Abstract
We have used gene-specific DNA fragments as hybridization probes to quantitate the levels of transcripts encoding several phycobilisome polypeptides in the cyanobacterium Fremyella diplosiphon in response to changes in the light environment. While the levels of transcripts encoding allophycocyanin, the core linker polypeptide, and the constitutive phycocyanin subunits are similar in F. diplosiphon grown either in red or green light, the levels of other transcripts change dramatically. Transcripts encoding the inducible phycocyanin subunits are barely detected in green light-grown cells and very abundant in red light-grown cells, while the level of phycoerythrin mRNA is approximately 10-fold more in green than red light-grown cells. Quantitation of the phycoerythrin and inducible phycocyanin transcripts after transfer of cultures from green to red light and red to green light demonstrate that both increase rapidly upon exposure of cells to inductive illumination. The decrease in the phycoerythrin mRNA level in red light is much slower than the decline in the levels of the inducible phycocyanin transcripts in green light. Since the half-lives of the inducible phycocyanin and phycoerythrin transcripts do not change when F. diplosiphon is exposed to red or green illumination, the steady state levels of these mRNAs are primarily controlled by the rate of transcription. Therefore, the high level of phycoerythrin mRNA maintained for several hours after cultures are transferred from green to red illumination must result from continued transcription of the phycoerythrin gene set. Differences in expression from the phycoerythrin and inducible phycocyanin gene sets in response to light quality are discussed in terms of possible mechanisms involved in their regulation.
Full text
PDF

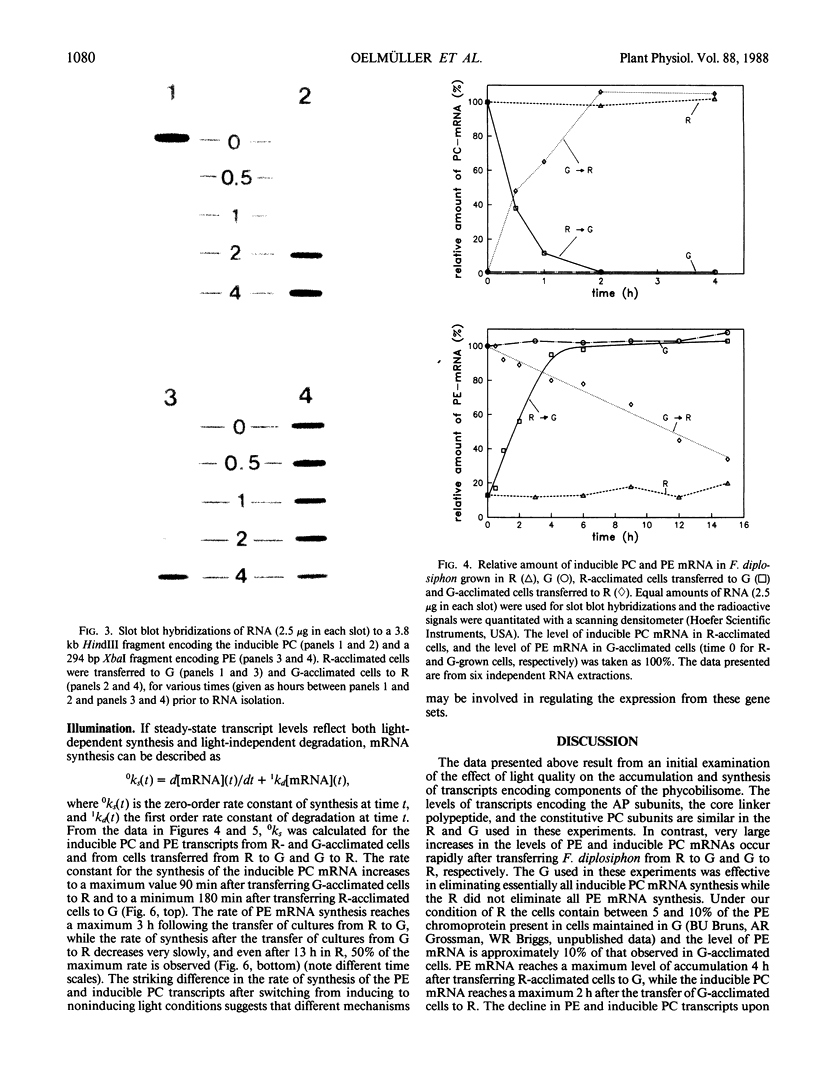
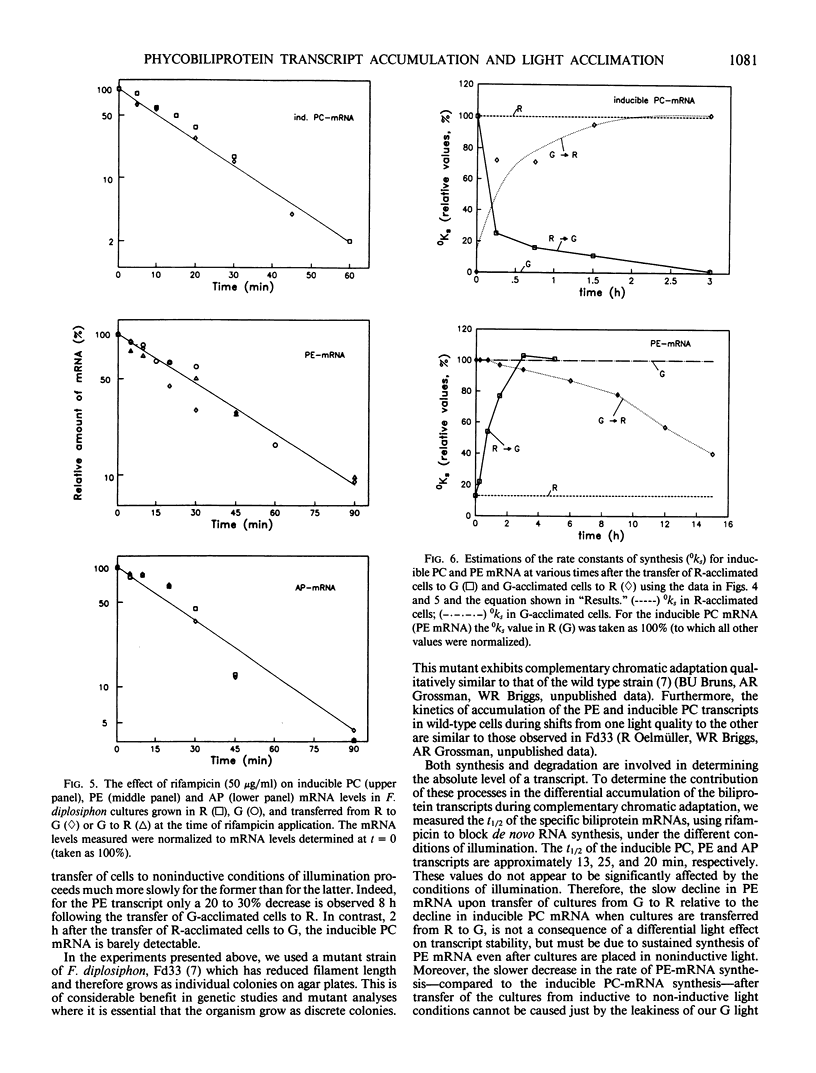


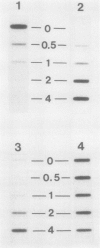
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belknap W. R., Haselkorn R. Cloning and light regulation of expression of the phycocyanin operon of the cyanobacterium Anabaena. EMBO J. 1987 Apr;6(4):871–884. doi: 10.1002/j.1460-2075.1987.tb04833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A., Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973 Aug;58(2):419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. A., Cohen-Bazire G. Effects of chromatic illumination on cyanobacterial phycobilisomes. Evidence for the specific induction of a second pair of phycocyanin subunits in Pseudanabaena 7409 grown in red light. Eur J Biochem. 1981 Oct;119(2):415–424. doi: 10.1111/j.1432-1033.1981.tb05624.x. [DOI] [PubMed] [Google Scholar]
- Bryant D. A. The photoregulated expression of multiple phycocyanin species. A general mechanism for the control of phycocyanin synthesis in chromatically adapting cyanobacteria. Eur J Biochem. 1981 Oct;119(2):425–429. doi: 10.1111/j.1432-1033.1981.tb05625.x. [DOI] [PubMed] [Google Scholar]
