Abstract
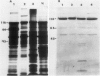
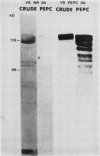
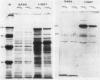
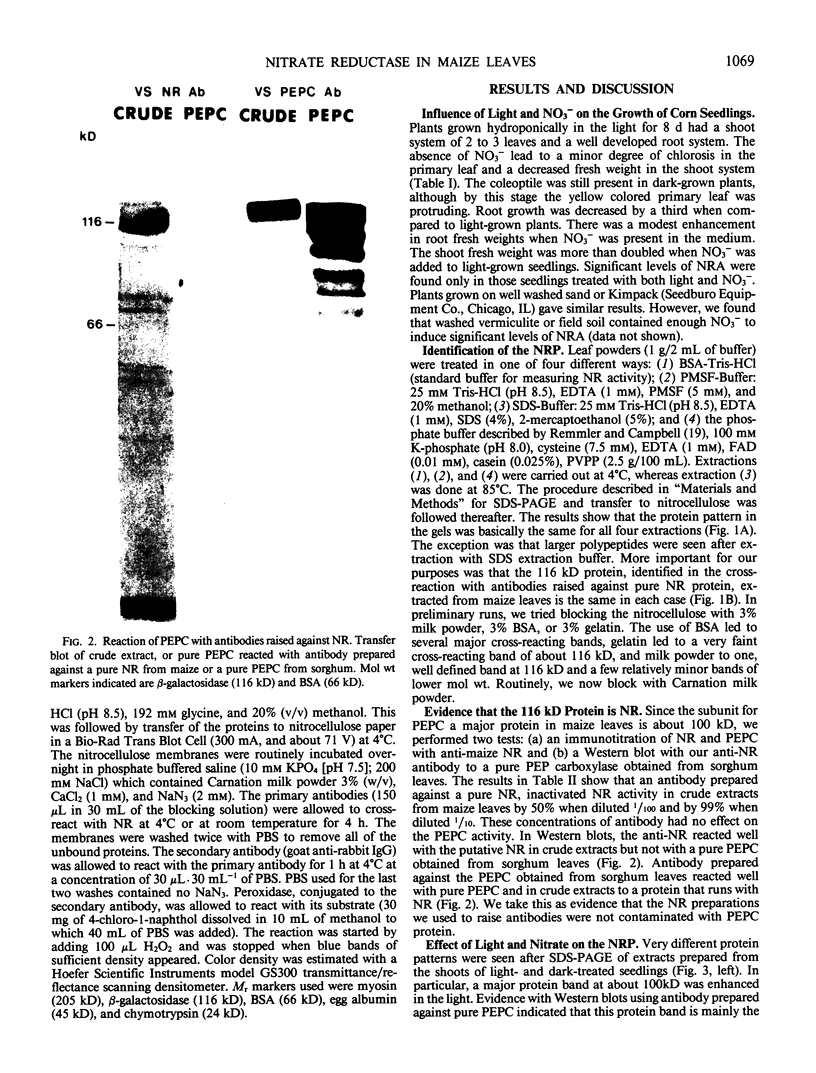
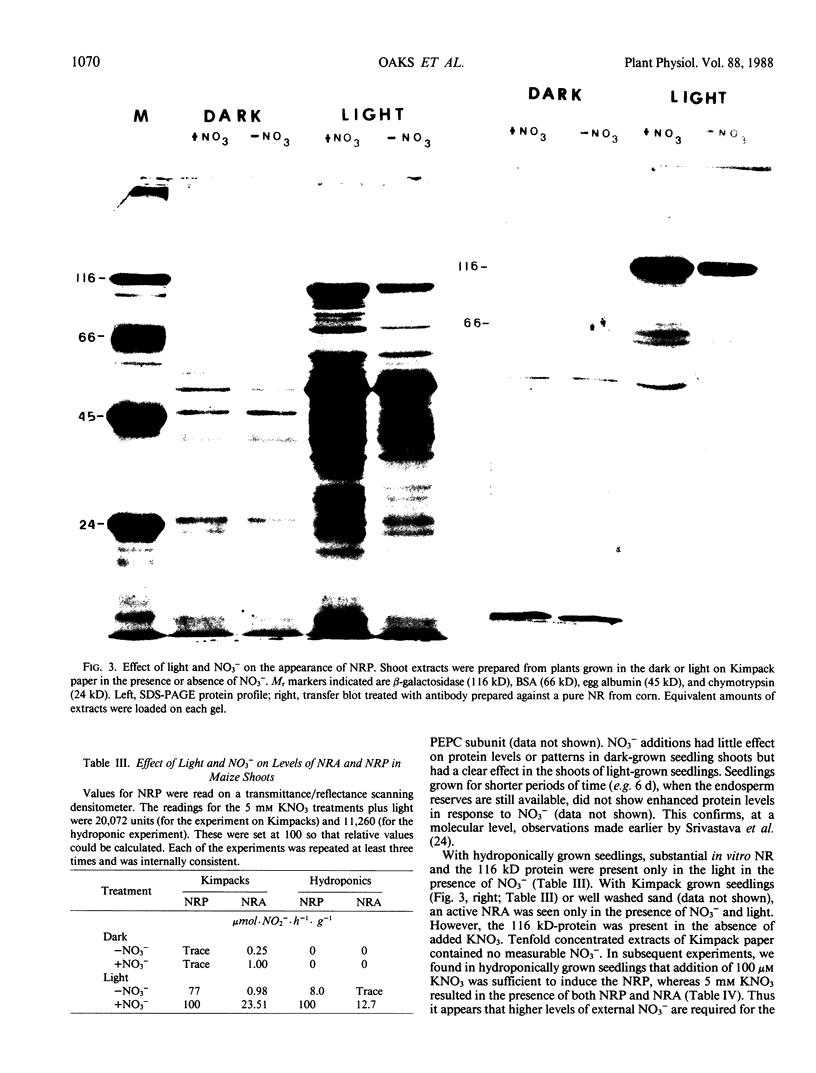
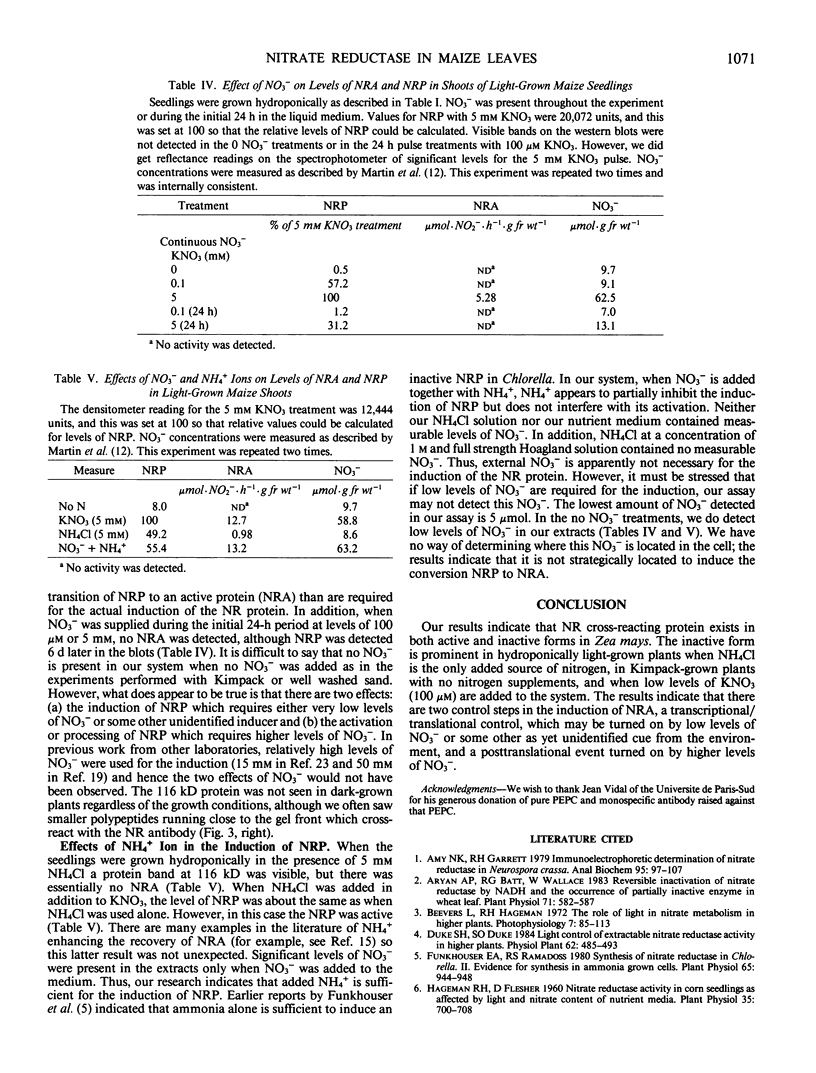
Corn seedlings (Zea mays cv W64A × W182E) were grown hydroponically, in the presence or absence of NO3−, with or without light and with NH4Cl as the only N source. In agreement with earlier results nitrate reductase (NR) activity was found only in plants treated with both light and NO3−. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by transfer of the proteins to nitrocellulose paper and reaction with antibodies prepared against a pure NR showed that crude extracts prepared from light-grown plants had a polypeptide of approximately 116 kilodaltons (the subunit size for NR) when NO3− was present in the growth medium. Crude extracts from plants grown in the dark did not have the 116 kilodalton polypeptide, although smaller polypeptides, which reacted with NR-immunoglobulin G, were sometimes found at the gel front. When seedlings were grown on Kimpack paper or well washed sand, NR activity was again found only when the seedlings were exposed to light and NO3−. Under these conditions, however, a protein of about 116 kilodaltons, which reacted with the NR antibody was present in light-grown plants whether NO3− was added to the system or not. The NR antibody cross-reacting protein was also seen in hydroponically grown plants when NH4Cl− was the only added form of nitrogen. These results indicate that the induction of an inactive NR-protein precursor in corn is mediated either by extremely low levels of NO3− or by some other unidentified factor, and that higher levels of NO3− are necessary for converting the inactive NR cross-reacting protein to a form of the enzyme capable of reducing NO3− to NO2−.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy N. K., Garrett R. H. Immunoelectrophoretic determination of nitrate reductase in Neurospora crassa. Anal Biochem. 1979 May;95(1):97–107. doi: 10.1016/0003-2697(79)90191-x. [DOI] [PubMed] [Google Scholar]
