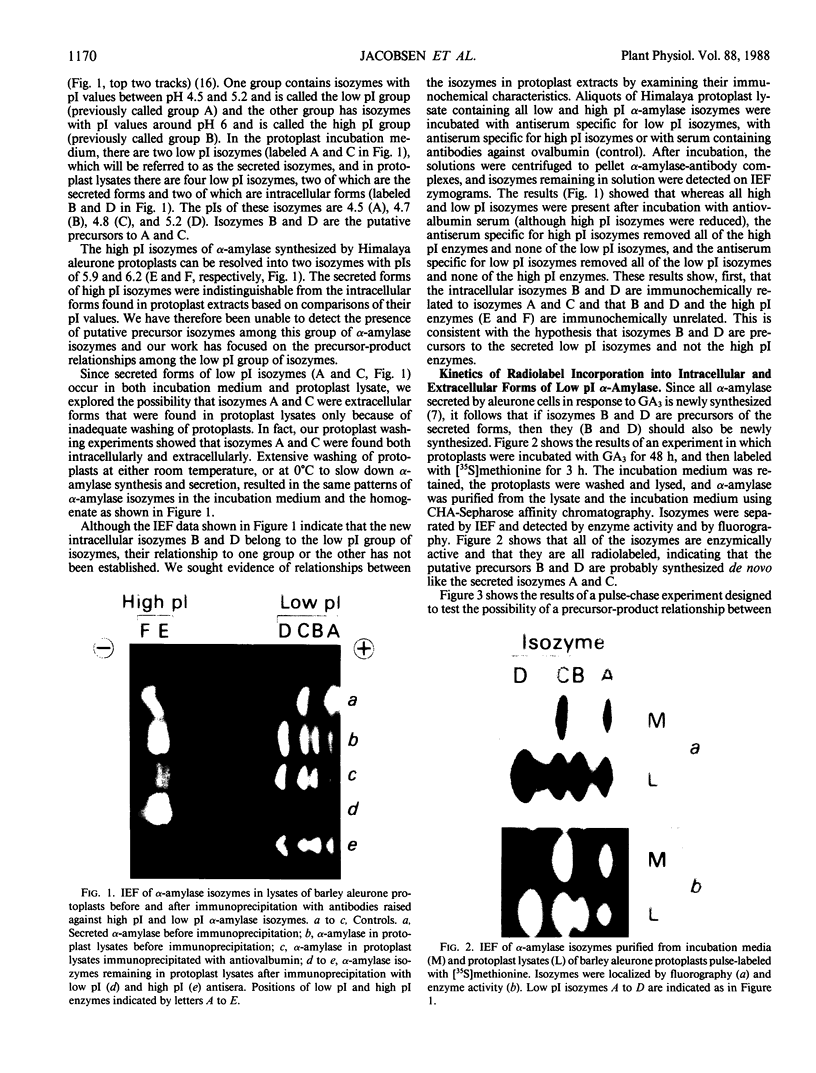
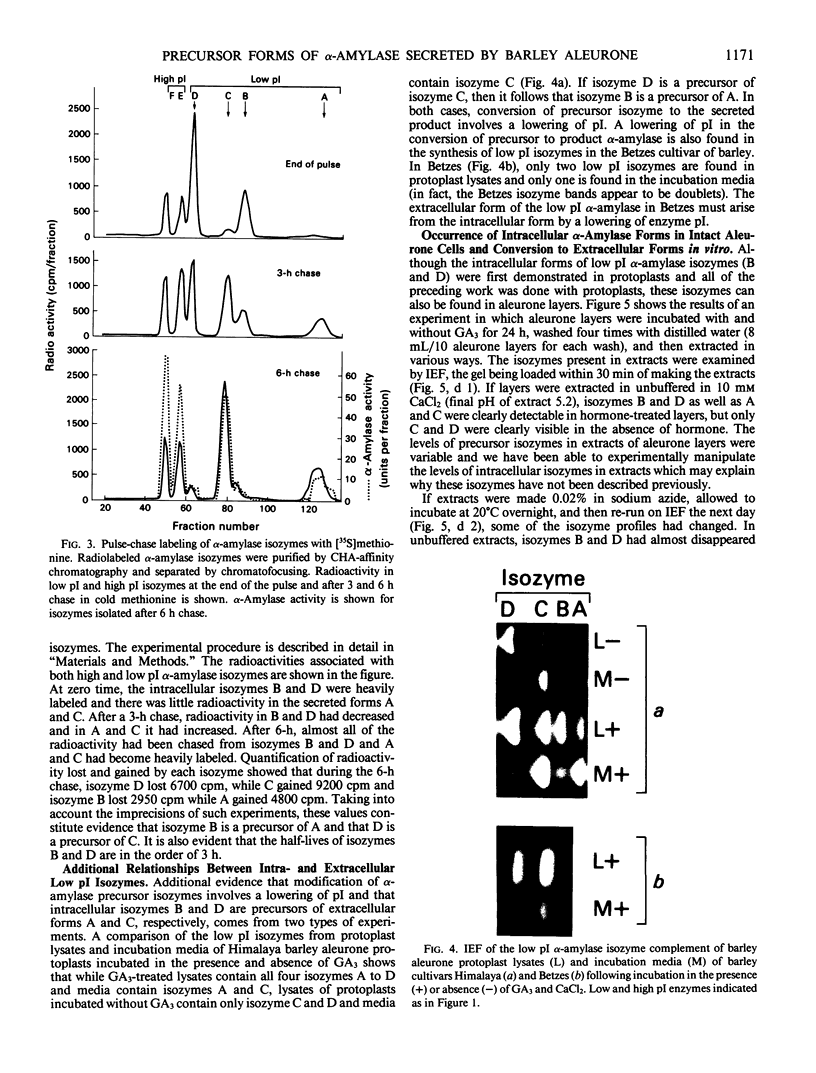
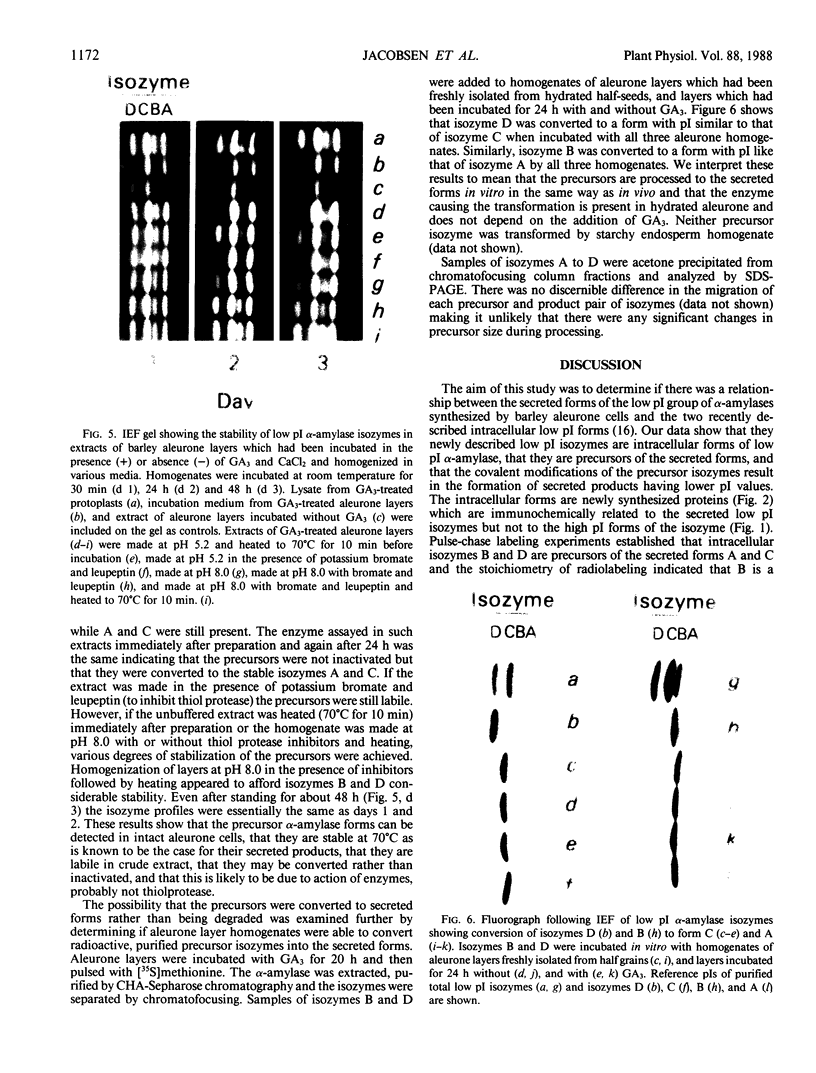
Abstract
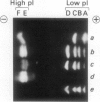
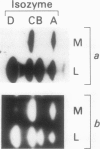
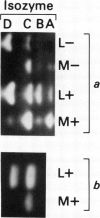
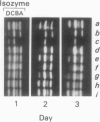
Gibberellin-treated barley (Hordeum vulgare L.) aleurone cell protoplasts have been shown previously to contain two α-amylase isozymes which are not secreted (JV Jacobsen, JA Zwar, PM Chandler 1985 Planta 13: 430-438). This report shows that these intracellular forms are immunochemically related to the low isoelectric point but not the high isoelectric point group of α-amylase isozymes and that they arise by new synthesis like the secreted forms. Pulse-chase studies show that the intracellular isozymes are precursors to the secreted isozymes. Conversion of the intra- to the extracellular forms involves decreases in isoelectric points with no change in size detectable by SDS-PAGE. The precursor isozymes were also detected in aleurone layer homogenates but they were unstable. They could be stabilized by various treatments including heating the homogenate to 70°C for 10 minutes indicating that the instability was enzymically mediated. Using purified radioactive precursor isozymes, it was shown that instability did not involve inactivation but the conversion to secreted forms. The nature of the covalent modification associated with conversion