Abstract
This study aims to investigate the effects of 1,25-dihydroxyvitamin D3 (1,25(OH)2VitD3) on osteogenic differentiation of human periodontal ligament stem cells (hPDLSCs) and the activity of hPDLSC sheets and the differences in the tissue regeneration activity of hPDLSC sheets on tooth root fragment treated by different methods. Healthy caries-free premolars were collected. The hPDLSCs were obtained by enzymatic digestion. Surface markers of stem cells were analyzed by flow cytometry and the multidirectional differentiation ability of hPDLSCs was detected. During the osteogenic differentiation of hPDLSCs, 1,25(OH)2VitD3 was added and the effect of 1,25(OH)2VitD3 on osteogenic differentiation of hPDLSCs was assessed using Western blotting, quantitative reverse transcription–polymerase chain reaction (qRT-PCR), enzyme-linked immunosorbent assay, cell staining, and immunofluorescence. After hPDLSC sheets were prepared, histology and immunofluorescence analysis of the effect of 1,25(OH)2VitD3 on sheet activity were performed. In addition, root fragments were prepared and treated with scaling, 24% EDTA (ethylenediamide tetraacetic acid), and Er,Cr:YSGG lasers, respectively, and the tissue regeneration activity of hPDLSC sheets on different root fragments were observed. 1,25(OH)2VitD3 promoted the high gene and protein expressions of osteogenic markers ALP (alkaline phosphatase), Runx2, and OPN (osteopontin antibody) in hPDLSCs, along with enhanced ALP activity and staining, alizarin red staining, and immunofluorescence staining, indicating that the osteogenic differentiation ability of hPDLSCs was improved. Extracellular matrix secretion was increased in hPDLSC sheets, along with the positive expressions of the protein markers fibronectin and collagen I, suggesting that 1,25(OH)2VitD3 could enhance these effects. In addition, the root fragments treated by Er,Cr:YSGG laser were more suitable for the attachment and regeneration of hPDLSC sheets, demonstrating that 1,25(OH)2VitD3 could improve the tissue regeneration performance of these sheets. 1,25(OH)2VitD3 can promote osteogenic differentiation of hPDLSCs and thus plays an active role in hPDLSC sheet formation and tissue regeneration. In addition, the Er,Cr:YSGG laser can be used as the recommended treatment method for the root surface regenerated by hPDLSCs.
Keywords: 1,25-Dihydroxyvitamin D3; human periodontal ligament stem cells; osteogenic differentiation; cell sheets; root surface treatment
Background
Periodontitis mainly causes gum inflammation and alveolar bone resorption, leading to progressive, irreversible damage to tooth supporting tissues 1 . Meanwhile, periodontitis is also closely related to cardiovascular and cerebrovascular diseases and diabetes2,3. The currently available treatments for periodontitis can successfully control the inflammation and facilitate the self-repair of periodontal tissues. However, how to promote periodontal tissue regeneration and finally achieve complete repair is challenging.
Human periodontal ligament stem cells (hPDLSCs) have the ability to proliferate and to differentiate into cementoblasts, osteoblasts, PDL cells, and other cells. They can be involved in alveolar bone regeneration and treatment of periodontitis, and thus have been regarded as a promising cell source for periodontal tissue engineering4 –6. However, some key issues concerning the culture and application of hPDLSCs are still hot research topics and dilemma, such as influencing factors of hPDLSCs activity, transplant vectors, and host status.
Vitamin D (VitD) is thought to be associated with a variety of diseases, such as periodontitis, diabetes, and atherosclerosis7 –9, and previous studies have also confirmed that VitD can improve both blood glucose control and inflammatory status and may assist in basic periodontal treatment in patients with type 2 diabetes mellitus complicated by chronic periodontitis. One of the aims of this study was to explore the effect of VitD on the osteogenic activity of hPDLSCs and thus identify the role of hPDLSCs in the treatment of periodontitis. At present, whether VitD promotes or inhibits osteogenesis is still controversial10,11, and there is an urgent need to clarify its exact role in osteogenesis.
Cell sheet technology, as an emerging tissue engineering technology, is quite different from cell vectors. It uses the extracellular matrix autocrined by the cell sheet as a vector to maximize the biological characteristics of cells 12 . Research has shown significant effects of cell sheets in promoting the regeneration of myocardium, cornea, and periodontal tissues13 –15. However, the PDL stem cells (PDLSCs) sheet biotechnology has a limited role in periodontal tissue regeneration due to the status of the implantation area and the properties of the sheets 16 . The other aim of this study was to determine whether VitD could improve the tissue regenerative activity of PDLSC sheets and whether there is a correlation between the statuses of different implantation areas and the regenerative activity of PDLSC sheets.
Materials and Methods
Ethical Statement
This study was approved by the Medical Research Ethics Committee of General Hospital of Ningxia Medical University (Protocol No. 2018-345). Patients who had their premolars extracted for orthodontic purpose at the Department of Stomatology, General Hospital of Ningxia Medical University were selected and provided informed consent.
