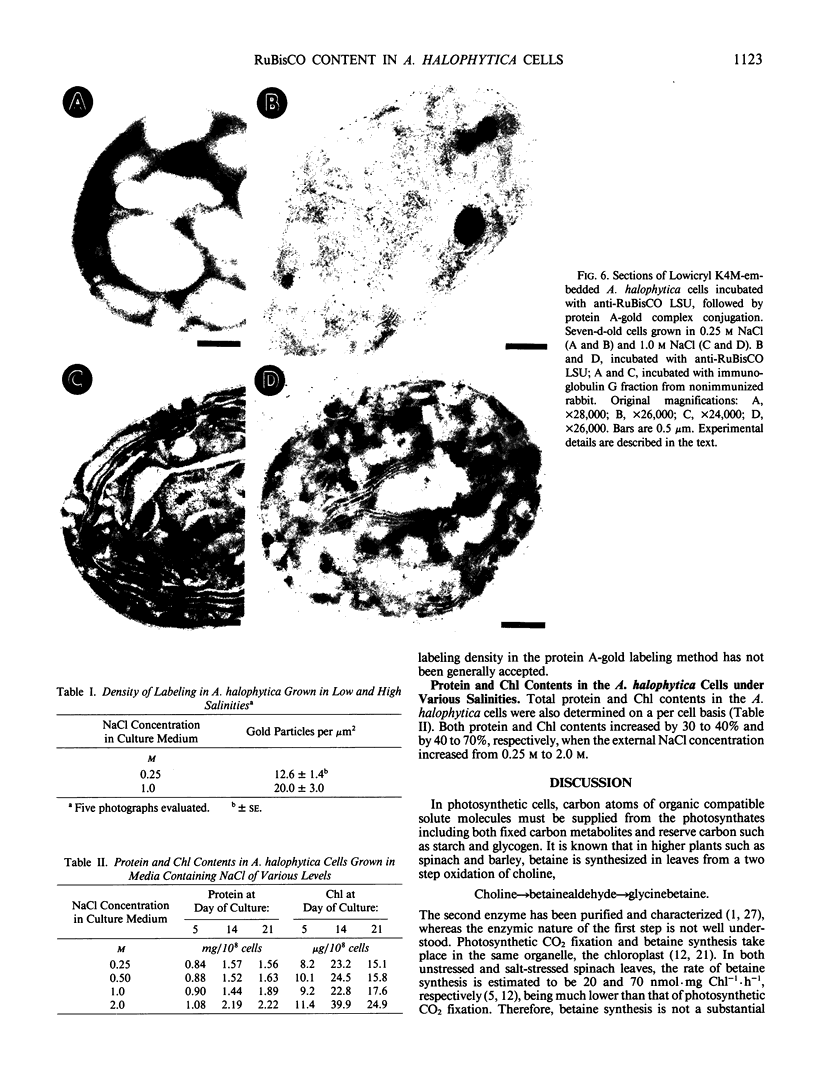
Abstract
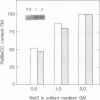
The growth of the halotolerant cyanobacterium Aphanothece halophytica, previously adapted to 0.5 molar NaCl, was optimal when NaCl concentration in culture medium was in the range 0.5 to 1.0 molar. The growth was delayed at either too low or too high salinities with lag time of ca. 0.5 day in 0.25 molar NaCl and ca. 2 days in 2 molar NaCl under the experimental conditions. However, the growth rates at the logarithmic phase were similar in the culture media containing NaCl in the range 0.25 to 2.0 molar. The capacity of photosynthetic CO2 fixation increased 3.7-fold in the cells at the logarithmic phase as NaCl concentration in the culture medium increased from 0.25 to 2.0 molar. The protein level of ribulose 1,5-bisphosphate carboxylase/oxygenase was also found to increase with increasing salinity using both an immunoblotting method and protein A-gold immunoelectron microscopy. These results indicate that high photosynthetic capacity and high ribulose 1,5-bisphosphate carboxylase/oxygenase content may entail an important role in betaine synthesis and adaptation of the A. halophytica cells to high NaCl level.
