Abstract
Duchenne muscular dystrophy is a severe, progressive, muscle-wasting disease that leads to difficulties with movement and, eventually, to the need for assisted ventilation and premature death. The disease is caused by mutations in DMD (encoding dystrophin) that abolish the production of dystrophin in muscle. Muscles without dystrophin are more sensitive to damage, resulting in progressive loss of muscle tissue and function, in addition to cardiomyopathy. Recent studies have greatly deepened our understanding of the primary and secondary pathogenetic mechanisms. Guidelines for the multidisciplinary care for Duchenne muscular dystrophy that address obtaining a genetic diagnosis and managing the various aspects of the disease have been established. In addition, a number of therapies that aim to restore the missing dystrophin protein or address secondary pathology have received regulatory approval and many others are in clinical development.
Duchenne muscular dystrophy (DMD) is a severe, progressive, muscle-wasting disease. The earliest symptoms are difficulties with climbing stairs, a waddling gate and frequent falls; patients present with these symptoms around 2–3 years of age1. Most patients become wheelchair dependent around 10–12 years of age and need assisted ventilation at around 20 years of age1. With optimal care, most patients with DMD die between 20 and 40 years of age from cardiac and/or respiratory failure1.
DMD is caused by mutations in DMD (encoding dystrophin) that prevent the production of the muscle isoform of dystrophin (Dp427m)2. Mutations in DMD can also cause Becker muscular dystrophy (BMD)2, which is a milder disease with a later onset and a slower progression than DMD1. The different spectra of the diseases can be explained by the ‘reading frame rule’3. In muscle, dystrophin links cytoskeletal F-actin with the extracellular matrix via its N-terminal and C-terminal domains. In DMD, frameshifting mutations (deletions or duplications that involve a number of nucleotides not divisible by three) or nonsense mutations (a point mutation where the code for a codon for an amino acid is changed into a stop codon) cause premature truncation of protein translation, leading to non-functional and unstable dystrophin. Nonsense-mediated decay does not seem to affect these dystrophin transcripts but epigenetic changes cause a reduction in transcript production4. By contrast, in BMD, mutations in the middle of the gene maintain the reading frame (deletions or duplications containing a number of nucleotides divisible by three) and allow the production of dystrophins with fewer spectrin-like repeats but that have both F-actin and extracellular matrix binding domains and are therefore partially functional (FIG. 1).
Fig. 1 |. Schematic depiction of DMD and dystrophin protein.

a | The ~2.4 Mb full-length DMD gene contains eight promoters and 79 exons. Three upstream promoters (Dp427b, Dp427m and Dp427p) produce the ~11.4 kb full-length cDNA and the 427 kDa full-length dystrophin protein. Four internal promoters (Dp260, Dp140, Dp116 and Dp71) generate N-terminal truncated non-muscle isoforms of dystrophin. Alternative splicing at the 3’-end and alternative polyadenylation (the addition of a poly(A) tail to RNA) yield additional isoforms of dystrophin such as Dp40. The full-length protein generated from Dp427m is the primary muscle isoform. b | Full-length dystrophin can be divided into four major domains, including the N-terminal F-actin-binding domain (ABD; encoded by exons 1–8), rod (R; encoded by exons 8–64), cysteine-rich (CR; encoded by exons 64–70) and C-terminal (CT; encoded by exons 71–79) domains. The rod domain can be further divided into 24 spectrin-like repeats and four interspersed hinges. c | In patients with Duchenne muscular dystrophy (DMD), protein production is prematurely truncated and the resulting protein is not functional. This leads to the loss of connections between the cytoskeleton and the extracellular matrix. d | By contrast, in patients with Becker muscular dystrophy, a partially functional dystrophin is produced that contains the crucial domains required to connect to F-actin and the extracellular matrix.
This Primer provides a comprehensive introduction to DMD, starting with epidemiology, inheritance pattern and pathophysiology. Also discussed are the standards of care for the diagnosis and multidisciplinary management of patients with DMD to enhance their quality of life (QOL) as much as possible. In addition, the therapeutic approaches that have been approved and others in clinical development are reviewed.
Epidemiology
Dystrophinopathies are X-linked recessive disorders affecting 1 in 5,000 to 1 in 6,000 live male births5–7. The prevalence of DMD is less than 10 cases per 100,000 males and seems to be the same between regions6–8. By contrast, the prevalence of BMD is less than 8 cases per 100,000 live male births7. Whether the prevalence of DMD has changed over time is not known owing to a lack of data.
With optimal care, patients with DMD can survive to live in their forties, mainly owing to the development of guidelines for care and management and to improved treatment for cardiopulmonary dysfunction6. The survival of patients with DMD has improved over time; indeed, a study in France found that the median life expectancy was 25.77 years for those born before 1970 and 40.95 years for those born after 1970 (REF.9).