Isolation and Culture of Cells
The freshly extracted healthy periodontal-free premolars were collected and washed with phosphate-buffered solution (PBS; Vivacell, China). The PDL was scraped and then digested with collagenase I (3 mg/ml; Sigma, Germany) for 30 min at 37°C. Subsequently, cells were inoculated into Dulbecco’s Modified Eagle’s Medium (DMEM) low-sugar medium (Vivacell, China) containing 10% fetal bovine serum (FBS; Gibco, USA) and cultured in an incubator at 37°C with 5% CO2 and saturated humidity. The medium was changed every 2 days. When the cells reached the confluence of 80%, they were passaged for subsequent experiments.
Characterization of hPDLSCs
The stem cell surface markers (CD34, 560941, BD; CD45, 560975, BD; CD90, 561970, BD; CD105, 561443, BD; CD146, 561013, BD; and STRO-1, VF3010041A, Invitrogen) were analyzed with a flow cytometer to characterize hPDLSCs.
Identification of Multidirectional Differentiation Ability of hPDLSCs
The adipogenic ability of hPDLSCs was identified using an adipogenic induction medium containing 10% FBS, 1 μmol/l dexamethasone, 0.5 mmol/l 3-isobutyl-1-methylxanthine, 10 μg/ml insulin, and 100 μmol/l indomethacin. hPDLCs were inoculated in 12-well plates containing 1 ml adipogenic induction medium for 14 days and stained with oil red O (G1262; Solarbio, China) to assess lipid-droplet formation. The osteogenic ability of hPDLSCs was identified using an osteogenic induction medium (OM) containing 10% FBS, 10 nmol/l dexamethasone, 10 mmol/l β-glycerophosphate, and 50 μg/ml ascorbic acid. hPDLCs were inoculated in 12-well plates containing 1 ml OM for 14 days and stained with Alizarin red S (G1262; Solarbio) to assess the formation of mineralized nodules.
Cell Viability Assay
hPDLSCs were seeded in 96-well plates at a density of 5×103 cells/well. After the cells became adherent, they were cultured in OM containing 0 nmol/l, 1 nmol/l, 10 nmol/l, 100 nmol/l, or 1,000 nmol/l 1,25-dihydroxyvitamin D3 (1,25(OH)2VitD3; HY-10002, MCE) for 1, 3, 5, and 7 days, respectively. Each well was added with 10 μl Cell Counting Kit-8 reagent (CCK-8; US Everbright), and then the cells were incubated at 37°C for 1 h. Optical density values were measured at 450 nm using a microplate reader (Multiskan GO; Thermo Fisher, USA).
Western Blotting
hPDLSCs were seeded in 6-well plates at a density of 1 × 105 cells/well and cocultured using OM and varying concentrations of 1,25(OH)2VitD3 for 7 days. The total protein was extracted using a whole protein extraction kit (KGP2100; KeyGEN, China), and the protein concentration was determined using the BCA Protein Assay Kit (KGP902; KeyGEN). Each protein sample (50 μg/lane) was separated on 10% SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and then transferred to PVDF (polyvinylidene fluoride) membranes (Millipore), which were blocked with 10% skim milk (PS112L; Epizyme, China) for 2 h at room temperature and incubated overnight at 4°C. The antibodies used included alkaline phosphatase (ALP) antibody (Affinity, China), Runt-associated transcription factor 2 (Runx2; Affinity), osteopontin antibody (OPN; Affinity), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (Proteintech). The next day, the goat anti-rabbit secondary antibody (ZB-2301; ZSGB-BIO, China) was added and inoculated for 1 h at room temperature. Western blotting was performed using the Super ECL Plus Detection Reagent (S6009L; US Everbright, USA) and Biosystems C600 System (Azure, USA). Finally, the protein bands were quantified using the Image J software (Bio-Rad, California, USA).
RNA Extraction and Quantitative Reverse Transcription–Polymerase Chain Reaction (qRT-PCR)
hPDLSCs were seeded in six-well plates at a density of 1 × 105 cells/well and cocultured using OM and 1,25(OH)2VitD3 for 7 days. Total RNA was extracted using the Total RNA Extraction Kit (R6834; Omega) and cDNA was reverse-transcribed with the PrimeScript RT Kit (RR036A; Takara, Japan). qRT-PCR was performed using the TB Green kit (RR820A; Takara) on the qTOWER3G real-time PCR system (Analytik Jena, Germany) to assess the messenger RNA (mRNA) levels of ALP, Runx2, and OPN. Gene-specific primers were synthesized by Tsingke, China, and the sequences are shown in Table 1. All data were analyzed by the 2−ΔΔCT method, with GAPDH as the reference gene.
Table l.
Specific Primer Sequences for Control and Target Genes.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| Runx2 | CACTGGCGCTGCAACAAGA | CATTCCGGAGCTCAGCAGAATA |
| OPN | TCCTAGCCCCACAGACCCTT | CACACTATCACCTCGGCCAT |
| GAPDH | GCACCGTCAAGGCTGAGAAC | TGGTGAAGACGCCAGTGGA |
ALP: alkaline phosphatase; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; OPN: osteopontin antibody.
