectal Cancer. Dis Colon Rectum. 2019;62(12):1485–93. doi: 10.1097/DCR.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 21.Boone K, Barber D, Brown B. Imaging with electricity: report of the european concerted action on Impedance Tomography. J Med Eng Technol. 1997;21(6):201–32. doi: 10.3109/03091909709070013. [DOI] [PubMed] [Google Scholar]
- 22.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 23.Chen XQ, Xue CR, Hou P, Lin BQ, Zhang JR. Lymphocyte-to-monocyte ratio effectively predicts survival outcome of patients with obstructive colorectal cancer. World J Gastroenterol. 2019;25(33):4970–84. doi: 10.3748/wjg.v25.i33.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato R, Oikawa M, Kakita T, Okada T, Abe T, Yazawa T, Tsuchiya H, Akazawa N, Sato M, Ohira T, et al. Preoperative change of modified Glasgow prognostic score after stenting predicts the long-term outcomes of obstructive colorectal cancer. Surg Today. 2020;50(3):232–9. doi: 10.1007/s00595-019-01862-1. [DOI] [PubMed] [Google Scholar]
- 25.Sato R, Oikawa M, Kakita T, Okada T, Abe T, Yazawa T, Tsuchiya H, Akazawa N, Sato M, Ohira T, et al. The prognostic value of the prognostic nutritional index and inflammation-based markers in obstructive colorectal cancer. Surg Today. 2020;50(10):1272–81. doi: 10.1007/s00595-020-02007-5. [DOI] [PubMed] [Google Scholar]
- 26.Brennan CA, Garrett WS. Gut microbiota, inflammation, and Colorectal Cancer. Annu Rev Microbiol. 2016;70:395–411. doi: 10.1146/annurev-micro-102215-095513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pernot S, Terme M, Voron T, Colussi O, Marcheteau E, Tartour E, Taieb J. Colorectal cancer and immunity: what we know and perspectives. World J Gastroenterol. 2014;20(14):3738–50. doi: 10.3748/wjg.v20.i14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picard E, Verschoor CP, Ma GW, Pawelec G. Relationships between Immune Landscapes, genetic subtypes and responses to Immunotherapy in Colorectal Cancer. Front Immunol. 2020;11:369. doi: 10.3389/fimmu.2020.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fahim M, Dijksman LM, van Kessel CS, Smeeing DPJ, Braaksma A, Derksen WJM, Smits AB. Promising results of a new treatment in patients with bowel obstruction in colorectal surgery. Eur J Surg Oncol. 2020;46(3):415–9. doi: 10.1016/j.ejso.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Thanikachalam K, Khan G. Colorectal Cancer and Nutrition. Nutrients 2019, 11(1). [DOI] [PMC free article] [PubMed]
- 32.Fahim M, Dijksman LM, Derksen WJM, Bloemen JG, Biesma DH, Smits AB. Prospective multicentre study of a new bowel obstruction treatment in colorectal surgery: reduced morbidity and mortality. Eur J Surg Oncol. 2021;47(9):2414–20. doi: 10.1016/j.ejso.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 33.Mamtani R, Lewis JD, Scott FI, Ahmad T, Goldberg DS, Datta J, Yang YX, Boursi B. Disentangling the association between statins, cholesterol, and Colorectal Cancer: a nested case-control study. PLoS Med. 2016;13(4):e1002007. doi: 10.1371/journal.pmed.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.