Full text
PDF
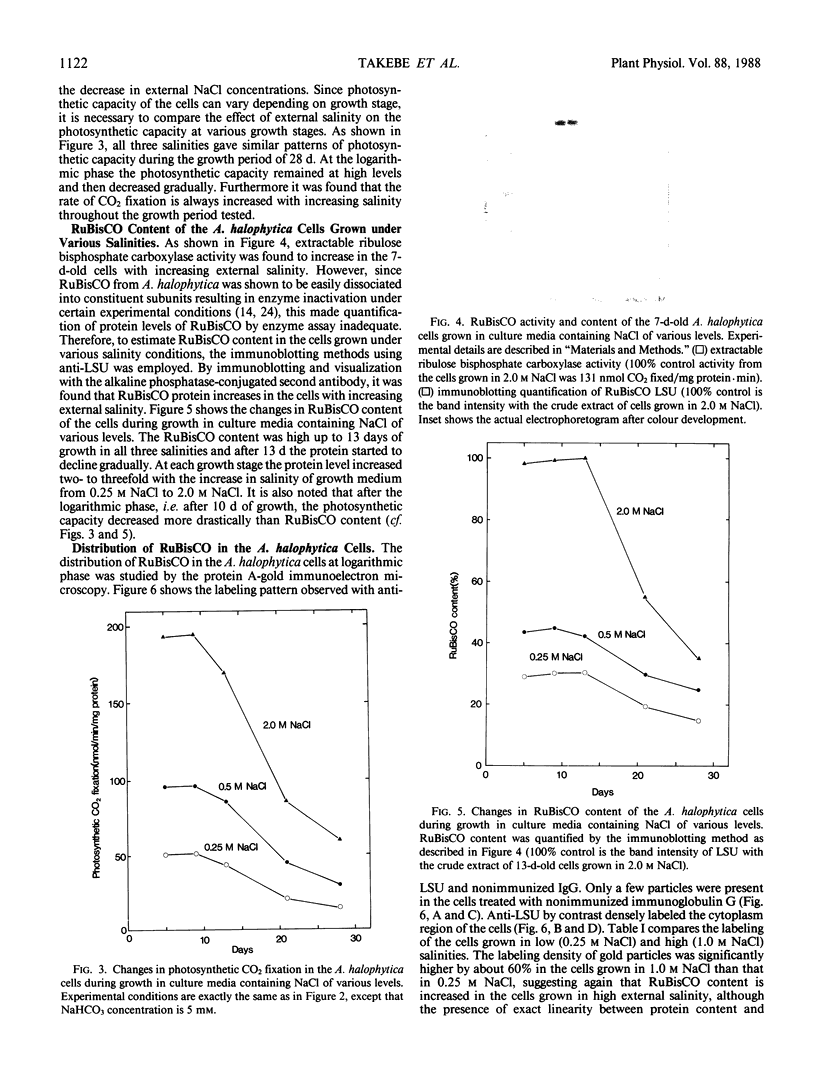

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa K., Takabe T., Sugiyama T., Akazawa T. Purification of betaine-aldehyde dehydrogenase from spinach leaves and preparation of its antibody. J Biochem. 1987 Jun;101(6):1485–1488. doi: 10.1093/oxfordjournals.jbchem.a122019. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Blumwald E., Tel-Or E. Salt adaptation of the cyanobacterium synechococcus 6311 growing in a continuous culture (turbidostat). Plant Physiol. 1984 Jan;74(1):183–185. doi: 10.1104/pp.74.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlick S., Oren A., Padan E. Occurrence of facultative anoxygenic photosynthesis among filamentous and unicellular cyanobacteria. J Bacteriol. 1977 Feb;129(2):623–629. doi: 10.1128/jb.129.2.623-629.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A. D., May A. M., Grumet R., Bode J., Jamieson G. C., Rhodes D. Betaine synthesis in chenopods: Localization in chloroplasts. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3678–3682. doi: 10.1073/pnas.82.11.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incharoensakdi A., Takabe T., Akazawa T. Effect of Betaine on Enzyme Activity and Subunit Interaction of Ribulose-1,5-Bisphosphate Carboxylase/Oxygenase from Aphanothece halophytica. Plant Physiol. 1986 Aug;81(4):1044–1049. doi: 10.1104/pp.81.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incharoensakdi A., Takabe T., Akazawa T. Factors affecting the dissociation and reconstitution of ribulose-1,5-bisphosphate carboxylase/oxygenase from Aphanothece halophytica. Arch Biochem Biophys. 1985 Mar;237(2):445–453. doi: 10.1016/0003-9861(85)90298-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landfald B., Strøm A. R. Choline-glycine betaine pathway confers a high level of osmotic tolerance in Escherichia coli. J Bacteriol. 1986 Mar;165(3):849–855. doi: 10.1128/jb.165.3.849-855.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabe T., Rai A. K., Akazawa T. Interaction of constituent subunits in ribulose 1,5-bisphosphate carboxylase from Aphanothece halophytica. Arch Biochem Biophys. 1984 Feb 15;229(1):202–211. doi: 10.1016/0003-9861(84)90145-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegmann K. Osmotic regulation of photosynthetic glycerol production in Dunaliella. Biochim Biophys Acta. 1971 Jun 15;234(3):317–323. doi: 10.1016/0005-2728(71)90197-6. [DOI] [PubMed] [Google Scholar]
- Weigel P., Weretilnyk E. A., Hanson A. D. Betaine aldehyde oxidation by spinach chloroplasts. Plant Physiol. 1986 Nov;82(3):753–759. doi: 10.1104/pp.82.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Yokota S., Tsuji H., Kato K. Localization of lysosomal and peroxisomal enzymes in the specific granules of rat intestinal eosinophil leukocytes revealed by immunoelectron microscopic techniques. J Histochem Cytochem. 1984 Mar;32(3):267–274. doi: 10.1177/32.3.6693757. [DOI] [PubMed] [Google Scholar]