- Bryant D. A., de Lorimier R., Lambert D. H., Dubbs J. M., Stirewalt V. L., Stevens S. E., Jr, Porter R. D., Tam J., Jay E. Molecular cloning and nucleotide sequence of the alpha and beta subunits of allophycocyanin from the cyanelle genome of Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1985 May;82(10):3242–3246. doi: 10.1073/pnas.82.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobley J. G., Miranda R. D. Mutations affecting chromatic adaptation in the cyanobacterium Fremyella diplosiphon. J Bacteriol. 1983 Mar;153(3):1486–1492. doi: 10.1128/jb.153.3.1486-1492.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. Molecular characterization and evolution of sequences encoding light-harvesting components in the chromatically adapting cyanobacterium Fremyella diplosiphon. J Mol Biol. 1988 Feb 5;199(3):447–465. doi: 10.1016/0022-2836(88)90617-1. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Lomax T. L., Grossman A. R. Genes encoding major light-harvesting polypeptides are clustered on the genome of the cyanobacterium Fremyella diplosiphon. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3924–3928. doi: 10.1073/pnas.83.11.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakoff S., Scheibe J. Action Spectra for Chromatic Adaptation in Tolypothrix tenuis. Plant Physiol. 1973 Feb;51(2):382–385. doi: 10.1104/pp.51.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gendel S., Ohad I., Bogorad L. Control of Phycoerythrin Synthesis during Chromatic Adaptation. Plant Physiol. 1979 Nov;64(5):786–790. doi: 10.1104/pp.64.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Glazer A. N., Lundell D. J., Yamanaka G., Williams R. C. The structure of a "simple" phycobilisome. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):159–180. doi: 10.1016/s0769-2609(83)80103-3. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Phycobilisomes: structure and dynamics. Annu Rev Microbiol. 1982;36:173–198. doi: 10.1146/annurev.mi.36.100182.001133. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Lemaux P. G., Conley P. B. Regulated synthesis of phycobilisome components. Photochem Photobiol. 1986 Dec;44(6):827–837. doi: 10.1111/j.1751-1097.1986.tb05543.x. [DOI] [PubMed] [Google Scholar]
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- Haury J. F., Bogorad L. Action Spectra for Phycobiliprotein Synthesis in a Chromatically Adapting Cyanophyte, Fremyella diplosiphon. Plant Physiol. 1977 Dec;60(6):835–839. doi: 10.1104/pp.60.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Leach C. K., Carr N. G. In vitro protein synthesis and measurement of the stability of messenger RNA in the blue-green alga, anabaena variabilis. J Gen Microbiol. 