- Aryan A. P., Batt R. G., Wallace W. Reversible Inactivation of Nitrate Reductase by NADH and the Occurrence of Partially Inactive Enzyme in the Wheat Leaf. Plant Physiol. 1983 Mar;71(3):582–587. doi: 10.1104/pp.71.3.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beevers L., Hageman R. H. The role of light in nitrate metabolism in higher plants. Photophysiology. 1972;(7):85–113. [PubMed] [Google Scholar]
- Funkhouser E. A. Synthesis of Nitrate Reductase in Chlorella: II. EVIDENCE FOR SYNTHESIS IN AMMONIA-GROWN CELLS. Plant Physiol. 1980 May;65(5):944–948. doi: 10.1104/pp.65.5.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman R. H., Flesher D. Nitrate Reductase Activity in Corn Seedlings as Affected by Light and Nitrate Content of Nutrient Media. Plant Physiol. 1960 Sep;35(5):700–708. doi: 10.1104/pp.35.5.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Losada M., Paneque A., Aparicio P. J., Vega J. M., Cárdenas J., Herrera J. Inactivation and repression by ammonium of the nitrate reducing system in chlorella. Biochem Biophys Res Commun. 1970 Mar 27;38(6):1009–1015. doi: 10.1016/0006-291x(70)90340-2. [DOI] [PubMed] [Google Scholar]
- Martin F., Winspear M. J., Macfarlane J. D., Oaks A. Effect of Methionine Sulfoximine on the Accumulation of Ammonia in C(3) and C(4) Leaves : The Relationship between NH(3) Accumulation and Photorespiratory Activity. Plant Physiol. 1983 Jan;71(1):177–181. doi: 10.1104/pp.71.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Poulle M., Oaks A. Characterization of Nitrate Reductase from Corn Leaves (Zea mays cv W64A x W182E) : Two Molecular Forms of the Enzyme. Plant Physiol. 1984 Jun;75(2):285–289. doi: 10.1104/pp.75.