DMD in females is very rare (<1 per million) and is limited to case reports of individuals with Turner syndrome10–12, a translocation involving DMD or those with bi-allelic DMD mutations13. Female carriers (that is, those with a DMD mutation on one X chromosome) are usually asymptomatic but, in rare cases, resemble BMD. Approximately 2.5–19% of carriers have skeletal muscle symptoms and 7.3–16.7% develop dilated cardiomyopathy14; carriers may also have additional cardiac symptoms, including an abnormal echocardiogram without muscle involvement. Up to 13.3% of female carriers with BMD mutations have skeletal muscle symptoms and dilated cardiomyopathy but respiratory defects do not usually occur14. A systematic analysis into how these manifestations affect the lifespan of carriers of DMD and BMD has not been carried out, but a single review study in Scotland suggested that lifespan was normal in carriers with cardiomyopathy15.
Genetics
Thousands of different mutations in DMD have been found in patients with DMD or BMD2,16. Approximately 60–70% of mutations in patients with DMD are deletions, 5–15% are duplications, and 20% are point mutations, small deletions or insertions2,17. By contrast, in patients with BMD, 60–70% of mutations are deletions, 20% are duplications, and 5–10% are point mutations, small deletions or insertions2,18,19. Deletions and duplications cluster in hotspot regions in DMD, which are located at exons 45–55 and 3–9; approximately 47% and 7% of patients with DMD have mutations in these hotspots, respectively20,21.
Although >99% of patients with DMD or BMD have a deletion, duplication or small mutation, larger genomic rearrangements between an X chromosome and an autosome (non-sex chromosome) have also been reported. A few cases of translocations involving DMD have been reported22; these translocations will cause DMD in both males and females, the latter owing to non-random X-inactivation of the unaffected X chromosome. In these cases, cells with inactivation of the mutated X chromosome (cells that could, in theory, produce dystrophin) are not viable owing to the inactivation effect of the chromosomal translocation on the autosome. Only the cells where the unaffected X chromosome is inactivated will be viable. However, these will not produce dystrophin owing to the chromosomal translocation affecting DMD. Therefore, females with these translocations are unable to produce any dystrophin.
De novo mutations are common in DMD and BMD; indeed, DMD and BMD are caused by de novo germline mutations in one-third of patients18,23–26. Notably, mothers who are not somatic carriers of DMD mutations but who have children with DMD or BMD are at risk of having another child with DMD or BMD owing to germline mosaicism (a percentage of her oocytes carries the mutation). The frequency of germline mosaicism in oocytes or sperms varies per individual but can be up to 14%27.
Mechanisms/pathophysiology
DMD and dystrophin
DMD is primarily a disease of muscle degeneration and necrosis28 (FIG. 2), although the mechanisms underlying muscle death have been elusive. Several hypotheses of muscle death were debated before the discovery of dystrophin, including muscle ischaemia, motor neuron abnormality, nutritional deficiency, metabolic defects, aberrant calcium regulation and sarcolemmal damage29. Of these, the sarcolemmal hypothesis gained the most popularity as it seems to explain many clinical findings of DMD. According to the sarcolemmal hypothesis, DMD is caused by structural and/or functional defects of a sarcolemmal protein owing to mutations in the encoding gene29; subsequent investigations identified dystrophin and DMD as the causative protein and gene, respectively30,31 (FIG. 1), although in contrast to the initial hypothesis, dystrophin is a subsarcolemmal rather than a sarcolemmal protein.
Fig. 2 |. Healthy muscle and DMD muscle histology.

Cross-sectional staining of healthy muscle (panels a–d) and skeletal muscle from a patient with Duchenne muscular dystrophy (DMD; panels e–h). Haematoxylin and eosin (HE) staining shows centrally nucleated myofibers, inflammatory cell infiltration, variable myofiber size, and endomysium and perimysium connective tissue deposition (panels a and e). Masson trichrome (MT) staining shows increased fibrosis (blue staining) in a patient with DMD compared with healthy muscle (panels b and f). Immunofluorescence labelling of dystrophin and laminin shows a lack of dystrophin in a patient with DMD compared with healthy muscle (panels c and g) and variation in myofiber size in DMD muscle (panels d and h).
DMD encodes the muscle-specific dystrophin (Dp427m) in addition to two other full-length isoforms from the promoters Dp427c and Dp427p32,33 (FIG. 1); these isoforms are expressed in cortical neurons and cerebellar Purkinje cells, respectively32,33. The Dp427p isoform was identified in mice and research suggests that Dp427p expression in human cerebellum is very low during embryonic development and postnatally34. In addition to the full-length dystrophin isoforms, shorter dystrophin isoforms are produced by four internal promoters. These isoforms are primarily expressed throughout different tissues; Dp260 is expressed primarily in the retina35, Dp140 is expressed in the central nervous system, kidney36 and at high levels in embryonic brain34, Dp116 is primarily expressed in peripheral nerves and Schwann cells37, and Dp71 is expressed ubiquitously but at higher levels in neuronal cells than in other cell types38. Another isoform, Dp40, arises from the same promoter as Dp71 but is polyadenylated in intron 70 (REF.39).