1974 Mar;81(1):47–58. doi: 10.1099/00221287-81-1-47. [DOI] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. R. Major light-harvesting polypeptides encoded in polycistronic transcripts in a eukaryotic alga. EMBO J. 1985 Aug;4(8):1911–1919. doi: 10.1002/j.1460-2075.1985.tb03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. Isolation and characterization of a gene for a major light-harvesting polypeptide from Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4100–4104. doi: 10.1073/pnas.81.13.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomax T. L., Conley P. B., Schilling J., Grossman A. R. Isolation and characterization of light-regulated phycobilisome linker polypeptide genes and their transcription as a polycistronic mRNA. J Bacteriol. 1987 Jun;169(6):2675–2684. doi: 10.1128/jb.169.6.2675-2684.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Glazer A. N. Molecular architecture of a light-harvesting antenna. Core substructure in Synechococcus 6301 phycobilisomes: two new allophycocyanin and allophycocyanin B complexes. J Biol Chem. 1983 Jan 25;258(2):902–908. [PubMed] [Google Scholar]
- Lundell D. J., Yamanaka G., Glazer A. N. A terminal energy acceptor of the phycobilisome: the 75,000-dalton polypeptide of Synechococcus 6301 phycobilisomes--a new biliprotein. J Cell Biol. 1981 Oct;91(1):315–319. doi: 10.1083/jcb.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lönneborg A., Lind L. K., Kalla S. R., Gustafsson P., Oquist G. Acclimation Processes in the Light-Harvesting System of the Cyanobacterium Anacystis nidulans following a Light Shift from White to Red Light. Plant Physiol. 1985 May;78(1):110–114. doi: 10.1104/pp.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazel D., Guglielmi G., Houmard J., Sidler W., Bryant D. A., Tandeau de Marsac N. Green light induces transcription of the phycoerythrin operon in the cyanobacterium Calothrix 7601. Nucleic Acids Res. 1986 Nov 11;14(21):8279–8290. doi: 10.1093/nar/14.21.8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüller R., Grossman A. R., Briggs W. R. Photoreversibility of the Effect of Red and Green Light Pulses on the Accumulation in Darkness of mRNAs Coding for Phycocyanin and Phycoerythrin in Fremyella diplosiphon. Plant Physiol. 1988 Dec;88(4):1084–1091. doi: 10.1104/pp.88.4.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlinger T., Gantt E. A M(r) 95,000 polypeptide in Porphyridium cruentum phycobilisomes and thylakoids: Possible function in linkage of phycobilisomes to thylakoids and in energy transfer. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5542–5546. doi: 10.1073/pnas.79.18.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandeau de Marsac N. Occurrence and nature of chromatic adaptation in cyanobacteria. J Bacteriol. 1977 Apr;130(1):82–91. doi: 10.1128/jb.130.1.82-91.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]