Abstract
Pregnancy and lactation hormones have been shown to mediate anatomical changes to the musculoskeletal system that generates animal movement. In this study, we characterize changes in the medial gastrocnemius muscle, its tendon and aponeuroses that are likely to have an effect on whole animal movement and energy expenditure, using the rat model system, Rattus norvegicus. We quantified muscle architecture (mass, cross‐sectional area, and pennation angle), muscle fiber type and diameter, and Young's modulus of stiffness for the medial gastrocnemius aponeuroses as well as its contribution to Achilles tendon in three groups of three‐month‐old female rats: virgin, primiparous pregnant, and primiparous lactating animals. We found that muscle mass drops by 23% during lactation but does not change during pregnancy. We also found that during pregnancy muscle fibers switch from Type I to IIa and during lactation from Type IIb to Type I. The stiffness of connective tissues that has a demonstrated role in locomotion, the aponeurosis and tendon, also changed. Pregnant animals had a significantly less stiff aponeurosis. However, tendon stiffness was most affected during lactation, with a significant drop in stiffness and interindividual variation. We propose that the energetic demands of locomotion may have driven the evolution of these anatomical changes in muscle‐tendon units during pregnancy and lactation to ensure more energy can be allocated to fetal development and lactation.
Keywords: biomechanics, female athlete, muscle mechanics, tendon mechanics
We examined the anatomy of a muscle‐tendon unit that is critical for walking, the medial gastrocnemius and its contribution to the Achilles tendon, in virgin non‐pregnant (NP), primiparous pregnant (P), and lactating (L) rats. We found that muscle fiber type composition varied among these stages, as a result of changes in both muscle fiber diameter and number of each fiber type. We also quantified Young's modulus of elasticity of two connective tissues, the muscle aponeurosis and the tendon. We found that the aponeurosis is significantly less stiff during pregnancy, but indistinguishable among the NP and lactating groups. However, Young's modulus of the muscle tendon showed high interindividual variation obstructing any statistically significant effect of reproductive condition, although there is a trend towards lower tendon stiffness during pregnancy that may persist through lactation. These changes in muscle‐tendon anatomy are likely to affect contractile properties such as muscle gearing ratio and elastic potential energy storage during walking or running.
1. INTRODUCTION
All eutherian mammals, including humans, have a prolonged gestation period followed by lactation, an even more metabolically expensive physiological state (Hammond & Diamond, 1992). Much of the limited literature on movement during pregnancy in mammals deals with changes in posture and the center of mass due to the fetal load. Pregnant humans tend to increase the contact time of the legs with the ground (duty factor) (Forczek & Staszkiewicz, 2012) and display increased lumbar lordosis to redistribute the fetal load over the supporting limbs (Whitcome et al., 2009). However, the effect of remodeling in the limb muscle that both supports the fetal load and ensures healthy movement in the mother has not been fully explored, despite the fact that muscle remodeling can directly impact locomotor performance (Zierath & Hawley, 2004).
The goal of this study was to assess whether and how the anatomy of a muscle‐tendon unit that is critical for walking and running, changes during pregnancy and lactation. The limited existing research into the effects of pregnancy and lactation on skeletal muscle is motivated by the muscle's susceptibility to insulin resistance in gestational diabetes (Lontay et al., 2015), meat quality in animal science (Lefaucheur & Ecolan, 1990) or pelvic floor dysfunction in humans (Catanzarite et al., 2018). However, a large gap remains in our understanding of how pregnancy and lactation affect the remodeling of tissues that are relevant to locomotion. A panel of 16 experts convened by the International Olympic Committee to review the current literature on the link between pregnancy, birthing, and exercise (Bø et al., 2016), found only one study that tracked the effects of pregnancy on muscle (Lontay et al., 2015), none on tendons, and no studies on muscle morphology during lactation (Bø et al., 2017). The gap in our knowledge of female physiology is not constrained to human biology, but is true for studies in ecology and evolution as well (Klein et al., 2015).
One of the reasons for this lack of information is likely to be the confounding effects of changing endocrine hormones during pregnancy and lactation. Although the cellular effects of such hormones on the musculoskeletal system have been documented (Dehghan et al., 2014; Ferlin et al., 2017; Hansen & Kjaer, 2017; Ireland & Ott, 2000), the effects on whole muscles have not been described or quantified well. Muscle fibers change biochemically, from slow oxidative to fast glycolytic (Lefaucheur & Ecolan, 1990; Lontay et al., 2015), and there is even evidence that new muscle fibers (Li et al., 2006; Rehage et al., 2007) are formed in the presence of relaxin, which are younger and thus produce higher forces (Mu et al., 2010). Additionally, some of the hormones, such as relaxin, have a vascularizing and vasodilating effect on muscle (Braun et al., 2012; Willcox et al., 2013), effects that have the potential to affect muscle performance during exercise.
The high rate of protein anabolism during milk production results in protein catabolism and hence sarcopenia in maternal skeletal muscles (Clowes et al., 2003; Clowes et al., 2005; Etienne et al., 1985; King et al., 1993; Lefaucheur & Ecolan, 1990; Lefaucheur et al., 1986). As a result, a reduction in muscle mass on the order of 15% is not unusual (Etienne et al., 1985). However, the muscle atrophy appears to be muscle specific because it is mediated by insulin receptor concentration (Lefaucheur et al., 1986; Lefaucheur & Vigneron, 1986; Lontay et al., 2015), which is highest in Type I fibers, lowest in Type IIb fibers and intermediate in Type IIa fibers. As a result, Type I muscle fibers are more sensitive to catabolism during the energetically demanding stages of pregnancy and lactation (Atinmo et al., 1976; Lontay et al., 2015). In the presence of estrogen, which remains elevated throughout pregnancy and lactation, Type I fibers change to Type IIa, and Type IIa changes to Type IIb (Lontay et al., 2015). However, because of the different concentration of insulin receptors in these fiber types, the transition from Type I to IIa is slower than the transition from Type IIa to IIb. As a result, muscles with higher proportions of Type I fibers, such as the longissimus dorsi muscle, see a larger percent change in fiber composition during pregnancy and lactation, from 45% Type IIa in non‐pregnant (NP) animals to 65% postpartum (Lefaucheur & Ecolan, 1990; Lontay et al., 2015).