Dystrophin-associated protein complex
The elucidation of dystrophin-binding partners involved a combination of studies in human cell models and animal models along with the study of biopsy and autopsy tissues from patients with muscular dystrophy. In striated muscle, dystrophin interacts with the sarcolemma, cytoskeleton (actin microfilaments, intermediate filaments, microtubules and other related structural proteins), channel proteins, and signalling or scaffolding proteins either directly or indirectly (FIG. 3). Collectively, dystrophin and its binding partners form the dystrophin-associated protein complex (DAPC)40. The traditional DAPC was discovered in the early 1990s and was called the dystrophin glycoprotein complex (DGC) as several components are glycoproteins. The DGC contains 11 proteins: dystrophin, the dystroglycan subcomplex (α-dystroglycan and β-dystroglycan), the sarcoglycan subcomplex (α-sarcoglycan, β-sarcoglycan, γ-sarcoglycan and δ-sarcoglycan), sarcospan, syntrophin, dystrobrevin and neuronal nitric oxide synthase (nNOS)41. Since the discovery of the DGC, our understanding on dystrophin-interacting partners has improved. More binding partners have been identified and the interaction domains of many of these proteins have been mapped. In addition, the dynamic nature of the DAPC is appreciated; different DAPCs exist in different tissues or cells and even in different regions of the same myocyte. Collectively, these data have improved our understanding of DMD pathogenesis.
Fig. 3 |. Dystrophin and binding partners.

Dystrophin binding partners can be categorized as cytoskeletal proteins, transmembrane proteins, extracellular proteins, cytosolic signalling and scaffolding proteins, endocytic proteins, and cardiac-specific interacting proteins. ABD, actin-binding domain; CNS, central nervous system; CR, cysteine-rich domain; CT, C-terminal domain; DG, dystroglycan; NCX, sodium-calcium exchanger; nNOS, neuronal nitric oxide synthase; NOX2, NADPH oxidase 2; PMCA, plasma membrane calcium ATPase.
Dystrophin interacts with the sarcolemma through four binding domains located in spectrin-like repeats R1–3 and R10–12 and in the cysteine rich (CR) and C-terminal (CT) domains42 (FIG. 3). Dystrophin–actin binding (mainly with filamentous γ-actin in the cytoplasm) occurs via the actin-binding domain (ABD) (by two calponin-homolog motifs) and R11–15 (by electrostatic interaction)43. The microtubule–dystrophin interaction occurs via R4–15 and R20–23 (REFS44,45). In addition, dystrophin directly associates with two intermediate filament proteins, keratin 19 (via the N-terminal ABD) and synemin (via R11–15 and the CR domain)46,47. In addition to synemin, the CR domain also interacts with the transmembrane protein β-dystroglycan48, membrane docking protein ankyrin49 and the intermediate filament-associated protein plectin50. The connection between dystrophin and signalling and scaffolding proteins is more complex as it involves direct and indirect connections. Dystrophin directly binds to PAR1b (also known as MARK2, via R8–10)51, nNOS (via R16 and R17)52, α-syntrophin (via two syntrophin-binding sites at the CT domain and R17), β1-syntrophin and β2-syntrophin (via the CT domain and R22), α1-dystrobrevin, α2-dystrobrevin and α3-dystrobrevin (via two coiled-coil motifs at the CT domain), calmodulin (via the CR domain)53, and myospryn (also known as CMYA5, via the CR and CT domains)54.
α-Syntrophin brings several other proteins to the DAPC: plasma membrane calcium ATPase (PMCA), channel proteins (sodium channels Nav1.4 and Nav1.5, potassium channels Kiv2 and Kiv4.1, calcium channels TRPC1 and TRPC4, and the water channel aquaporin 4) and other signalling proteins (calmodulin, G proteins, Grb2, myocilin, diacylglycerol kinase-ζ, SAPK3 (also known as MAPK12 or p38γ MAPK) and ARMS (also known as KIDINS220))40,55,56. α-Syntrophin also works with R16/17 to jointly anchor nNOS to the sarcolemma in skeletal muscle55–57. β1-syntrophin recruits the signalling and scaffold proteins p38γ MAPK and CARM1 (also known as PRMT4) to establish satellite cell polarity58. β2-Syntrophin engages the signalling and scaffold proteins MAST205 and SAST59. α-Dystrobrevin links intermediate filament-associated protein plectin, intermediate filament protein syncoilin and synemin, coiled-coil protein dysbindin, signalling protein α-catulin, and sarcoglycan–sarcospan complex to the DAPC55,56. Myospryn interacts with dysbindin, microfilament protein α-actinin, intermediate filament protein desmin, and signalling protein calcineurin and protein kinase A55,56,60.
In addition to these proteins, the dystroglycan subcomplex and sarcoglycan–sarcospan complex bring in additional tertiary interacting proteins to the DAPC. These proteins include extracellular matrix proteins (such as laminin, agrin, perlecan and neurexin61), the extracellular signalling or structural protein biglycan, the transmembrane signalling or structural protein integrin, signalling proteins (such as Src, Grb2, Tks5 and rapsyn), and endocytic proteins (caveolin 3 and dynamin 1)40,55,56,61.
Consequences of dystrophin deficiency
Dystrophin deficiency results in the disassembly of the DAPC and in loss of the interaction between F-actin and the extracellular matrix. As the DAPC has important mechanical and signalling roles in maintaining muscle cell structural integrity and contractile activity, DAPC disassembly leads to wide-ranging consequences on muscle cell function.
