Abstract
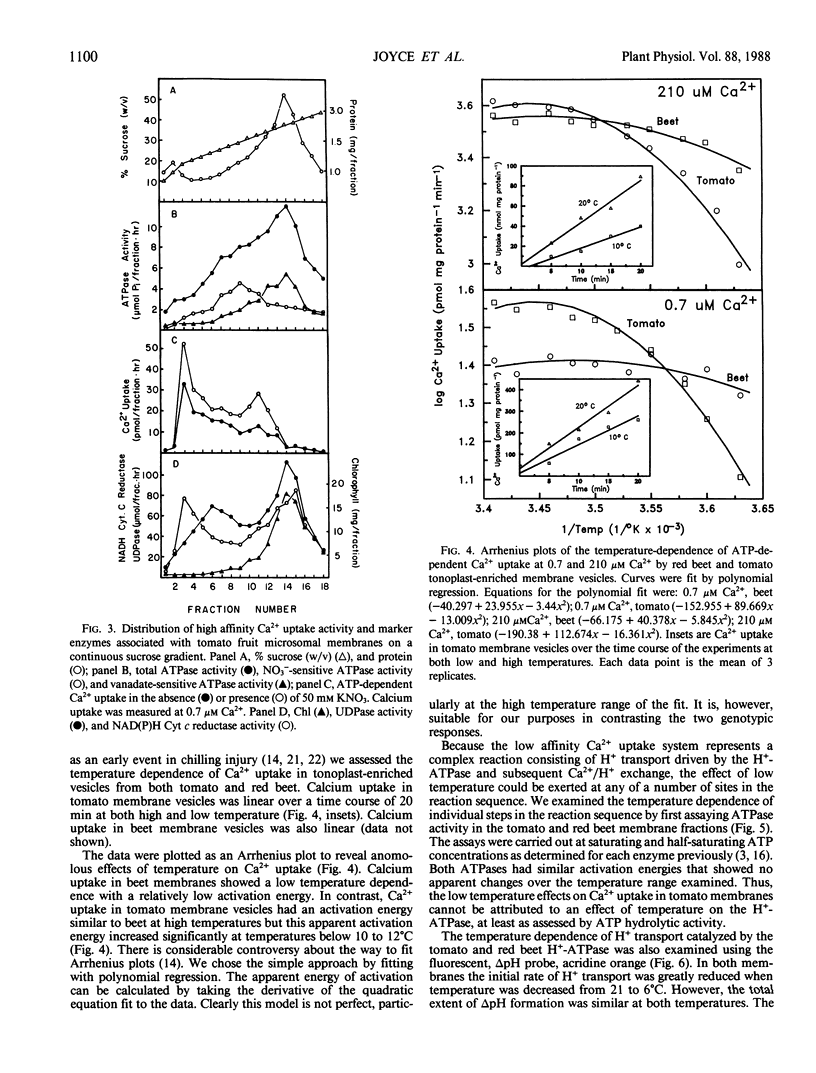
Calcium transport into tomato (Lycopersicon esculentum Mill, cv Castlemart) fruit tonoplast vesicles was studied. Calcium uptake was stimulated approximately 10-fold by MgATP. Two ATP-dependent Ca2+ transport activities could be resolved on the basis of sensitivity to nitrate and affinity for Ca2+. A low affinity Ca2+ uptake system (Km > 200 micromolar) was inhibited by nitrate and ionophores and is thought to represent a tonoplast localized H+/Ca2+ antiport. A high affinity Ca2+ uptake system (Km = 6 micromolar) was not inhibited by nitrate, had reduced sensitivity to ionophores, and appeared to be associated with a population of low density endoplasmic reticulum vesicles that contaminated the tonoplast-enriched membrane fraction. Arrhenius plots of the temperature dependence of Ca2+ transport in tomato membrane vesicles showed a sharp increase in activation energy at temperatures below 10 to 12°C that was not observed in red beet membrane vesicles. This low temperature effect on tonoplast Ca2+/H+ antiport activity could only by partially ascribed to an effect of low temperature on H+-ATPase activity, ATP-dependent H+ transport, passive H+ fluxes, or passive Ca2+ fluxes. These results suggest that low temperature directly affects Ca2+/H+ exchange across the tomato fruit tonoplast, resulting in an apparent change in activation energy for the transport reaction. This could result from a direct effect of temperature on the Ca2+/H+ exchange protein or by an indirect effect of temperature on lipid interactions with the Ca2+/H+ exchange protein.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A. B., O'neill S. D., Eilmann M., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: III. Modulation of ATPase Activity by Reaction Substrates and Products. Plant Physiol. 1985 Jul;78(3):495–499. doi: 10.1104/pp.78.3.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Poole R. J. Kinetics of Ca/H Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris L. Plant Physiol. 1986 Mar;80(3):727–731. doi: 10.1104/pp.80.3.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush D. R., Sze H. Calcium transport in tonoplast and endoplasmic reticulum vesicles isolated from cultured carrot cells. Plant Physiol. 1986 Feb;80(2):549–555. doi: 10.1104/pp.80.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson I. B., Reid M. S., Romani R. J. Effects of low temperature and respiratory inhibitors on calcium flux in plant mitochondria. Plant Physiol. 1985 Apr;77(4):877–880. doi: 10.1104/pp.77.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka P. W. Defense strategies against hypoxia and hypothermia. Science. 1986 Jan 17;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Oleski N., Mahdavi P., Peiser G., Bennett A. B. Transport Properties of the Tomato Fruit Tonoplast : I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1987 Aug;84(4):993–996. doi: 10.1104/pp.84.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. J., Wayne R. O., Datta N. Role of calcium ions in phytochrome responses: an update. Physiol Plant. 1986;66:344–348. doi: 10.1111/j.1399-3054.1986.tb02430.x. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. A Ca/H Antiport System Driven by the Proton Electrochemical Gradient of a Tonoplast H-ATPase from Oat Roots. Plant Physiol. 1985 Dec;79(4):1111–1117. doi: 10.1104/pp.79.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Zocchi G., Hanson J. B. Calcium influx into corn roots as a result of cold shock. Plant Physiol. 1982 Jul;70(1):318–319. doi: 10.1104/pp.70.1.318. [DOI] [PMC free article] [PubMed] [Google Scholar]