Sex hormones, including relaxin and estrogen, also have a significant effect on the elastic connective tissues that along with muscle fibers determine the function of the whole muscle or muscle‐tendon unit (Biewener & Roberts, 2000; Holt et al., 2016; Roberts & Azizi, 2011). The role of relaxin in loosening the pubic symphysis during pregnancy has long been known (Samuel et al., 1998). In vitro and epidemiological studies have shown that relaxin, in particular, has an antifibrotic effect on collagenous tissues (Hansen & Kjaer, 2017; Kang et al., 2017) reducing their stiffness. The stiffness of tissues such as muscle aponeuroses and tendons has the potential to affect a muscle‐tendon unit's range of motion, gear ratio, and storage of elastic potential energy (Konow et al., 2020). If an increased aponeurosis stiffness can cause a loss of variable muscle gear ratio (Holt et al., 2016), a decreased aponeurosis stiffness could potentially lead to higher range of muscle gear ratios, offering fine‐tuning of the muscle's properties to its locomotor demands. Similarly, an increase in tendon stiffness resulted in a reduction in elastic potential energy usage, which is likely to be a factor in the increased metabolic cost of locomotion in the elderly (Mian et al., 2006). Additionally, increased stretching of a more compliant muscle before contraction may affect muscle‐tendon unit performance (Seiberl et al., 2021). However, the link between these morphological and biochemical changes in muscle‐tendon units during pregnancy and lactation, and locomotor performance remains unclear.
In this study, we explore the effects of pregnancy and lactation on the morphology of the medial gastrocnemius muscle, its aponeuroses and its main tendon, the Achilles, in the rat Rattus norvegicus, an animal model of terrestrial locomotion that is extensively used in both comparative and biomedical contexts. The gastrocnemius muscle is critical for powering walking (Huang et al., 2015; Robertson & Sawicki, 2014), and its tendon was shown to store elastic energy (Konow et al., 2020; Roberts et al., 1997). Given that the gastrocnemius muscle in rats is high in Type I muscle fibers (Eng et al., 2008), we would expect to see a large shift in fiber type during pregnancy and lactation as well. Although the link between fiber type and insulin resistance has been appreciated for a while because of the risks associated with gestational diabetes (Narvaez‐Sanchez et al., 2019), the functional implications of muscle fiber type changes in animal locomotion remain completely unexplored, despite the fact that differences in muscle fiber proportion of similar magnitude can have significant functional repercussions (Thompson, 2017). Together, the increased blood circulation, new muscle fibers, and fiber type changes suggest there could be significant effects on the organism's capacity for prolonged aerobic activity during pregnancy and lactation.
2. MATERIALS AND METHODS
We used young healthy Sprague Dawley rats Rattus norvegicus (Charles River) that were 3–6 months old and focused on three physiological stages: virgin female animals (NP); primiparous pregnant animals (P) between days 14–17 of a 21 day‐long pregnancy; lactating animals (L) that are primiparous and have completed the normal length of lactation for the rat, which is 21 days. For the material properties, we also included young (3–6 months) male samples for comparison.
2.1. Muscle morphology
The length of the left medial gastrocnemius was measured in situ, and the muscle was then dissected from each animal and weighted while fresh. The Achilles tendon from the left leg was also removed. The tissues were then stored in mammalian physiological saline for up to 6 months. The right muscle was fixed in place with an injection of 10% formalin while the leg was kept in a constant position, with the knee and ankle at 90° for consistency. After 60 min, the muscle was dissected out and fixed overnight in formalin and transferred to 70% ethanol for storage. Fiber pennation angle and length from the fixed muscle were measured from images taken with a Leica M165C microscope equipped with a Leica Microsystems 80 mm analyzer.
2.2. Uniaxial materials testing
Proximal aponeuroses and tendons were isolated from muscle samples previously frozen. The peritenon of the Achilles tendon was removed and an individual muscle tendon used for testing. Samples were placed along the longitudinal axis of the tissue and mounted on spring‐loaded clamps in a Univert uniaxial testing unit (CellScale™), equipped with a 10 N load cell. For each aponeurosis, rectangular sections of approximately 4 mm × 10 mm were used. Tissues were preloaded with ~0.2 N of force and then stretched with a ramp signal by 5% for tendon and 10%, 12%, and 15% for aponeuroses only, at 5 Hz for 1 s, to simulate in vivo strain rates (Horner et al., 2011). Each tissue was preconditioned with one cycle before measurements were taken. Length increases were chosen to gradually find the maximal length that the tissue could be deformed to without damaging it, especially since we had no a priori way to know how our conditions would affect the mechanical properties of the tissue. Length changes for aponeuroses were larger than for tendons based on previous work (Danos et al., 2016; Holt et al., 2016). Resting length, L0, was calculated as the length of the tissue at 0.2 N force and strain was calculated as the increase in length divided by L0. Cross‐sectional area of the tissue was calculated from the mass of the tissue between the clamps and the density of collagenous tissues (Mendez & Keys, 1960). We used tissue cross‐sectional area to normalize the force, by diving measured force normalized by cross‐sectional area.
A quadratic function was fit to these data and the energy lost during the stretch and recovery phase (hysteresis) was calculated, as the difference in the area under the stretch curve and the recovery curve as a percent of the area under the stretch curve. Young's modulus was calculated as the tangent slope of the stress–strain curve at 5% strain for tendons and 7.5% strain for aponeuroses, from the first stretch cycle after preconditioning. ANOVA and Tukey tests (α = 0.05) were done on each variable calculated per condition to determine statistical differences (RStudio, 2022, version 2022.02.1.461).
2.3. Histology
The frozen medial gastrocnemius was defrosted and cut across the muscle belly perpendicular to the muscle's line of action. One‐half of the muscle was covered in OCT, refrozen using liquid nitrogen, and mounted on a cryostat specimen disc. The muscle sample was then placed in a ‐4°C freezer to allow excess 2‐methyl‐butane to evaporate until sectioning, which was done at 10 μm thickness and at −20°C and placed on poly‐L‐lysine coated slides. For each rat, three muscle sections were stained with a standard hematoxylin and eosin dye to check if sectioning was done perpendicularly to the long axis of most fibers. Well‐sectioned fibers were then stained for fast twitch fiber protein Myosin IIa [SC‐71; dilution factor of 1:30] and Myosin IIb [BF‐F3; dilution factor of 1:150] and for myosin heavy chain Type I protein [BA‐D5; dilution factor of 1:1000] using a modified protocol (Ehmsen et al., 2019) (Table 1). We also stained for laminin to outline each cell membrane (dilution factor of 1:1000). The stained muscle samples were then imaged under a Leica EVOS microscope.
TABLE 1.
Anatomical structure and the relationship to its Primary Antibody, Secondary Antibody, Excitation Wavelength, Emission Wavelength, and Color.
| Anatomical structure | 1° antibody | 2° antibody | Excitation wavelength (nm) | Emission wavelength (nm) | Color |
|---|---|---|---|---|---|
| Type I | BA‐D5 (DSHB*) | DyLight 405 (Jackson Laboratories) | 399 | 434 | Blue |
| Type IIa | SC‐71 (DHSB) | Alexa Fluor® IGG1‐488 (Jackson Laboratories) | 493 | 519 | Green |
| Type IIb | BF‐F3 (DHSB) | Alexa Fluor® IGM‐594 (Jackson Laboratories) | 590 | 617 | Orange/Red |
| Laminin |
Anti‐Lamini‐2 (Millipore Sigma) |
Alexa Fluor® IGC‐647 (Thermo Fisher Scientific) | 651 | 667 | Red |
DSHB is Developmental Studies Hybridoma Bank, The University of Iowa.