Sarcolemma weakening.
Force is generated through repeated cycles of contraction and relaxation of the sarcomere, which requires the efficient handling of mechanical stress by the sarcolemma. In healthy muscle, the integrity of the sarcolemma is maintained via the linkages between the cytoskeleton, sarcolemma and extracellular matrix through the DAPC and the integrin complex. In DMD, DAPC disassembly weakens the sarcolemma, which becomes highly susceptible to contraction damage (BOX 1). This mechanism is supported by several observations. For example, sarcolemmal tears (so-called ‘delta’ lesions) have been detected using electron microscopy in muscle from patients with DMD62. In addition, sarcolemma rupture can be detected by passive leaking-in of circulating proteins or dyes, such as albumin, IgG and Evans blue, and leaking-out of muscle enzymes, such as creatine kinase (CK) from muscle into the blood, especially after exercise in both humans and mice63. Moreover, muscle damage correlates with muscle stress64; the most stressed muscles, such as the diaphragm, are affected earlier and more severely than less-stressed muscles. Finally, muscle disease is significantly attenuated in animal models by even a partial restoration of cytoskeleton, sarcolemma and extracellular matrix linkage using a highly abbreviated micro-dystrophin protein65.
Box 1 |. Muscle activity and DMD pathogenesis.
Duchenne muscular dystrophy (DMD) cannot be explained by a single mechanism218; indeed, overlapping yet distinctive pathogenetic processes are likely involved at different stages of the disease and account for different clinical presentations in various organ systems.
Muscle tissue is specialized for contraction and forms the basis of body movement, ventilation and pumping blood. Contractile activity also generates substantial mechanical stress that, if not properly managed, will damage muscle. The failure to manage muscle activity-induced mechanical stress constitutes a central theme in DMD pathogenesis.
Several lines of evidence suggest the involvement of muscle activity in the initiation and progression of muscle disease in DMD. For example, dystrophin is expressed early in human gestation219; however, patients have minimal symptoms until 2–3 years of age when they start to walk. Similarly, the onset of muscle necrosis coincides with increased cage activity in dystrophin-deficient mdx mice and limb immobilization prevents muscle degeneration in mdx mice220. In addition, the diaphragm is the primary ventilation muscle at rest and is the most severely affected muscle. Dystrophin expression patterns and dystrophin-associated protein complex (DAPC) composition further point to the importance of muscle activity. Specifically, dystrophin is highly enriched at muscle sites that receive the most mechanical stress such as the myotendinous junction, the costamere (which connects the sarcomere to the sarcolemma) and the Z-disc network. The DAPC connection at the costamere is further strengthened by additional interactions through α-actinin, ankyrin, desmin, syncoilin and synemin.
Functional ischaemia.
Small clusters of muscle degeneration and regeneration are the first observable muscle lesions in patients with DMD and are readily detected in haematoxylin and eosin-labelled cross sections on a skeletal muscle biopsy in the first year of life before symptom onset66,67. This finding is explained by the discovery that dystrophin (specifically dystrophin spectrin-like repeats R16 and R17) anchors nNOS to the sarcolemma to produce and release nitric oxide to the surrounding vasculature in exercising muscle, leading to vasodilation52,68 and ensuring blood perfusion in contracting muscles. In the absence of dystrophin, nNOS is mislocalized to the sarcoplasm and the total cellular nNOS level is reduced, thereby compromising protective vasodilation and leading to ischaemic damage to muscle69. Importantly, R16-containing and R17-containing dystrophins effectively restore sarcolemmal nNOS and ameliorate muscle pathology in mouse and dog models of DMD52,70,71.
Free-radical damage.
Muscles from animal models and patients with DMD produce significantly more free radicals than normal muscle. A major source of free radicals in muscle is the production of reactive oxygen species (ROS) by NADPH oxidase 2 (NOX2). NOX2 is activated by the microtubule-associated protein Rac1 upon mechanical stretching of the muscle, leading to ROS production by NOX2 (REFS72,73). The process of NOX2-mediated ROS production is significantly enhanced in DMD as the microtubule lattice becomes dense and disorganized due to the loss of dystrophin–microtubule interaction44,72,73. Reactive nitrogen species, generated by the cytosolic activation of delocalized nNOS, are another important source of free radicals in DMD muscle74. In addition, infiltrating inflammatory cells and dysfunctional mitochondria produce further free radicals in DMD muscle. Collectively, this free-radical production leads to increased markers of protein, lipid and DNA oxidation in DMD muscle, indicating ongoing and recurring oxidative damage75,76. The dystrophic muscle is also more vulnerable than healthy muscle to oxidative stress77. Reduced glutathione is one of the most abundant and important antioxidants that protects muscle against harmful oxidative injury; however, glutathione levels are significantly decreased in dystrophic muscle78,79 and therefore the ability to cope with oxidative stress is greatly reduced in DMD muscle. Despite the accumulating evidence implying oxidative stress in DMD pathogenesis, generic antioxidant therapy has not yielded convincing clinical benefits in patients75.