ALP Staining and Detection
After hPDLSCs were cocultured using OM and 1,25(OH)2VitD3 for 7 days, ALP staining was performed, followed by ALP activity analysis to evaluate the osteogenic level of hPDLSCs. hPDLSCs were rinsed with PBS three times and fixed with 4% paraformaldehyde for 15 min. After the prepared ALP incubation solution (G1480; Solarbio) was added dropwise, the cells were inoculated in a dark moist chamber for 15 to 20 min. ALP staining was observed under microscope. To detect ALP activity, cells were first lysed with a protein extraction kit (KGP2100; KeyGEN); after the lysate was obtained, ALP levels in cells were detected following the kit instructions (A059-2; Nanjing, China). To avoid interference by proteins in cells, the protein concentration was simultaneously detected using a BCA protein assay kit (KGP902; KeyGEN) to correct for ALP levels.
Alizarin Red S Staining
After hPDLSCs were cocultured using OM and 1,25(OH)2VitD3 for 7 days, calcification of hPDLSCs was observed using alizarin red S staining solution (G1452; Solarbio). After the culture medium was removed, the cells were fixed in 4% paraformaldehyde for 15 min. Then, 1% alizarin red S solution was added to evenly cover the cells for staining for 5 min and the mineralized nodules were observed under microscope.
Immunofluorescence Detection of Cells
After hPDLSCs were cocultured using OM and 1,25(OH)2VitD3, the cells were fixed in 4% paraformaldehyde solution for 20 min. Then, the cells were incubated in 0.3% TritonX-100 (T8200; Solarbio) for 20 min, followed by cell blocking with 5% BSA (bovine serum albumin) for 30 min. ALP antibody (1:200; Affinity) and Runx2 antibody (1:200; Affinity) were added to incubate cells at 4°C overnight. The next day, cells were stained with the Actin-Tracker Green-488 (microfilament green fluorescent probe) and the Actin-Tracker Red-594 (microfilament red fluorescent probe; 1:100; Beyotime, China) and kept in dark for 1 h. Subsequently, CoraLite594 goat anti-rabbit antibody and CoraLite488 goat anti-rabbit antibody (1:100; Proteintech) were incubated at room temperature in dark for 1 h. Finally, the nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI; AR1177; Boster, China). The fluorescence images were photographed using the Zeiss LSM 800 Confocal Laser Scanning Microscope (Zeiss, Germany).
Preparation of hPDLSC Sheets
hPDLSCs were seeded in 100-mm Petri dishes at a density of 1 × 106 cells/well and cocultured with 10 ml of induction medium (i.e., low-glucose DMEM containing 50 μg/ml ascorbic acid and 10% FBS) and 1,25(OH)2VitD3. After 10 days of incubation, white membrane-like material became visible, which were the monolayer cell sheets (MCSs) of hPDLSCs. MCSs were carefully peeled off with a cell scraper, superimposed on another unpeeled sheet, coverslip-pressurized for 1 h, and incubated at 37°C with 5% CO2 and saturated humidity in an incubator for 24 h. Finally, the growth of multilayer cell sheets (MUCSs) of hPDLSCs was observed.
Histological Analysis
The acquired hPDLSCs diaphragm was fixed with 4% paraformaldehyde, dehydrated in an alcohol gradient and embedded with paraffin, and HE stained after sectioning to assess histological changes.
Histoimmunofluorescence Assay
The hPDLSC sheets were fixed, dehydrated, optimal cutting temperature–embedded, sliced 5 μm using a CM1860 cryotome (Leica, Germany), and incubated overnight at 4°C with antibodies, including ALP, fibronectin (ABclonal, China), and Collagen I (ABclonal). The next day, the sheets were inoculated with goat anti-rabbit antibodies, including CoraLite594, CoraLite488, and CoraLite 647 (1:100; Proteintech, USA) at room temperature in dark for 1 h. Cellular nuclei were labeled with DAPI. The fluorescence images were photographed using the Zeiss LSM 800 Confocal Laser Scanning Microscope (Zeiss, Germany).
Scanning Electron Microscopy (SEM)
Ex vivo teeth with periodontitis were selected for the preparation of tooth root fragments. Plaque and tartar visible in the experimental area were scraped off in an imbricated pattern. Root fragments (5 mm × 5 mm × 1 mm) were harvested from 2 mm below the adjacent enamel boundary in a direction toward the tooth root and then treated with 24% ethylenediamide tetraacetic acid (EDTA) and Er,Cr:YSGG laser (Biolase, USA), respectively. Subsequently, the root fragments were placed in a 6-well plate containing hPDLSCs, enabling the sheets to completely wrap around the root fragments. The complex was incubated in an incubator at 37°C, with 5% CO2 and saturated humidity for another 7 days. The root fragment/sheet complex was fixed with 3% glutaraldehyde buffer, refixed with osmium acid, dehydrated, and sputter coated with an ultrathin layer of gold. An S-3400N scanning electron microscope (Hitachi, Japan) was used to observe the growth of hPDLSC sheets on the root fragments.