Image analysis was performed using FIJI (Schindelin et al., 2012). We outlined each muscle fiber and measured its cross‐sectional area as well the number of fibers per image (1127.75 by 845.81 μm) for each fiber type. We then summed all the fiber cross‐sectional area across all types to obtain total muscle cross‐sectional area, so we could calculate the proportion of total muscle cross‐sectional area occupied by each fiber type. We assumed a circular cross‐sectional shape for each fiber and calculated a fiber diameter, to facilitate comparisons with other published values. Between one and three sections were measured for each individual and the individual mean calculated. The proportion of each muscle fiber type was transformed using an arcsine function before performing an ANOVA on the effect of condition on muscle fiber type proportion.
Number and diameter for Type I, Type IIa, and Type IIb were combined as response variables in a MANOVA with Condition and Individual as the predictor variable.
3. RESULTS
3.1. Muscle morphology
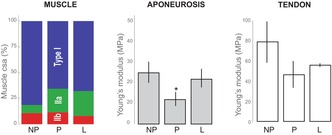
The mass of the medial gastrocnemius muscle (Figure 1) in lactating animals was significantly lower than in non–pregnant and pregnant animals which were indistinguishable from each other (ANOVA d.f. = 10, F = 11.917, p = 0.002; Tukey HSD p = 0.006). Body mass, however, was significantly higher during pregnancy, but indistinguishable before (NP) and at the end of lactation (L), as expected (ANOVA d.f. = 10, F = 5.898, p = 0.02; Tukey HSD NP‐P p = 0.025 and P‐L p = 0.044). As a result, the Muscle mass: Body mass ratio was higher in NP animals but similarly low in P and L, despite a nearly 27% increase in body mass during pregnancy.
FIGURE 1.
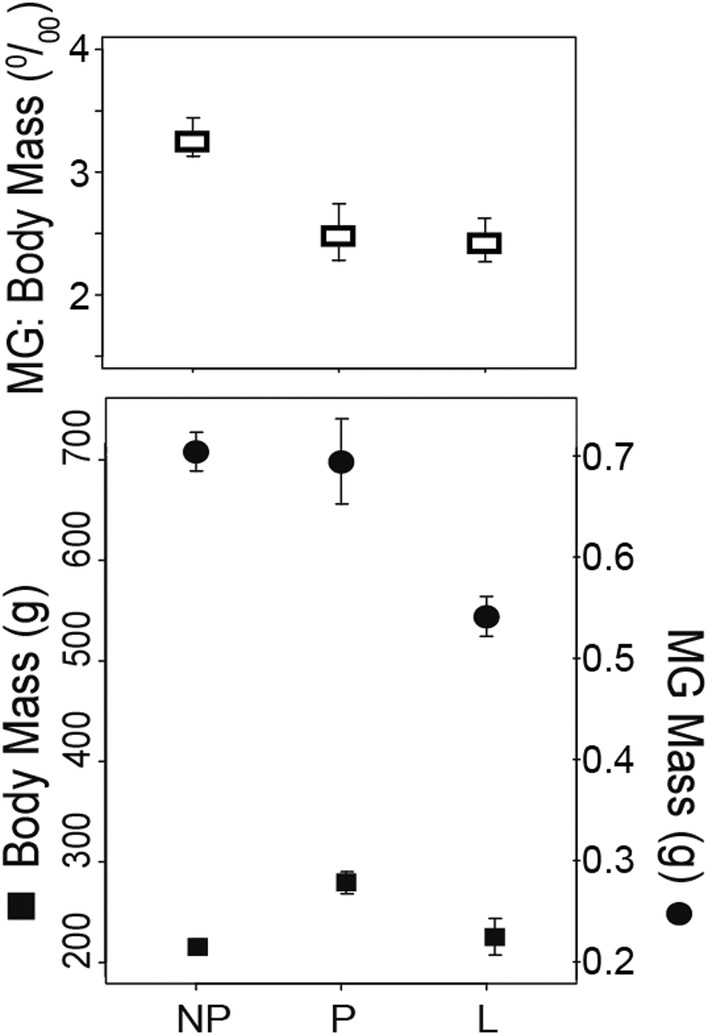
Muscle and body mass. Mean±SEM absolute and relative mass of the medial gastrocnemius in non‐pregnant (NP, N = 4), pregnant (P, N = 4) and postpartum (L, N = 5) rats.
Pennation angle did not differ significantly among the conditions examined (23.2 ± 0.4°).
3.2. Material properties
Mean tendon and aponeurosis Young's modulus as well as hysteresis values are reported in Table 2. Variation in the tendon Young's modulus was not explained by Condition (ANOVA, F = 1.06, d.f. = 3, p = 0.39). However, visual inspection of the stress–strain curves resulting from tendon uniaxial tests (Figure 2) showed noticeably lower variation in the traces from lactating animals compared to the other samples. Therefore, we used Welch's two‐sample t‐tests to test for differences in the means among groups, since this test is less sensitive to unequal variances than an ANOVA. We performed three tests, comparing each of the female groups to the male group. Only the mean Young's modulus from Lactating group differed from that of the male group (Welch t‐test, t = 4.59, d.f. = 4.19, p = 0.009; Figure 3).
TABLE 2.
Tendon and aponeurosis Young's modulus (E) and hysteresis as proportion of energy lost.
| NP | P | L | Male | ||
|---|---|---|---|---|---|
| Tendon | E (MPa) | 78.67 ± 20.38 | 46.64 ± 12.86 | 55.78 ± 1.48 | 82.65 ± 10.50 |
| Hysteresis | 0.18 ± 0.040 | 0.20 ± 0.028 | 0.22 ± 0.017 | 0.19 ± 0.016 | |
| Aponeurosis | E (MPa) | 7.92 ± 2.04 | 8.07 ± 2.13 | 9.79 ± 2.73 | 9.89 ± 2.56 |
| Hysteresis | 0.51 ± 0.067 | 0.43 ± 0.061 | 0.38 ± 0.031 | 0.42 ± 0.040 |
Note: N = 5 per group, mean ± SEM.
FIGURE 2.

Elastic properties of connective tissues in the muscle‐tendon unit. Stretch component of a ramp stretch‐relax cycle from a uniaxial tensile stress on the contribution of the medial gastrocnemius tendon to the Achilles. Note the low variation in stress–strain curves in lactating animals.
FIGURE 3.