Cytosolic calcium overloading.
The resting cytosolic (especially subsarcolemmal) calcium concentration in muscle from DMD models and in patient-derived cell models is higher than that of healthy muscle80. Calcium overloading-induced mitochondrial dysfunction results in metabolic defects and calcium overloading also directly contributes to muscle death by triggering degradative pathways such as calcium-dependent calpain protease and phospholipase A2 and mitochondrial-dependent necrosis81.
Calcium overloading is partially caused by abnormal sarcolemmal calcium entry through the calcium channels (stretch-activated, store-operated, voltage-gated and receptor-operated channels), plasma membrane Ca2+ ATPase (PMCA), sodium-calcium exchanger (NCX) and microtears on the sarcolemma56. Interestingly, stretch-activated calcium channels, store-operated calcium channels and PMCA are DAPC-associated proteins and NCX is linked to the DAPC via calmodulin56. Despite these data, calcium channel blockers have demonstrated almost no clinical benefit in patients with DMD82, suggesting that calcium channel-mediated entry may only play a minor role in pathogenesis.
The involvement of the sarcoplasmic reticulum (SR) in DMD has gained attention. Calcium is released from the SR via the ryanodine receptor (RyR) during muscle contraction and is pumped back to the SR by sarco/endoplasmic reticulum calcium ATPase (SERCA) during relaxation. In DMD, RyR is nitrosylated and phosphorylated owing to nitrosative/oxidative stress and abnormal signalling (such as via the DAPC member protein kinase A)83,84, respectively, which reduce the binding of calstabin (a protein that stabilizes the RyR channel)84. The disassociation of calstabin increases RyR channel opening, leading to calcium leakage from the SR85. By contrast, RyR stabilizers significantly reduced muscle pathology in the mdx mouse model of DMD84,86. SERCA activity is significantly reduced in dystrophic muscle largely due to the increased expression of sarcolipin, a small peptide that negatively regulates SERCA activity. Overexpressing SERCA or depleting sarcolipin effectively ameliorated skeletal muscle disease and cardiomyopathy in symptomatic DMD mouse models87,88.
Regeneration failure.
The failure to regenerate underlies muscle wasting, fibrosis and fat replacement in DMD. Some studies have suggested that the DAPC plays a direct role in muscle regeneration58,89. Indeed, in healthy muscle, injury is repaired by the asymmetric division of satellite cells and the interactions of DPAC proteins (dystrophin–PAR1b and β1-syntrophin–p38γ/Carm1) are essential for this process58,89. DAPC disassembly compromises the myogenic commitment of activated satellite cells, thus impairing regeneration. Although this theory reveals a potential direct effect of the loss of dystrophin on regeneration, more work is needed to demonstrate how impaired asymmetric satellite cell division leads to regeneration failure in both animal models and patients. In addition to the potential defect in myogenic commitment, failed regeneration occurs due to the indirect consequences of changes in the microenvironment from the loss of dystrophin such as matrix restructuring, epigenetic changes and chronic inflammation (reviewed in REFS90–92).
Consequences of muscle damage.
DAPC disassembly-induced muscle damage (such as sarcolemmal leakage, ischaemic injury, oxidative and nitrosative stress, and aberrant activation of calcium-dependent protease and phospholipase) causes defective organelles and altered cellular constituents in dystrophic muscle. In normal muscle, defective organelles and protein aggregates are removed by autophagy93; however, autophagy is severely repressed in DMD owing to the activation of Akt, a potent autophagy inhibitor94. DAPC disassembly contributes to Akt activation through the ROS-mediated activation of the Src/PI3 kinase pathway95. Integrin upregulation and integrin–laminin binding also contribute to Akt activation through the PI3 kinase pathway96. Impaired autophagy leads to the unchecked accumulation of dysfunctional proteins and defective organelles and, eventually, to muscle cell degeneration97.
Degenerated and damaged muscle cells are sensed and cleared by infiltrating inflammatory cells. In young patients with DMD (2–8 years of age), these infiltrates mainly consist of macrophages and T cells98. At the early phase of inflammation, pro-inflammatory M1 macrophages promote muscle cell lysis through the production of inducible NOS. In later phases, M1 macrophages are replaced by anti-inflammatory M2 macrophages, which promote regeneration and fibrosis99. CD4+ helper T cells assist other immune cells by producing inflammatory cytokines and CD8+ cytotoxic T cells trigger muscle cell death through a perforin-mediated mechanism99. Neutrophils, mast cells and eosinophils also contribute to inflammation and immune-mediated pathology in dystrophic muscle98,99. During the early phase of the disease, degenerated muscle is repaired by regeneration; however, in later phases, dead muscle cells are ultimately replaced by fatty and fibrotic tissue owing to the impaired regenerative capacity and upregulation of TGFβ in the chronic inflammatory milieu of DMD muscle100.