Statistical Analysis
Data were analyzed and plotted using the GraphPad 8.0 software. Data are presented as mean±standard deviation. Differences were measured by t test and one-way analysis of variance. A P value of less than 0.05 was considered statistically significant.
Results
Isolation and Identification of hPDLSCs
hPDLSCs grew in long spindle-shaped clones (Fig. 1A). Alizarin red S staining showed mineralized nodules (Fig. 1B) and oil red O staining showed lipid-droplet formation (Fig. 1C), indicating that hPDLSCs had multiple differentiation potentials. Flow cytometry revealed that hPDLSCs expressed almost no hematopoietic stem cell markers (CD34, 1.31% and CD45, 0.39%), but had positive expressions of mesenchymal stem cell surface markers (CD90, 99.9%; CD105, 95.4%; CD146, 82.8%; and STRO-1, 14.0%; Fig.1D).
Figure 1.

Isolation and identification of hPDLSCs. (A) hPDLSCs were successfully isolated and cultured, showing long spindle shape under light microscope. Scale bar: 200 μm. (B) Alizarin red S staining revealed a large number of mineralized nodules in hPDLSCs. Scale bar: 200 μm. (C) Oil red O staining revealed a large number of lipids in hPDLSCs. Scale bar: 200 μm. (D) Flow cytometry revealed that hPDLSCs expressed almost no CD34 or CD45 but had positive expressions of CD90, CD105, CD146, and STRO-1. hPDLSCs: human periodontal ligament stem cells.
1,25(OH)2VitD3 Promoted Osteogenic Differentiation of hPDLSCs
On days 1, 3, 5, and 7 of culture, different concentrations of 1,25(OH)2VitD3 showed different effects on the activity of hPDLSCs (Fig. 2A). Low-concentration 1,25(OH)2VitD3 exhibited inhibitory effects on cell activity, and the 100-nM concentration had less effect on the activity. Western blotting showed that the expressions of osteogenic markers ALP, Runx2, and OPN increased in a stepped manner as the concentration of 1,25(OH)2VitD3 increased (Fig. 2B, C). Therefore, 100 nM was chosen as the most suitable concentration. qRT-PCR results on day 7 of coculture revealed significantly elevated mRNA levels for ALP, Runx2, and OPN, and 1,25(OH)2VitD3 further increased the expression levels of these genes (Fig. 2D). Seven days after osteogenesis induction, ALP activity was higher in the 1,25(OH)2VitD3 group (Fig. 2E), along with wider ALP-stained area (Fig. 2F). Correspondingly, the mineralized nodules stained with alizarin red were more concentrated in the 1,25(OH)2VitD3 group (Fig.2G). In addition, the confocal microscopy displayed the expressions of ALP and Runx2 in cells (Figs. 3 and 4). Compared with the OM group, the 1,25(OH)2VitD3 group showed no significant change in the morphology of hPDLSCs but with significantly enhanced immunofluorescence signals of ALP and Runx2, which was consistent with the Western blotting results.
Figure 2.
1,25(OH)2VitD3 promotes osteogenic differentiation of hPDLSCs. (A) The viability of hPDLSCs on days 1, 3, 5, and 7 of coculture with OM and varying concentrations of 1,25(OH)2VitD3 (CCK-8 assay). (B and C) Western blot analysis and band grayscale of ALP, Runx2, and OPN protein levels after 7 days of coculture. (D) qRT-PCR of the gene expression levels of ALP, Runx2, and OPN after 7 days of coculture with osteogenesis induction medium (OM) and 1,25(OH)2VitD3 (100nM). (E and F) ALP staining and ALP activity assay for evaluating the osteogenesis levels after 7 days of coculture with OM and 1,25(OH)2VitD3 (100 nM). Scale bar: 200 μm. (G) Alizarin red S staining after 7 days of coculture with OM and 1,25(OH)2VitD3. Scale bar: 200 μm. The graphs represent the mean ± standard deviation (n = 3–5). ALP: alkaline phosphatase; hPDLSCs: human periodontal ligament stem cells; OPN: osteopontin antibody; qRT-PCR: quantitative reverse transcription–polymerase chain reaction. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, compared with the control group.
Figure 3.
Immunofluorescence analysis of ALP expression in hPDLSCs after coculture with OM and of 1,25(OH)2VitD3 ALP was stained with red fluorescence (CoraLite594), actin was stained with green fluorescence (Actin-Tracker Green-488), and nuclei are stained with blue fluorescence (DAPI). Scale bar: 20 μm. ALP: alkaline phosphatase; DAPI: 4′,6-diamidino-2-phenylindole; hPDLSCs: human periodontal ligament stem cells; OM: osteogenic induction medium.
Figure 4.
Immunofluorescence analysis of Runx2 expression in hPDLSCs after coculture with OM and 1,25(OH)2VitD3 Runx2 was stained with green fluorescence (CoraLite488), actin was stained with red fluorescence (Actin-Tracker Red-594), and nuclei are stained with blue fluorescence (DAPI). Scale bar: 20 μm. DAPI: 4′,6-diamidino-2-phenylindole; hPDLSCs: human periodontal ligament stem cells; OM: osteogenic induction medium.