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa H., Yonemura Y., Yamamoto H., Sato T., Ogura N., Sato R. Spinach nitrate reductase: purification, molecular weight, and subunit composition. Plant Physiol. 1985 Jan;77(1):124–128. doi: 10.1104/pp.77.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulle M., Oaks A., Bzonek P., Goodfellow V. J., Solomonson L. P. Characterization of Nitrate Reductases from Corn Leaves (Zea mays cv W64AxW182E) and Chlorella vulgaris: Sensitivity to a Proteinase Extracted from Corn Roots. Plant Physiol. 1987 Oct;85(2):375–378. doi: 10.1104/pp.85.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmler J. L., Campbell W. H. Regulation of Corn Leaf Nitrate Reductase : II. Synthesis and Turnover of the Enzyme's Activity and Protein. Plant Physiol. 1986 Feb;80(2):442–447. doi: 10.1104/pp.80.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarrelli J., Campbell W. H. Immunological approach to structural comparisons of assimilatory nitrate reductases. Plant Physiol. 1981 Dec;68(6):1226–1230. doi: 10.1104/pp.68.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P. Purification of NADH-Nitrate Reductase by Affinity Chromatography. Plant Physiol. 1975 Dec;56(6):853–855. doi: 10.1104/pp.56.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomonson L. P., Vennesland B. Properties of a nitrate reductase of Chlorella. Biochim Biophys Acta. 1972 Jun 23;267(3):544–557. doi: 10.1016/0005-2728(72)90183-1. [DOI] [PubMed] [Google Scholar]
- Somers D. A., Kuo T. M., Kleinhofs A., Warner R. L., Oaks A. Synthesis and degradation of barley nitrate reductase. Plant Physiol. 1983 Aug;72(4):949–952. doi: 10.1104/pp.72.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischner R. Light-mediated Activation of Nitrate Reductase in Synchronous Chlorella. Plant Physiol. 1978 Aug;62(2):284–286. doi: 10.1104/pp.62.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]