Genotype–phenotype correlation
Genotype–phenotype correlation studies have provided unique perspectives on DMD pathogenesis. Studies in patients with BMD have corroborated basic research findings on the relationship between dystrophin structure and function. For example, patients with BMD who lack the ABD or R16/R17 nNOS-binding domain often have more severe disease than patients with BMD who have other variants2,101. Genotype–phenotype correlation studies have also implicated regions of dystrophin that are important for cardiac disease (such as R16–R19) and cognitive deficiency (such as the CT domain)102–104. The mechanism behind these findings remains unclear.
Our understanding on DMD and BMD pathogenesis has also benefited from studying patients with BMD (even within the same family) that have the same mutation but have variations in severity and from studying patients with DMD who all lack dystrophin but show variations in the speed of disease progression. These studies have highlighted the importance of genetic modifiers, whereby variations in genes involved in, for example, inflammation or fibrosis formation, can influence disease outcome. For example, genetic variations in a region close to CD40 influences the expression of CD40, which is involved in T cell polarization. The variant causing increased expression of CD40 was associated with a slower disease progression, where patients carrying the protective allele of this variant lost ambulation significantly later in five cohorts of patients with DMD105.
Six potential genetic modifiers that slow down disease progression have been identified. These include variants that affect the expression and function of SPP1, LTBP4, CD40, THBS1, ACTN3 and TCTEX1D90,106. More genetic modifiers are likely to be identified in the future. As DMD is a rare disease, identifying these genetic modifiers is challenging and it is important to validate the findings in independent cohorts, as with the CD40 modifier, to exclude false positive results due to small sample sizes.
Cardiomyopathy and cognitive manifestations
The heart is the most stressed muscle in the body but cardiomyopathy is a late manifestation of DMD. This observation is likely due to differences in dystrophin expression and DAPC composition. Specifically, only in the heart is the Dp427m form of dystrophin expressed in t-tubules107, dystrophin directly binds to contractile apparatus (α-actin) at the Z-discs108, and dystrophin interacts with cypher, AHNAK1, cavin 1 and CRYAB, four cardioprotective proteins109.
As previously mentioned, mutations that prevent the production of muscle dystrophin also prevent the production of two other full-length and, depending on the location of the mutation, one or more shorter brain isoforms of dystrophin. It is therefore not surprising that many patients with DMD have cognitive deficits. Indeed, one-third of patients with DMD have cognitive impairment and learning difficulties and behavioural issues are also common110. Cognitive impairment is apparent from an early age but is not progressive. MRI of 29 patients with DMD revealed no gross abnormalities in the brain, although quantitative analysis revealed reduced grey matter volume, white matter abnormalities and reduced cerebral perfusion compared with age-matched controls111. In the brain, dystrophin is expressed at the postsynaptic inhibitory GABAergic neurons in the amygdala, hippocampus and cerebellar Purkinje cells112,113. Some studies have correlated cognitive impairment with mutations that affect the shorter isoforms of dystrophin (such as Dp140)114 . Furthermore, the differences observed by MRI were more apparent in patients with DMD who lacked Dp140 than in those with Dp140 expression111. However, little is known about the exact roles of the different dystrophin isoforms expressed in the brain. The relationship between DAPC disassembly and cognitive impairment is even less clear115,116. Interestingly, in the brain, DAPC has distinctive compositions and includes several unique components such as neurexin and γ-syntrophin112. Future studies are needed to clarify the connection between dystrophin deficiency and cognitive manifestations.
Diagnosis, screening and prevention
Obtaining an accurate genetic diagnosis
Guidelines for the genetic diagnosis of DMD should be followed117. DMD should be suspected in young males (aged 2–4 years old) who present with delayed motor milestones, muscle weakness, hypertrophic calves and the Gowers sign (where patients use their hands to ‘walk’ on their lower limbs when getting up from the floor to compensate for weakness in the upper leg and hip muscles). As mentioned earlier, 30% of patients with DMD have cognitive impairment at presentation and speech delay is common; therefore, these findings should also prompt suspicion of DMD118.
Plasma CK should be measured in boys with symptoms of DMD as CK levels are generally substantially increased in patients with DMD from birth, with levels of >20,000 U/L not uncommon. Notably, plasma aspartate transaminase (AST) and alanine transaminase (ALT) levels are also increased in patients with DMD owing to ongoing muscle damage and the high expression of these enzymes in skeletal muscle; however, AST and ALT levels are not usually assessed as part of a standard diagnostic work-up of patients with suspected DMD as CK levels are a more specific marker of muscle damage. DMD is likely in young males who present with typical DMD symptoms and elevated plasma CK levels; it is crucial to identify the DMD mutation in those individuals to confirm diagnosis119. In addition, identifying the causative mutation is required to initiate multidisciplinary care, to permit carrier analysis of the mother and genetic counselling, and to determine whether the patient is a candidate for the mutation-specific therapies currently approved in several regions120. Of note, as elevation of plasma CK is a non-specific marker for skeletal muscle damage, DMD cannot be diagnosed based only on elevated plasma CK levels; genetic confirmation is crucial, as indicated in the guidelines.