1,25(OH)2VitD3 Promoted the Formation of MUCS Structure
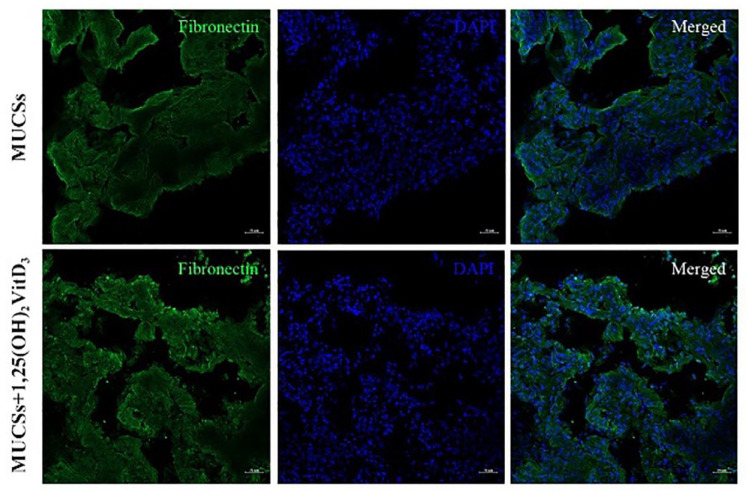
After 10 days of incubation, inverted microscopy showed that the cells in the sheets were densely arranged, with obvious polarity, and the extracellular matrix was rich (Fig. 5A). Morphologically, MCSs shrank to form irregular shapes after peeling, with good ductility (Fig. 5B), and MUCSs were tightly bound and less ductile (Fig. 5C). Histology revealed that MCSs contained a large amount of extracellular matrix; however, they were relatively thin, with the presence of some cavity structures (Fig. 5D). The extracellular matrix of MUCSs was more abundant and more tightly bound. The presence of 1,25(OH)2VitD3 increased matrix secretion, making the cell arrangement denser (Figure 5D). The structural proteins fibronectin and collagen I of the extracellular matrix were further detected by immunofluorescence method, and the results showed that these proteins were strongly positively expressed in MUCSs, and the presence of 1,25(OH)2VitD3 enhanced the expressions of these proteins as well as the expression of ALP (Figs. 6–8).
Figure 5.
1,25(OH)2VitD3 promotes the formation of MUCS structure. (A) Inverted microscopy of hPDLSC sheets. Scale bar: 200 μm. (B) Morphologies of MCSs 10 days after culture. (C) Morphologies of MUCSs. (D) Histology of hPDLSC sheets in different groups. Scale bar: 100 μm. hPDLSCs: human periodontal ligament stem cells; MCSs: monolayer cell sheets; MUCSs: multilayer cell sheets.
Figure 6.
Immunofluorescence analysis of COL-I expression in hPDLSC sheets: COL-I was stained with red fluorescence (CoraLite594) and nuclei were stained with blue fluorescence (DAPI). Scale bar: 20 μm. COL-1: type 1 collagen; DAPI: 4′,6-diamidino-2-phenylindole; hPDLSCs: human periodontal ligament stem cells.
Figure 7.
Immunofluorescence analysis of fibronectin expression in hPDLSC sheets: Fibronectin was stained with green fluorescence (CoraLite488) and nuclei were stained with blue fluorescence (DAPI). Scale bar: 20 μm. DAPI: 4′,6-diamidino-2-phenylindole; hPDLSCs: human periodontal ligament stem cells.
Figure 8.
Immunofluorescence analysis of ALP expression in hPDLSC sheets: ALP was stained with purple-red fluorescence (CoraLite647) and nuclei were stained with blue fluorescence (DAPI). Scale bar: 20 μm. ALP: alkaline phosphatase; DAPI: 4′,6-diamidino-2-phenylindole; hPDLSCs: human periodontal ligament stem cells.
Treatments With 1,25(OH)2VitD3 and Er,Cr:YSGG Laser–Enhanced Tissue Regenerative Activity of MUCSs
The SEM findings of different root fragment treatment methods are shown in Fig. 9. In the scaling group, the surface of the tooth root was uneven, a residue of smear layer was visible, and the dentinal tubule orifices were less exposed. In the 24% EDTA treatment group, the surface of the tooth root was more even, there was less mineralized matrix deposition, and the dentinal tubule orifices were more obvious. The Er,Cr:YSGG laser treatment group had similar effectiveness to 24% EDTA treatment group: the surface of the tooth root was relatively even, there was a small amount of irregular mineralized matrix deposition, and the dentinal tubule orifices were visible but not completely exposed. After the root fragments were cocultured with MUCSs, the tissue regeneration performance of MUCSs was, however, not consistent due to the differences in local root surface state and the failure of homogeneous MUCS dissociation. In general, the dentinal root surface in the scaling group was organically combined with the extracellular matrix of MUCSs, and the mineralized matrix deposition was visible. The mineralized matrix deposition in the 24% EDTA treatment group was more obvious, and mineralized nodules were visible. Compared with these two groups, the Er,Cr:YSGG laser treatment group had clustered mineralized matrix deposition, and the mineralized matrix was regularly arranged, with better tissue regeneration ability of MUCSs. The presence of 1,25(OH)2VitD3 improved the regeneration performance of MUCSs to different degrees. The dentinal root surface was more closely bound to the extracellular matrix of MUCSs. The structure of the root fragment/sheet complex was denser. The mineralized matrix deposition increased, with a large number of mineralized nodules arranged in clusters.