was not determined but available data indicate that it does not involve glycosylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beintema J. J., Gaastra W., Scheffer A. J., Welling G. W. Carbohydrate in pancreatic ribonucleases. Eur J Biochem. 1976 Apr 1;63(2):441–448. doi: 10.1111/j.1432-1033.1976.tb10246.x. [DOI] [PubMed] [Google Scholar]
- Cardelli J. A., Golumbeski G. S., Dimond R. L. Lysosomal enzymes in Dictyostelium discoideum are transported to lysosomes at distinctly different rates. J Cell Biol. 1986 Apr;102(4):1264–1270. doi: 10.1083/jcb.102.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J., Varner J. E. Gibberellic Acid-enhanced synthesis and release of alpha-amylase and ribonuclease by isolated barley and aleurone layers. Plant Physiol. 1967 Mar;42(3):398–406. doi: 10.1104/pp.42.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland C. S., Doms R. W., Bolzau E. M., Webster R. G., Helenius A. Assembly of influenza hemagglutinin trimers and its role in intracellular transport. J Cell Biol. 1986 Oct;103(4):1179–1191. doi: 10.1083/jcb.103.4.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filner P., Varner J. E. A test for de novo synthesis of enzymes: density labeling with H2O18 of barley alpha-amylase induced by gibberellic acid. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1520–1526. doi: 10.1073/pnas.58.4.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorecki M., Zeelon E. P. Cell-free synthesis of rat parotid preamylase. J Biol Chem. 1979 Jan 25;254(2):525–529. [PubMed] [Google Scholar]