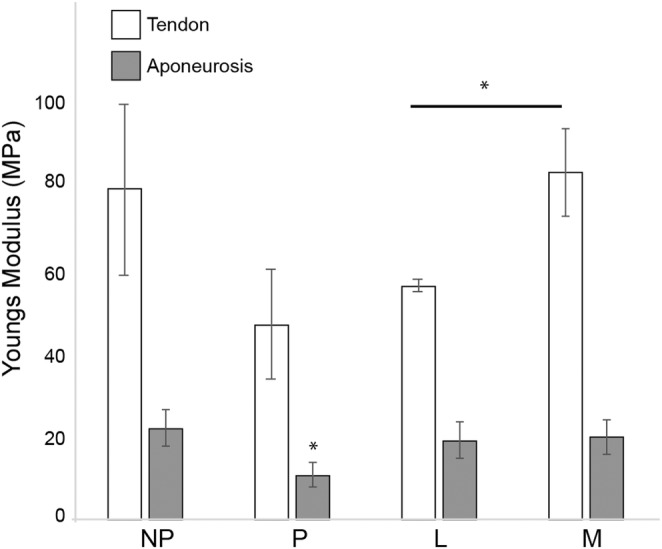
Comparison of Young's modulus for the Achilles tendon and aponeurosis of the medial gastrocnemius. Young's modulus was lower for lactating mammals than male animals, but indistinguishable among samples from all the female rats. However, Young's modulus of the aponeurosis was only significantly lower during pregnancy.
There was no significant difference in mean tendon hysteresis values among groups (20.0 ± 2.5%, mean ± SEM.; Table 2).
An ANOVA did not find a significant effect of condition on Young's modulus of the medial gastrocnemius aponeurosis (Figure 3; Table 2; ANOVA, F = 1.34, d.f. = 3, p = 0.30). A visual inspection of the mean aponeurosis Young's modulus (Figure 3) made us wonder if Young's modulus for the pregnant group was lower than the values for non–pregnant and lactating groups. A one‐sided Welch t‐test between the values from non–pregnant and pregnant groups indicated that Young's modulus of is significantly lower at the alpha = 0.10 level (Welch's t‐test, t = −1.62, d.f. = 6.5, p = 0.076).
Aponeurosis hysteresis, the amount of energy lost during each stretch‐release cycle, did not differ among our groups (46 ± 10%).
3.3. Muscle fiber types
All muscle fiber types were affected by Condition (Tables 3 and 4; Figure 4). The mean (±SEM) number (Figure 4(b)) of Type I fibers per slide increased significantly during pregnancy compared to the NP condition and returned to pre‐pregnancy levels during lactation (ANOVA, F = 5.09, d.f. = 2, p = 0.027; Tukey HSD). The number of Type IIa fibers showed a significant increase during pregnancy and a return to NP numbers during (ANOVA, F = 5.09, d.f. = 2, p = 0.027; Tukey HSD). The number of Type IIb fibers did not change during pregnancy but decreased significantly during lactation (ANOVA, F = 4.77, d.f. = 2, p= 0.032; Tukey HSD).
TABLE 3.
MANOVA results for fiber type composition variables (number of fibers and fiber diameter (μm)) with Condition (NP, P, L; d.f. = 2) as the predictor variable.
| Type I | Type IIa | Type IIb | ||||
|---|---|---|---|---|---|---|
| Number | Diameter | Number | Diameter | Number | Diameter | |
| F‐statistic | 5.092 | 10.718 | 3.263 | 6.331 | 4.77 | 7.696 |
| p‐value | 0.027* | 0.0026* | 0.077** | 0.0148* | 0.032* | 0.008* |
Note: All variables were significantly affected by Condition at the 0.10 level (*) and all except the number of Type IIa fibers at the 0.05 level (**).
TABLE 4.
Overview of the effects of pregnancy and lactation on the mass and fiber type composition of the medial gastrocnemius (MG).
| NP (n = 5) | P (n = 4) | L (n = 5) | |
|---|---|---|---|
| Massbody (g) | 215.4 ± 7 | 280.0 ± 10 | 225.3 ± 17 |
| MassMG (g) | 0.704 ± 0.02 | 0.695 ± 0.04 | 0.541 ± 0.02 |
| Type I | |||
| Number of fibers | 217 ± 16.6 | 105.3 ± 19.8 | 165.2 ± 31.4 |
| Diameter (µm) | 72.2 ± 5.2 | 33.0 ± 7.9 | 34.5 ± 7.6 |
| Proportion (%) | 81.4 ± 2 | 65.0 ± 12 | 68.0 ± 6 |
| csa (mm2) | 60.9 | 48.7 | 42.8 |
| Type IIa | |||
| Number of fibers | 72.4 ± 10.2 | 129.7 ± 27.0 | 90.6 ± 9.3 |
| Diameter (µm) | 17.5 ± 0.5 | 15.4 ± 0.5 | 18.0 ± 0.5 |
| Proportion (%) | 8.0 ± 0.7 | 22.9 ± 9 | 24.1 ± 5 |
| csa (mm2) | 6.0 | 17.0 | 15.2 |
| Type IIb | |||
| Number of fibers | 37.8 ± 7.0 | 44.4 ± 5.1 | 19.3 ± 5.1 |
| Diameter (µm) | 27.8± 1.9 | 19.9 ± 1.2 | 23.3 ± 0.7 |
| Proportion (%) | 10.6 ± 2 | 11.5 ± 4 | 7.9 ± 2 |
| csa (mm2) | 7.9 | 8.5 | 5.0 |
Note: Mean ± SEM. is reported for all variables except for each fiber type cross‐sectional area (csa), which is an estimate calculated by multiplying the mean number of fibers by the mean fiber csa for each type.
FIGURE 4.

Fiber type composition as determined by antibody staining for Type I, IIa and IIb myosin heavy chains. (a). Images of triple‐stained muscle cross‐sections from non‐pregnant (NP), pregnant (P) and lactating (L) animals (top). (b). The mean (±SEM) number of Type I (blue) fibers decreased significantly during pregnancy, while the number of Type IIa (green) fibers increased during pregnancy. The number of Type IIb (red) fibers, however, remained unchanged during pregnancy but significantly decreased during Lactation. (c). Type I fibers became smaller in diameter during pregnancy and remained small during lactation. However, Type IIa and IIb decreased during pregnancy but returned to non‐pregnant size during lactation. (d). As a result of the combined number and diameter changes during pregnancy and lactation, the muscle becomes more glycolytic during pregnancy and lactation. Asterisk (*) indicates p < 0.05, Tukey HSD.
Type I fibers became smaller in diameter (Figure 4(c)) during pregnancy and remained small during lactation (ANOVA, F = 10.7, d.f. = 2, p = 0.003; Tukey HSD). However, the diameter of Type IIa and IIb decreased during pregnancy but returned to NP size during lactation (Type IIa ANOVA, F = 6.3, d.f. = 2 p = 0.015; Type IIb ANOVA, F = 7.7, d.f. = 2, p = 0.008).