Figure 9.
Treatments with 1,25(OH)2VitD3 and Er,Cr:YSGG laser–enhanced tissue regenerative performance of MUCSs. After the tooth root fragments were treated in the three different ways: scaling, scaling + 24% EDTA, and scaling + Er, Cr:YSGG laser, they were cocultured with hPDLSCs sheets for 7 days before SEM. ALP: alkaline phosphatase; hPDLSCs: human periodontal ligament stem cells; MUCSs: multilayer cell sheets; SEM: scanning electron microscopy.
Discussion
As one of the seed cells for periodontal tissue engineering, hPDLSCs possess the self-renewal and multidirectional differentiation abilities of mesenchymal stem cells, among which osteogenic differentiation is the key point in the treatment of periodontitis-associated bone defects. Thus, how to promote the osteogenic differentiation of hPDLSCs has long been a research hotspot and dilemma.
Some studies have proposed that VitD can promote osteogenic differentiation of human tooth germ stem cells and umbilical cord mesenchymal stem cells17,18; however, few studies have explored the role of VitD in promoting osteogenic differentiation of hPDLSCs. In this study, hPDLSCs were successfully isolated and cultured by enzymatic digestion and their multidirectional differentiation abilities were confirmed. The addition of VitD during osteogenic differentiation further promoted the increase of osteogenic gene markers, such as ALP, Runx2, and OPN in hPDLSCs, suggesting an increase in osteogenic capacity; similarly, another study also found that VitD increased the expression level of ALP in fat-derived stem cells 19 . Previous studies reported that serum VitD level was generally low in periodontitis patients, and appropriate VitD supplementation during periodontal treatment is more conducive to the recovery from periodontitis. It is therefore envisaged that if hPDLSCs are used clinically for the treatment of periodontitis in the future, VitD supplementation may be a potential adjunct treatment. However, excessive VitD intake has been reported to lead to hypercalcemia and renal failure 20 , and the blood level of VitD should be monitored regularly during its supplementation.
As a part of tissue engineering technology, cell sheets are characterized by the construction of endogenous scaffolds and microenvironments through their own extracellular matrix. No exogenous scaffold substances are needed during this process, which preserves the autocrine properties and connections among cells. Cell sheets have been applied to the repair of bone defects caused by infection, malignant tumors, or trauma 21 . Compared with MCSs, MUCSs prepared in our study had higher viability and richer extracellular matrix, so that they had better endogenous scaffold strength and microenvironment. Meanwhile, fibronectin and collagen I proteins were highly expressed. These proteins are considered to be the main structural proteins of the extracellular matrix and are closely related to the adhesion and differentiation of mesenchymal stem cells22,23. Surprisingly, VitD has a certain role in promoting the formation of MUCSs structure. The increased protein expressions of fibronectin and collagen I, and the higher content of extracellular matrix are conducive to increasing endogenous scaffold strength and intercellular signaling, thus facilitating the attachment of MUCSs and the construction of microenvironment. In addition, SEM of the tooth root fragment/sheet complex also revealed that the presence of VitD improved the regeneration performance of MUCSs to varying degrees. MUCSs were more tightly attached to the root fragments, with more mineralized matrix deposition, suggesting VitD make MUCSs have stronger tissue regeneration activity.
In patients with periodontitis, the accumulation of inflammatory factors in the periodontal microenvironment, the presence of plaque and tartar, and the destruction of root structure are not conducive to the attachment and growth of MUCSs. Scaling can remove plaque and tartar and improve the periodontal microenvironment to some extent; however, it cannot completely remove the smear layer on the root surface due to the limitations in anatomical location and operational techniques 24 . Therefore, the application of some adjuvant therapies (e.g., chemical agents and laser treatments that have emerged in recent years) can help remove adverse factors and further improve the periodontal microenvironment. EDTA root surface treatment could remove the smear layer through chelation 25 . As a painless and minimally invasive technique, Er,Cr:YSGG laser has been widely used in oral treatment, such as root surface treatment and soft tissue repair in recent years26,27. In the control group of our study, 24% EDTA and Er,Cr:YSGG laser treatments were more effective in improving the state of the root surface, with comparable effectiveness. However, periodontal tissue regeneration is a dynamic process involving the compatibility between root fragments and sheets. The surface conditions of the root fragments include the degree of dentinal tubular openness, bone morphogenetic proteins, and mineralized matrix deposition, which can affect the regenerative activity of MUCSs to varying degrees28,29. In this study, the MUCSs showed different degrees of regeneration performance after inoculation with root slices treated with different methods. In particular, MUCSs in the Er,Cr:YSGG laser treatment group were closely attached to the root surface, along with massive mineralized matrix deposition, suggesting that these cells had better tissue regeneration activity. This may be explained by the better tissue compatibility of the root fragments treated by the Er,Cr:YSGG laser, which makes it easier for MUCSs to be attached to the root surface and exert better tissue regeneration activity.