- Jacobsen J. V., Higgins T. J. Characterization of the alpha-Amylases Synthesized by Aleurone Layers of Himalaya Barley in Response to Gibberellic Acid. Plant Physiol. 1982 Dec;70(6):1647–1653. doi: 10.1104/pp.70.6.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. J., Kauffman D. L., Allan B. J., Williams B. L. Further studies on the structural differences between the isoenzymes of human parotid -amylase. Biochemistry. 1971 Dec 21;10(26):4867–4874. doi: 10.1021/bi00802a006. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Rogers J. C. Two barley alpha-amylase gene families are regulated differently in aleurone cells. J Biol Chem. 1985 Mar 25;260(6):3731–3738. [PubMed] [Google Scholar]
- Schwaiger H., Tanner W. Effects of gibberellic acid and of tunicamycin on glycosyl-transferase activities and on alpha-amylase secretion in barley. Eur J Biochem. 1979 Dec 17;102(2):375–381. doi: 10.1111/j.1432-1033.1979.tb04252.x. [DOI] [PubMed] [Google Scholar]
- Silvanovich M. P., Hill R. D. Affinity chromatography of cereal alpha-amylase. Anal Biochem. 1976 Jun;73(2):430–433. doi: 10.1016/0003-2697(76)90191-3. [DOI] [PubMed] [Google Scholar]
- Wold F. In vivo chemical modification of proteins (post-translational modification). Annu Rev Biochem. 1981;50:783–814. doi: 10.1146/annurev.bi.50.070181.004031. [DOI] [PubMed] [Google Scholar]