As a result of the combined number and diameter changes during pregnancy and lactation, the proportion of the overall muscle cross‐sectional area changed as well (Table 4; Figure 4(d)). However, a power analysis (pwr.anova.test, library pwr, RStudio) showed that we did not have a high enough sample size to detect a large effect even at a significance level of 0.10. However, the trends we saw were toward a larger proportion of the muscle cross‐sectional area occupied by Type I fiber in NP animals than in pregnant and lactating animals. Type IIa fibers occupied the least cross‐sectional area in NP animals and the highest in lactating animals. The proportions of the whole muscle cross‐sectional area of Type IIb fibers peaked during pregnancy and dropped below the NP levels during lactation.
4. DISCUSSION
4.1. Muscle morphology and fiber type composition
There was an overall drop in muscle mass by 23% by the end of lactation (Figure 1) reflected in a nearly three‐fold reduction of fiber diameter across all fiber types (Figure 4(c)). As a result of the combined number and diameter changes during pregnancy and lactation, the muscle becomes more glycolytic during pregnancy and lactation (Figure 4(d)). Lee et al. (2015) found that mice with a Tbx15 mutation have reduced number of glycolytic (Type IIb) muscle fibers; there is approximately a 12% reduction in oxygen consumption (Lee et al., 2015). A similar shift is observed in endurance athletes, who after training show an increase in Type IIa fast oxidative muscle fibers and a decrease in Type I slow oxidative and Type IIb fast glycolytic muscle fibers. In pregnancy, it appears that the transcriptional regulator smoothelin‐like protein 1 (SMTNL1) mediates fiber switching during pregnancy, altering oxidative muscle fibers to glycolytic ones (Lontay et al., 2015). Pregnancy and lactation are two of the most metabolically demanding physiological states for vertebrates (Hammond et al., 1996). With muscle mass making up nearly one‐third of an individual's body mass (Janssen et al., 2000), changes in the metabolic efficiency of skeletal muscle cells could have adaptively significant implications.
We saw a significant a decrease in the number of Type I fibers and an increase in Type IIa but not in Type IIb fibers during pregnancy (Figure 4(b)). These results support the existing model of fiber type switching during pregnancy. Previous studies indicate that pregnancy activates certain enzymes and contractile proteins that are associated with Type IIb fibers, a process mediated by the smoothelin‐like protein 1 (SMTNL 1) transcription factor (Lontay et al., 2015). Part of the transition could be due to immobilization, since it has been shown that muscle atrophy targets Type I fibers and forms more Type IIb fibers (Ehmsen et al., 2019). Steroid sex hormones have also been implicated in muscle fiber type changes during pregnancy (Edwards, 2005). The molecular underpinnings of these anatomical changes deserve further investigation.
We did not see an increase in Type IIb fibers during pregnancy (Figure 4(b)), potentially because the duration that we examined (14–21 days of pregnancy) was too short for this transition. However, after birth and the conclusion of a normal lactation period, as muscle remodeling was no longer under the influence of pregnancy hormones, it was the Type IIb fibers that switched biochemical character to Type I (Figure 4(b)). Breastfeeding results in improved glucose handling via a decrease in insulin production, improved insulin sensitivity, and a drop in β‐cell proliferation. In addition, lipid metabolism is decreased in metabolically active tissues and lipid stores are mobilized to facilitate lipid transport to the mammary gland for milk synthesis (Hyatt et al., 2017).
4.2. Tendon and aponeurosis mechanical properties
We found that there is significantly more inter‐individual variation in the mechanical properties of the Achilles tendon in virgin female animals than in our male group. This is an important finding since most direct experimental data on which muscle‐tendon modeling is based comes from male animals (e.g. Danos et al., 2016). As a result, exercise and rehabilitation advice based on these models are likely to be ineffective for a large population of women. The large variation in mechanical properties was somewhat anticipated, given a prevalent belief in the scientific community that the menstrual cycle affects connective tissue, and is often the reason invoked for not including female animals in studies. However, we were surprised that the large variation persisted in animals that were all in a similar reproductive, and hence hormonal, stage during pregnancy.
Perhaps the most surprising results was the low variation in Young's modulus measured in lactating animals. This points to lactation, and not pregnancy, being the reproductive stage at which hormones have the biggest effect on connective tissue stiffness. Healthy Achilles tendon function is critical to the energy efficiency of walking and running (Cavagna et al 1990; Ishikawa et al., 2005; Lichtwark & Wilson, 2006) as well as the optimization of muscle contraction force and velocity (Farris & Sawicki, 2012). This lack of information is alarming when we consider that Achilles tendon injuries are one of the most commonly seen issues in sports medicine, with the extreme condition of tendon rupture reaching a frequency of 2.1 in 100,000 in the US (Lemme et al., 2018).
Information from the effects of pregnancy and lactation on muscle tendons can be important not only in relation to athletic performance. Women report higher rates of all types of musculoskeletal problems (USBJI, 2020, Table 9A.1) than men, and among women who run for exercise, tendinopathies are the most consistently reported problem, with the knee and ankle joints particularly susceptible (Francis et al., 2018). A review found that athletes and non‐athletes alike suffer from pelvic girdle and lower back pain during pregnancy, at indistinguishable prevalence rates estimated by some at 50% of all pregnant women and 25% of postpartum women. For half of these women, the pain was strong enough to seek medical help (Wu et al., 2004). Experimental administration of relaxin and estrogen in a guinea pig model resulted in weaker anterior cruciate ligament (Dragoo et al., 2009). Therefore, the findings of this study could have implications for skeletal muscles and connective tissues in other parts of the body as well. Neuropathy associated with carpal tunnel syndrome is also associated with breastfeeding in women with no pre‐eclampsia history and is alleviated relatively soon after breastfeeding stops (Osterman et al., 2012).
How do pregnancy and lactation hormones interact? Relaxin receptors are found in the Achilles tendon (Kim et al., 2016) suggesting that this is the pathway through which tendon stiffness is altered since this hormone is antifibrotic (Bathgate et al., 2013). Although relaxin serum levels are low during lactation, expression of relaxin receptors has a synergistic effect with estrogen and progesterone (Leblanc et al., 2017) and this interaction extends to mammary gland development (Sherwood et al., 1993). The antifibrotic, myogenic, and vasodilating effects of relaxin, along with its numerous receptors and hormonal interactions, warrant a closer look on muscle‐tendon remodeling during pregnancy and lactation (Braun et al., 2012; Li et al., 2006; Mu et al., 2010; Willcox et al., 2013).
4.3. Functional implications
The stiffness of tissues such as muscle aponeuroses and tendons has the potential to affect a muscle‐tendon unit's gear ratio (Konow et al., 2020), range of motion and storage of elastic potential energy (Danos et al., 2016). Our previous work has shown how critical the tuning of muscle‐tendon properties can be for healthy muscle function and movement. Age‐induced increased aponeurosis stiffness in the gastrocnemius can completely eliminate a muscle's ability to instantaneously adapt to a variety of force demands because the stiff aponeurosis inhibits muscle bulging causing it to operate at a constant gear ratio (Holt et al., 2016). If an increased aponeurosis stiffness can cause a loss of variable muscle gear ratio, a decreased aponeurosis stiffness could potentially lead to higher range of muscle gear ratios, offering improved fine‐tuning of the muscle's properties to its locomotor demands. Fine‐tuning muscle properties to functional demands optimizes muscle control and hence metabolic cost.