VitD can promote osteogenic differentiation of hPDLSCs and thus plays an active role in MUCSs formation and tissue regeneration. Meanwhile, the potential of Er,Cr:YSGG laser in periodontal tissue engineering should also be further explored. Our future research will also focus on the molecular mechanisms through which VitD exerts its effects on hPDLSCs and MUCSs and on how the Er,Cr:YSGG laser improves the complex process of root surface-sheet compatibility, thus further advancing the clinical application of periodontal tissue engineering techniques.
Footnotes
Author Contributions: J.W. and C.Z. have made contributions to the writing of the manuscript and the design of the figures. S.G. and X.X. contributed to the study design and revision of the manuscript. All authors have approved the submitted version of the article and have agreed to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors read and approved the final manuscript.
Data Availability Statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethical Approval: This study was approved by the institutional review board.
Statement of Human and Animal Rights: This article does not contain any studies with human or animal subjects.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Key Research and Development Program of Ningxia, China (2019BEG03035).
ORCID iD: Chenglei Zhang  https://orcid.org/0000-0001-6494-0040
https://orcid.org/0000-0001-6494-0040
References
- 1. Herrera D, Sanz M, Kebschull M, Jepsen S, Sculean A, Berglundh T, Papapanou PN, Chapple I, Tonetti MS. EFP Workshop Participants and Methodological Consultant. Treatment of stage IV periodontitis: the EFP S3 level clinical practice guideline. J Clin Periodontol. 2022;49(Suppl. 24):4–71. [DOI] [PubMed] [Google Scholar]
- 2. Sanz M, Marco Del Castillo A, Jepsen S, Gonzalez-Juanatey JR, D’Aiuto F, Bouchard P, Chapple I, Dietrich T, Gotsman I, Graziani F, Herrera D, et al. Periodontitis and cardiovascular diseases: consensus report. J Clin Periodontol. 2020;47:268–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baeza M, Morales A, Cisterna C, Cavalla F, Jara G, Isamitt Y, Pino P, Gamonal J. Effect of periodontal treatment in patients with periodontitis and diabetes: systematic review and meta-analysis. J Appl Oral Sci. 2020;28:e20190248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang Y, Luo W, Zheng L, Hu J, Nie L, Zeng H, Tan X, Jiang Y, Li Y, Zhao T, Yang Z, He T-C, et al. Efficient bone regeneration of BMP9-stimulated human periodontal ligament stem cells (hPDLSCs) in decellularized bone matrix (DBM) constructs to model maxillofacial intrabony defect repair. Stem Cell Res Ther. 2022;13:535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ren S, Zhou Y, Zheng K, Xu X, Yang J, Wang X, Miao L, Wei H, Xu Y. Cerium oxide nanoparticles loaded nanofibrous membranes promote bone regeneration for periodontal tissue engineering. Bioact Mater. 2021;7:242–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Daghrery A, Ferreira JA, Xu J, Golafshan N, Kaigler D, Bhaduri SB, Malda J, Castilho M, Bottino MC. Tissue-specific melt electrowritten polymeric scaffolds for coordinated regeneration of soft and hard periodontal tissues. Bioact Mater. 2022;19:268–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seyyar SA, Tıskaoğlu NS, Onder Tokuc E, Mercanlı M, Doğan L. Is serum vitamin D associated with diabetic retinopathy and its severity or with diabetes itself? Clin Exp Optom. 2023;106:612–18. [DOI] [PubMed] [Google Scholar]
- 8. Ketharanathan V, Torgersen GR, Petrovski BÉ, Preus HR. Radiographic alveolar bone level and levels of serum 25-OH-Vitamin D3 in ethnic Norwegian and Tamil periodontitis patients and their periodontally healthy controls. BMC Oral Health. 2019;19:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tay HM, Yeap WH, Dalan R, Wong SC, Hou HW. Increased monocyte-platelet aggregates and monocyte-endothelial adhesion in healthy individuals with vitamin D deficiency. FASEB J. 2020;34(8):11133–42. [DOI] [PubMed] [Google Scholar]