Although exome sequencing or gene sequencing will likely be routine for all patients with suspected inherited diseases in the future, at present, it is more economical to perform a genetic diagnosis for dystrophinopathies using a step-wise approach117,119 (FIG. 4). First, the presence and abundance of the 79 DMD exons is evaluated using generally multiplex ligation-dependent probe amplification (MLPA) or array comparative genome hybridization (array CGH). MLPA is used by most genetic diagnostic laboratories as is it easier to implement owing to the availability of commercially available kits121. As ~75% of patients harbour DMD deletions or duplications2, MLPA or array CGH will detect the causative mutation in most patients. Of note, if MLPA identifies a single exon deletion, it is important to confirm this finding with a secondary test to rule out whether one of the probes was unable to bind due to a small mutation. If MLPA or array CGH fail to identify a mutation, small mutation analysis using Sanger sequencing is required, during which each of the 79 exons and flanking intronic regions are sequenced.
Fig. 4 |. Diagnostic decision tree to confirm the genetic diagnosis of dystrophinopathies.
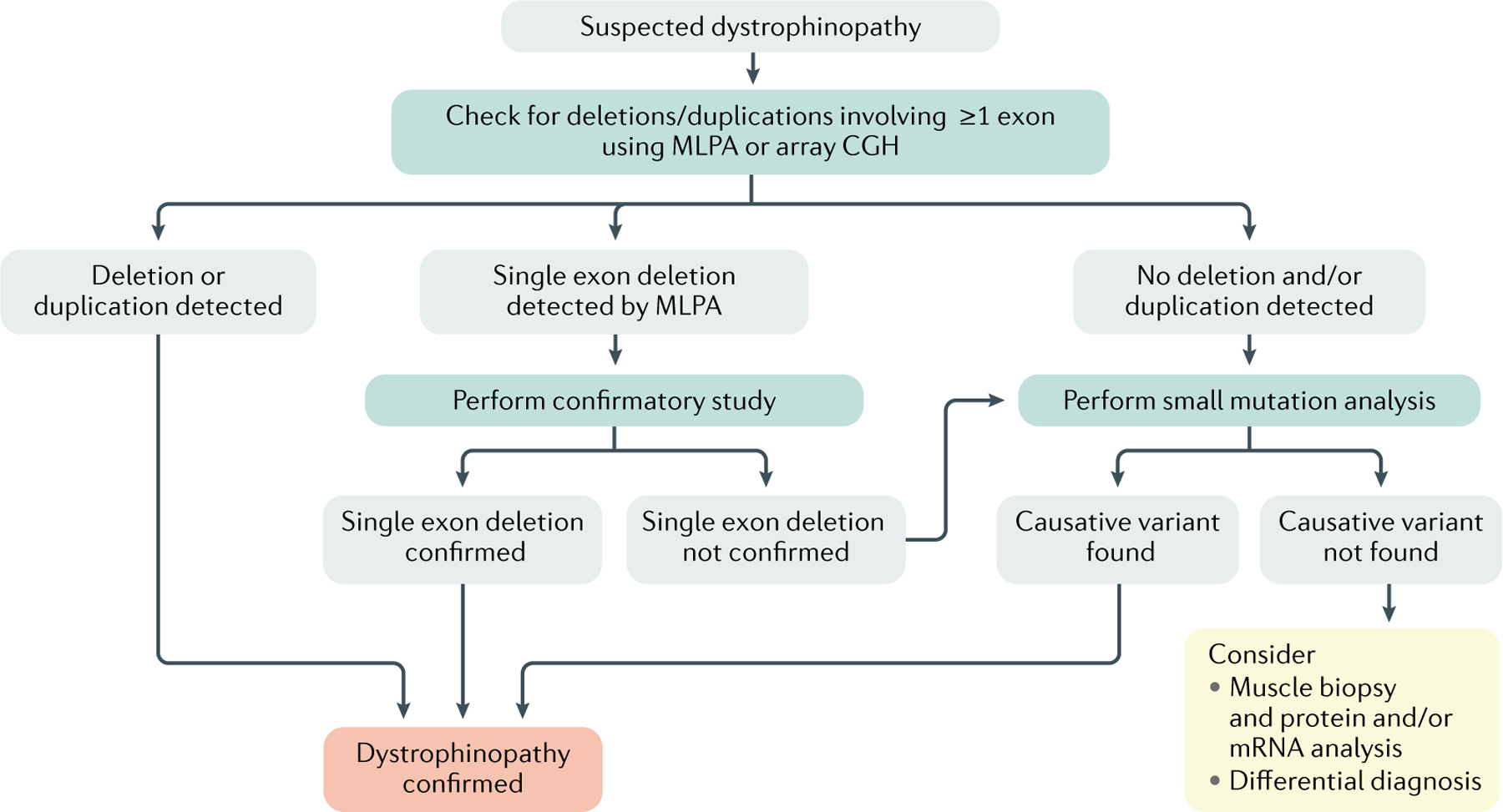
Boys with typical symptoms of a dystrophinopathy and increased plasma creatine kinase (CK) levels should undergo genetic testing to confirm the diagnosis. Initially, multiplex ligation-dependent probe amplification (MLPA) or array comparative genome hybridization (CGH) should be used to identify causative DMD variants; however, if a causative variant is not identified (which occurs in ~25% of patients), small mutation analysis should be used. Boys without an identified causative variant after small mutation analysis should be referred for muscle biopsy and protein or mRNA analysis.