Similarly, an age‐related increase in tendon stiffness resulted in a reduction in elastic potential energy usage and reduce the range of motion around joints (Danos et al., 2016), that is likely to be a factor in the increased metabolic cost of locomotion in the elderly (Mian et al., 2006). Conversely, elastic potential energy usage may increase when tendon stiffness is reduced. Elastic energy is stored in stretched connective tissues, such as tendons and aponeuroses, during the first half of limb loading (stance) and released during the second half contributing to the work done on the center of mass as it passes over the foot. EPE usage is metabolically efficient because it does not require ATP, unlike forces produced by muscles. This process can be repeated with minimal energy lost in each stretch‐release cycle (Thorpe et al., 2013). Additionally, increased stretching of a more compliant muscle before contraction may affect muscle‐tendon unit performance (Seiberl et al., 2021). Together these changes in function could contribute to lower metabolic costs of movement.
5. CONCLUSIONS
Providing experimental data from validated models that capture the muscle‐tendon remodeling that occurs during pregnancy and lactation is critical for providing evidence‐based support for women who exercise during those physiological stages. This study also begins to answer the question of anatomical variation in muscle‐tendon properties due to interindividual variation without controlling for menstrual phase (NP animals in this study). It will be important to validate our results in larger population samples. Additionally, our data also suggest that although body mass returns to pre‐pregnancy stages by the end of the lactation stage, muscle‐tendon remodeling is still happening. Therefore, mothers at this stage may face different locomotor challenges. More data on the effects of lactation on other connective tissues critical for locomotion are needed, as are better epidemiological studies on the musculoskeletal challenges faced by new mothers. Additionally, we still do not know if muscle‐tendon units ever return to pre‐pregnancy morphology, or whether they persist as has been shown in metabolic studies (Hyatt et al., 2017). Although several studies have shown how gait changes with pregnancy in humans (Krkeljas & Moss, 2015; Whitcome et al., 2009), there has been no attempt to differentiate between the effects of fetal load and morphological changes to muscle‐tendon units. Assuming that all gait or even postural changes during pregnancy are due to the additional mass carried by the female, leads to a miscalculation of the true metabolic cost of pregnancy in human and comparative studies and provides the false impression that soon after birth, any changes should be reversed. By not examining this life stage and its accompanying morphological changes, we miss out on a unique opportunity to study the form‐function relationship for muscle‐tendon units within an individual, without the phylogenetic or anatomical constraints of comparative studies. Not only is this information critical for a complete understanding of mammalian evolution but it will be key to providing evidence‐based support for mother athletes.
ACKNOWLEDGMENTS
The monoclonal antibodies used in this study were developed by J. Ehmsen, Höke Laboratory, Johns Hopkins Medical Institute and were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242.
Danos, N. , Patrick, M. , Barretto, J. , Bilotta, F. & Lee, M. (2023) Effects of pregnancy and lactation on muscle‐tendon morphology. Journal of Anatomy, 243, 860–869. Available from: 10.1111/joa.13916
DATA AVAILABILITY STATEMENT
Data will be shared upon request.
REFERENCES
- Atinmo, T. , Baldijāo, C. , Pond, W.G. & Barnes, R.H. (1976) Maternal protein malnutrition during gestation alone and its effects on plasma insulin levels of the pregnant pig, its fetuses and the developing offspring. The Journal of Nutrition, 106, 1647–1653. [DOI] [PubMed] [Google Scholar]
- Biewener, A.A. & Roberts, T.J. (2000) Muscle and tendon contributions to force, work, and elastic energy savings: a comparative perspective. Exercise and Sport Sciences Reviews, 28, 99–107. [PubMed] [Google Scholar]
- Bathgate, R.A.D. , Halls, M.L. , van der Westhuizen, E.T. , Callander, G.E. , Kocan, M. & Summers, R.J. (2013) Relaxin family peptides and their receptors. Physiological Reviews, 93, 405–480. [DOI] [PubMed] [Google Scholar]
- Bø, K. , Artal, R. , Barakat, R. , Brown, W. , Davies, G.A.L. , Dooley, M. et al. (2016) Exercise and pregnancy in recreational and elite athletes: 2016 evidence summary from the IOC expert group meeting, Lausanne. Part 1—exercise in women planning pregnancy and those who are pregnant. British Journal of Sports Medicine, 50, 571–589. [DOI] [PubMed] [Google Scholar]
- Bø, K. , Artal, R. , Barakat, R. , Brown, W.J. , Davies, G.A.L. , Dooley, M. et al. (2017) Exercise and pregnancy in recreational and elite athletes: 2016/17 evidence summary from the IOC expert group meeting, Lausanne. Part 3—exercise in the postpartum period. British Journal of Sports Medicine, 51, 1516–1525. [DOI] [PubMed] [Google Scholar]
- Braun, B.C. , Vargas, A. & Jewgenow, K. (2012) The molecular detection of relaxin and its receptor RXFP1 in reproductive tissue of Felis catus and Lynx pardinus during pregnancy. Reproduction, 143, 399–410. [DOI] [PubMed] [Google Scholar]
- Catanzarite, T. , Bremner, S. , Barlow, C.L. , Bou‐Malham, L. , O'Connor, S. & Alperin, M. (2018) Pelvic muscles' mechanical response to strains in the absence and presence of pregnancy‐induced adaptations in a rat model. American Journal of Obstetrics and Gynecology, 218, 512.e1–512.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavagna, G.A. , Heglund, N.C. & Taylor, C.R. (1990) Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 233, 1–19. [DOI] [PubMed] [Google Scholar]
- Clowes, E.J. , Aherne, F.X. & Baracos, V.E. (2005) Skeletal muscle protein mobilization during the progression of lactation. American Journal of Physiology‐Endocrinology and Metabolism, 288, E564–E572. [DOI] [PubMed] [Google Scholar]
- Clowes, E.J. , Aherne, F.X. , Foxcroft, G.R. & Baracos, V.E. (2003) Selective protein loss in lactating sows is associated with reduced litter growth and ovarian function1. Journal of Animal Science, 81, 753–764. [DOI] [PubMed] [Google Scholar]