- 10. Kelder C, Hogervorst JMA, Wismeijer D, Kleverlaan CJ, de Vries TJ, Bakker AD. Burst, short, and sustained vitamin D3 applications differentially affect osteogenic differentiation of human adipose stem cells. Int J Mol Sci. 2020;21:3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mokhtari-Jafari F, Amoabediny G, Dehghan MM, Helder MN, Zandieh-Doulabi B, Klein-Nulend J. Short pretreatment with calcitriol is far superior to continuous treatment in stimulating proliferation and osteogenic differentiation of human adipose stem cells. Cell J. 2020;22(3):293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imashiro C, Shimizu T. Fundamental technologies and recent advances of cell-sheet-based tissue engineering. Int J Mol Sci. 2021;22:425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo R, Morimatsu M, Feng T, Lan F, Chang D, Wan F, Ling Y. Stem cell-derived cell sheet transplantation for heart tissue repair in myocardial infarction. Stem Cell Res Ther. 2020;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bousnaki M, Beketova A, Kontonasaki E. A review of in vivo and clinical studies applying scaffolds and cell sheet technology for periodontal ligament regeneration. Biomolecules. 2022;12:435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hasenzahl M, Müsken M, Mertsch S, Schrader S, Reichl S. Cell sheet technology: influence of culture conditions on in vitro-cultivated corneal stromal tissue for regenerative therapies of the ocular surface. J Biomed Mater Res B Appl Biomater. 2021;109(10):1488–1504. [DOI] [PubMed] [Google Scholar]
- 16. Magalhães FD, Sarra G, Carvalho GL, Pedroni ACF, Marques MM, Chambrone L, Gimenez T, Moreira MS. Dental tissue-derived stem cell sheet biotechnology for periodontal tissue regeneration: a systematic review. Arch Oral Biol. 2021;129:105182. [DOI] [PubMed] [Google Scholar]
- 17. Posa F, Di Benedetto A, Colaianni G, Cavalcanti-Adam EA, Brunetti G, Porro C, Trotta T, Grano M, Mori G. Vitamin D effects on osteoblastic differentiation of mesenchymal stem cells from dental tissues. Stem Cells Int. 2016;2016:9150819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Padwa BL, Zhou S, Mullokandova J, LeBoff MS, Glowacki J. Synergistic effect of 1α,25-dihydroxyvitamin D3 and 17β-estradiol on osteoblast differentiation of pediatric MSCs. J Steroid Biochem Mol Biol. 2018;177:103–108. [DOI] [PubMed] [Google Scholar]
- 19. Sattary M, Rafienia M, Kazemi M, Salehi H, Mahmoudzadeh M. Promoting effect of nano hydroxyapatite and vitamin D3 on the osteogenic differentiation of human adipose-derived stem cells in polycaprolactone/gelatin scaffold for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2019;97:141–55. [DOI] [PubMed] [Google Scholar]
- 20. Auguste BL, Avila-Casado C, Bargman JM. Use of vitamin D drops leading to kidney failure in a 54-year-old man. CMAJ. 2019;191:E390–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. You Q, Lu M, Li Z, Zhou Y, Tu C. Cell sheet technology as an engineering-based approach to bone regeneration. Int J Nanomedicine. 2022;17:6491–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schumacher D, Curaj A, Staudt M, Simsekyilmaz S, Kanzler I, Boor P, Klinkhammer BM, Li X, Bucur O, Kaabi A, Xu Y, et al. Endogenous modulation of extracellular matrix collagen during scar formation after myocardial infarction. Int J Mol Sci. 2022;23:14571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dalton CJ, Lemmon CA. Fibronectin: molecular structure, fibrillar structure and mechanochemical signaling. Cells. 2021;10:2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cobb CM, Sottosanti JS. A re-evaluation of scaling and root planing. J Periodontol. 2021;92(10):1370–78. [DOI] [PubMed] [Google Scholar]
- 25. Issa DR, Abdel-Ghaffar KA, Al-Shahat MA, Hassan AAA, Iacono VJ, Gamal AY. Guided tissue regeneration of intrabony defects with perforated barrier membranes, simvastatin, and EDTA root surface modification: a clinical and biochemical study. J Periodontal Res. 2020;55(1):85–95. [DOI] [PubMed] [Google Scholar]
- 26. Chemaly N, Franzen R, Daou M, Karam M, Mhanna R, Kozlova Y, Habre P. Er, Cr:YSGG laser surface modification effect on dentin bonding to zirconia: an in vitro study. Photobiomodul Photomed Laser Surg. 2022;40(8):573–79. [DOI] [PubMed] [Google Scholar]
- 27. Talmac AC, Yayli NZA, Calisir M, Ertugrul AS. Comparing the efficiency of Er, Cr:YSGG laser and diode laser for the treatment of generalized aggressive periodontitis. Ir J Med Sci. 2022;191:1331–39. [DOI] [PubMed] [Google Scholar]
- 28. Ranc V, Žižka R, Chaloupková Z, Ševčík J, Zbořil R. Imaging of growth factors on a human tooth root canal by surface-enhanced Raman spectroscopy. Anal Bioanal Chem. 2018;410(27):7113–20. [DOI] [PubMed] [Google Scholar]
- 29. Liu X-X, Tenenbaum HC, Wilder RS, Quock R, Hewlett ER, Ren Y-F. Pathogenesis, diagnosis and management of dentin hypersensitivity: an evidence-based overview for dental practitioners. BMC Oral Health. 2020;20:220. [DOI] [PMC free article] [PubMed] [Google Scholar]