- Danos, N. , Holt, N.C. , Sawicki, G.S. & Azizi, E. (2016) Modeling age‐related changes in muscle‐tendon dynamics during cyclical contractions in the rat gastrocnemius. Journal of Applied Physiology, 121, 1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehghan, F. , Haerian, B.S. , Muniandy, S. , Yusof, A. , Dragoo, J.L. & Salleh, N. (2014) The effect of relaxin on the musculoskeletal system. Scandinavian Journal of Medicine and Science in Sports, 24, e220–e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoo, J.L. , Padrez, K. , Workman, R. & Lindsey, D.P. (2009) The effect of relaxin on the female anterior cruciate ligament: analysis of mechanical properties in an animal model. The Knee, 16, 69–72. [DOI] [PubMed] [Google Scholar]
- Edwards, D.P. (2005) Regulation of signal transduction pathways by estrogen and progesterone. Annual Review of Physiology, 67, 335–376. [DOI] [PubMed] [Google Scholar]
- Ehmsen, J.T. , Kawaguchi, R. , Mi, R. , Coppola, G. & Höke, A. (2019) Longitudinal RNA‐Seq analysis of acute and chronic neurogenic skeletal muscle atrophy. Scientific Data, 6, 179–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng, C.M. , Smallwood, L.H. , Rainiero, M.P. , Lahey, M. , Ward, S.R. & Lieber, R.L. (2008) Scaling of muscle architecture and fiber types in the rat hindlimb. The Journal of Experimental Biology, 211, 2336–2345. [DOI] [PubMed] [Google Scholar]
- Etienne, M. , Noblet, J. & Desmoulin, B. (1985) Mobilisation des réserves corporelles chez la truie primipare en lactation. Reproduction Nutrition Développement, 25, 341–344. [PubMed] [Google Scholar]
- Farris, D.J. & Sawicki, G.S. (2012) Human medial gastrocnemius force‐velocity behavior shifts with locomotion speed and gait. Proceedings of the National Academy of Sciences of the United States of America, 109, 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlin, A. , Toni, L.D. , Sandri, M. & Foresta, C. (2017) Relaxin and insulin‐like peptide 3 in the musculoskeletal system: from bench to bedside. British Journal of Pharmacology, 174, 1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forczek, W. & Staszkiewicz, R. (2012) Changes of kinematic gait parameters due to pregnancy. Acta Bioeng Biomechanics Wrocław Univ Technology, 14, 113–119. [PubMed] [Google Scholar]
- Francis, P. , Whatman, C. , Sheerin, K. , Hume, P. & Johnson, M.I. (2018) The proportion of lower limb running injuries by gender, anatomical location and specific pathology: a systematic review. Journal of Sports Science & Medicine, 18, 21–31. [PMC free article] [PubMed] [Google Scholar]
- Hammond, K.A. & Diamond, J. (1992) An experimental test for a ceiling on sustained metabolic rate in lactating mice. Physiological and Biochemical Zoology, 65, 952–977. [Google Scholar]
- Hammond, K.A. , Lloyd, K.C. & Diamond, J. (1996) Is mammary output capacity limiting to lactational performance in mice? The Journal of Experimental Biology, 199, 337–349. [DOI] [PubMed] [Google Scholar]
- Hansen, M. & Kjaer, M. (2017) Metabolic influences on risk for tendon disorders. Advances in Experimental Medicine and Biology, 920, 139–149. [DOI] [PubMed] [Google Scholar]
- Holt, N.C. , Danos, N. , Roberts, T.J. & Azizi, E. (2016) Stuck in gear: age‐related loss of variable gearing in skeletal muscle. Journal of Experimental Biology, 219, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner, A.M. , Russ, D.W. & Biknevicius, A.R. (2011) Effects of early‐stage aging on locomotor dynamics and hindlimb muscle force production in the rat. Journal of Experimental Biology, 214, 3588–3595. [DOI] [PubMed] [Google Scholar]
- Huang, T.‐W.P. , Shorter, K.A. , Adamczyk, P.G. & Kuo, A.D. (2015) Mechanical and energetic consequences of reduced ankle plantar‐flexion in human walking. Journal of Experimental Biology, 218, 3541–3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt, H.W. , Zhang, Y. , Hood, W.R. & Kavazis, A.N. (2017) Lactation has persistent effects on a mother's metabolism and mitochondrial function. Scientific Reports, 7, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland, M.L. & Ott, S.M. (2000) The effects of pregnancy on the musculoskeletal system. Clinical Orthopaedics and Related Research, 372, 169–179. [DOI] [PubMed] [Google Scholar]
- Ishikawa, M. , Komi, P.V. , Grey, M.J. , Lepola, V. & Bruggemann, G.‐P. (2005) Muscle‐tendon interaction and elastic energy usage in human walking. Journal of Applied Physiology, 99, 603–608. [DOI] [PubMed] [Google Scholar]
- Janssen, I. , Heymsfield, S.B. , Wang, Z. & Ross, R. (2000) Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. Journal of Applied Physiology, 89, 81–88. [DOI] [PubMed] [Google Scholar]
- Kang, Y.M. , Lee, H.M. , Moon, S.H. , Kang, H. & Choi, Y.R. (2017) Relaxin modulates the expression of MMPs and TIMPs in fibroblasts of patients with carpal tunnel syndrome. Yonsei Medical Journal, 58, 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.H. , Lee, S.K. , Lee, S.K. , Kim, J.H. & Fredericson, M. (2016) Relaxin receptor RXFP1 and RXFP2 expression in ligament, tendon, and shoulder joint capsule of rats. Journal of Korean Medical Science, 31, 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, R.H. , Toner, M.S. , Dove, H. , Atwood, C.S. & Brown, W.G. (1993) The response of first‐litter sows to dietary protein level during lactation. Journal of Animal Science, 71, 2457–2463. [DOI] [PubMed] [Google Scholar]
- Klein, S.L. , Schiebinger, L. , Stefanick, M.L. , Cahill, L. , Danska, J. , De Vries, G.J. et al. (2015) Opinion: sex inclusion in basic research drives discovery. Proceedings of the National Academy of Sciences, 112, 5257–5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konow, N. , Collias, A. & Biewener, A.A. (2020) Skeletal muscle shape change in relation to varying force requirements across locomotor conditions. Frontiers in Physiology, 11, 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krkeljas, Z. & Moss, S.J. (2015) Correlating mechanical work with energy consumption during gait throughout pregnancy. BMC Pregnancy and Childbirth, 15, 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc, D.R. , Schneider, M. , Angele, P. , Vollmer, G. & Docheva, D. (2017) The effect of estrogen on tendon and ligament metabolism and function. The Journal of Steroid Biochemistry and Molecular Biology, 172, 106–116. [DOI] [PubMed] [Google Scholar]
- Lee, K.Y. , Singh, M.K. , Ussar, S. , Wetzel, P. , Hirshman, M.F. , Goodyear, L.J. et al. (2015) Tbx15 controls skeletal muscle fibre‐type determination and muscle metabolism. Nature Communications, 6, 8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur, L. & Ecolan, P. (1990) Changes in muscle fiber populations and muscle enzyme activities in the primiparous lactating sow. Reproduction, Nutrition, Development, 30, 523–531. [DOI] [PubMed] [Google Scholar]
- Lefaucheur, L. , le Peuch, C. , Barenton, B. & Vigneron, P. (1986) Characterization of insulin binding to slices of slow and fast twitch skeletal muscles in the rabbit. Hormone and Metabolic Research, 18, 725–729. [DOI] [PubMed] [Google Scholar]
- Lefaucheur, L. & Vigneron, P. (1986) Post‐natal changes in some histochemical and enzymatic characteristics of three pig muscles. Meat Science, 16, 199–216. [DOI] [PubMed] [Google Scholar]
- Lemme, N.J. , Li, N.Y. , DeFroda, S.F. , Kleiner, J. & Owens, B.D. (2018) Epidemiology of achilles tendon ruptures in the United States: athletic and nonathletic injuries from 2012 to 2016. Orthopaedic Journal of Sports Medicine, 6, 232596711880823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. , Negishi, S. , Sakamoto, M. , Usas, A. & Huard, J. (2006) The use of relaxin improves healing in injured muscle. Annals of the New York Academy of Sciences, 1041, 395–397. [DOI] [PubMed] [Google Scholar]
- Lichtwark, G.A. & Wilson, A.M. (2006) Interactions between the human gastrocnemius muscle and the Achilles tendon during incline, level and decline locomotion. The Journal of Experimental Biology, 209, 4379–4388. [DOI] [PubMed] [Google Scholar]
- Lontay, B. , Bodoor, K. , Sipos, A. , Weitzel, D.H. , Loiselle, D. , Safi, R. et al. (2015) Pregnancy and Smoothelin‐like protein 1 (SMTNL1) deletion promote the switching of skeletal muscle to a glycolytic phenotype in human and mice. Journal of Biological Chemistry, 290, 17985–17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez, J. & Keys, A. (1960) Density and composition of mammalian muscle. Metabolism, 9, 184–188. [Google Scholar]
- Mian, O.S. , Thom, J.M. , Ardigò, L.P. , Narici, M.V. & Minetti, A.E. (2006) Metabolic cost, mechanical work, and efficiency during walking in young and older men. Acta Physiologica (Oxford, England), 186, 127–139. [DOI] [PubMed] [Google Scholar]
- Mu, X. , Urso, M.L. , Murray, K. , Fu, F. & Li, Y. (2010) Relaxin regulates MMP expression and promotes satellite cell mobilization during muscle healing in both young and aged mice. The American Journal of Pathology, 177, 2399–2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narvaez‐Sanchez, R. , Calderón, J.C. , Vega, G. , Trillos, M.C. & Ospina, S. (2019) Skeletal muscle as a protagonist in the pregnancy metabolic syndrome. Medical Hypotheses, 126, 26–37. [DOI] [PubMed] [Google Scholar]
- Osterman, M. , Ilyas, A.M. & Matzon, J.L. (2012) Carpal tunnel syndrome in pregnancy. Management of Compressive Neuropathies of the Upper Extremity, 43, 515–520. [DOI] [PubMed] [Google Scholar]
- Rehage, M. , Mohan, S. , Wergedal, J.E. , Bonafede, B. , Tran, K. , Hou, D. et al. (2007) Transgenic overexpression of pregnancy‐associated plasma protein‐a increases the somatic growth and skeletal muscle mass in mice. Endocrinology, 148, 6176–6185. [DOI] [PubMed] [Google Scholar]
- Roberts, T.J. & Azizi, E. (2011) Flexible mechanisms: the diverse roles of biological springs in vertebrate movement. Journal of Experimental Biology, 214, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, T.J. , Marsh, R.L. , Weyand, P.G. & Taylor, C.R. (1997) Muscular force in running turkeys: the economy of minimizing work. Science, 275, 1113–1115. [DOI] [PubMed] [Google Scholar]
- Robertson, B.D. & Sawicki, G.S. (2014) Exploiting elasticity: modeling the influence of neural control on mechanics and energetics of ankle muscle‐tendons during human hopping. Journal of Theoretical Biology, 353, 121–132. [DOI] [PubMed] [Google Scholar]
- Samuel, C.S. , Coghlan, J.P. & Bateman, J.F. (1998) Effects of relaxin, pregnancy and parturition on collagen metabolism in the rat pubic symphysis. The Journal of Endocrinology, 159, 117–125. [DOI] [PubMed] [Google Scholar]
- Schindelin, J. , Arganda‐Carreras, I. , Frise, E. , Kaynig, V. , Longair, M. , Pietzsch, T. et al. (2012) Fiji: an open‐source platform for biological‐image analysis. Nature Methods, 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiberl, W. , Hahn, D. , Power, G.A. , Fletcher, J.R. & Siebert, T. (2021) Editorial: the stretch‐shortening cycle of active muscle and muscle‐tendon complex: what, why and how it increases muscle performance? Frontiers in Physiology, 12, 693141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood, O.D. , Downing, S.J. , Guico‐Lamm, M.L. , Hwang, J.J. , O'Day‐Bowman, M.B. & Fields, P.A. (1993) The physiological effects of relaxin during pregnancy: studies in rats and pigs. Oxford Reviews of Reproductive Biology, 15, 143–189. [PubMed] [Google Scholar]
- Thompson, M.A. (2017) Physiological and biomechanical mechanisms of distance specific human running performance. Integrative and Comparative Biology, 57, 293–300. [DOI] [PubMed] [Google Scholar]
- Thorpe, C.T. , Klemt, C. , Riley, G.P. , Birch, H.L. , Clegg, P.D. & Screen, H.R.C. (2013) Helical sub‐structures in energy‐storing tendons provide a possible mechanism for efficient energy storage and return. Acta Biomaterialia, 9, 7948–7956. [DOI] [PubMed] [Google Scholar]
- USBJI . (2020) United States Bone and Joint Initiative: the burden of musculoskeletal diseases in the United States (BMUS), fourth edition, 2020. IL: Rosemont. [Google Scholar]
- Whitcome, K.K. , Shapiro, L.J. & Lieberman, D.E. (2009) Fetal load and the evolution of lumbar lordosis in bipedal hominins. Nature, 450, 1075–1078. [DOI] [PubMed] [Google Scholar]
- Willcox, J.M. , Summerlee, A.J.S. & Murrant, C.L. (2013) Relaxin induces rapid, transient vasodilation in the microcirculation of hamster skeletal muscle. The Journal of Endocrinology, 218, 179–191. [DOI] [PubMed] [Google Scholar]
- Wu, W.H. , Meijer, O.G. , Uegaki, K. , Mens, J.M.A. , van Dieën, J.H. , Wuisman, P.I.J.M. et al. (2004) Pregnancy‐related pelvic girdle pain (PPP), I: Terminology, clinical presentation, and prevalence. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society, 13, 575–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierath, J.R. & Hawley, J.A. (2004) Skeletal muscle fiber type: influence on contractile and metabolic properties. PLOS Biology, 2, e348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be shared upon request.