MLPA is implemented in diagnostic laboratories around the world in middle-income and high-income countries; however, small mutation analysis is often available only in specialized laboratories. In low-income countries, only multiplex PCR may be available. Multiplex PCR sets are available that assess the presence or absence of frequently deleted DMD exons based on the way deletions cluster in two hotspots122. However, as these sets do not assess all DMD exons, this analysis only reveals that some exons are missing and other exons are present and it is unclear exactly which exons are involved in the deletion. For example, when it is known that exon 43 and exon 46 are present while exon 45 is absent, the deletion can involve only exon 45 (out-of-frame) or exon 44 and exon 45 (in-frame)119. Thus, this approach does not permit the assessment of whether the deletion is in-frame or out-of-frame or whether a patient is eligible for a mutation-specific treatment. Furthermore, this test only reliably allows the identification of deletions and not of other mutation types and, accordingly, multiplex PCR is not recommended for the genetic diagnosis for DMD and BMD. However, if MLPA, array CGH and small mutation analysis are not available, multiplex PCR is preferable to not conducting any genetic analysis.
When interpreting the effect of the mutation, one has to bear in mind that the clinical symptoms are leading in deciding whether someone has DMD or BMD. In >90% of cases, in-frame mutations will lead to BMD and out-of-frame mutations to DMD2; however, exceptions have been reported. In the case of discordance between symptoms and genetic mutations, such as an in-frame mutation and symptoms of DMD, one could consider a muscle biopsy and protein analysis to assess, for example, whether alternative splicing can explain the discordant phenotype.
In cases whereby a causative mutation has been identified in the patient, the mother should undergo testing to assess whether she is a carrier. If the mother is confirmed as a carrier, her mother and sisters and, potentially, maternal aunts and cousins may also be carriers and should be offered counselling. Mothers who are carriers have a 50% chance of having another son with DMD or a daughter who is also a carrier. Notably, mothers who are not carriers have a risk of up to 14% of having another son with DMD owing to germline mosaicism27.
A muscle biopsy is not required for a diagnosis of DMD for most patients and is indicated only when no mutations are detected using MLPA, array CGH or Sanger sequencing. In these cases, the purpose of muscle biopsy is to assess whether dystrophin is properly localized (using immunofluorescence analysis) or absent/reduced (using Western blotting and immunofluorescence, absent levels are diagnostic of DMD) or has an altered size (using Western blotting, an altered size is diagnostic of BMD). Often, these cases are caused by deep intronic mutations and mRNA can be isolated from the biopsy for further analysis119. Care can be initiated in those without an identified causative mutation; however, genetic counselling is more challenging and it is unclear whether a patient is eligible for mutation-specific therapies. Notably, dystrophin protein analysis by Western blotting or immunofluorescence analyses is challenging owing to the large molecular size and low abundance of dystrophin123. As such, it is not widely available in a diagnostic setting in low-income and middle-income countries.
Newborn screening
The measurement of plasma CK levels from dried blood spots is feasible and can be used for DMD newborn screening (DMD-NS)5,124,125. In those with elevated CK levels, testing for DMD mutations should be used to confirm the diagnosis5.
Of note, screening for CK elevation can identify other muscle diseases. Indeed, in a review of 10 pilot DMD-NS studies, ~20% of positive results were due to non-DMD disorders, including BMD and limb girdle and congenital muscular dystrophies126. The ability to detect disorders other than DMD using this method highlights the need for follow-up testing algorithms to evaluate patients who lack DMD mutations. The rate of false negatives using this screening method was low (20 per 1,800,000)126.
Aside from local or regional pilot studies in, for example, Wales, Germany and the USA, no DMD-NS programme has been adopted. In general, neonatal screening is considered for neonatal-onset disorders for which early treatment shows strong evidence of improved outcome. Although DMD does not fully meet these criteria, advocacy groups are in favour of an early diagnosis to enable early management and interventions. Emerging therapies that might be more effective when used in an early stage of the disease before irreversible muscle damage has occurred strengthen their request to include DMD in newborn screening127. In addition, an early diagnosis of DMD should result in a prompt referral for genetic counselling and will identify the carrier status of the mother. Reproductive choices, such as pre-implantation genetic diagnosis or prenatal genetic testing through chorionic villus or amniotic fluid sampling, can be offered to female carriers, limiting the risk of DMD recurrence in these families. Furthermore, female carriers should be informed about the recommendations related to the higher risk of developing a cardiomyopathy117.
Prenatal screening for DMD
The adoption in routine prenatal care of non-invasive prenatal screening based on the analysis of cell-free DNA in the maternal serum raises new challenges. This technique detects fetal aneuploidies, typically trisomy 21, 13 and 18 (REF.128), but also reveals maternal copy-number variations (CNVs), the implications of which need to be assessed. CNVs in DMD are among the most commonly observed maternal CNVs, some of which have unknown significance129. The prediction of the phenotype caused by the CNV and subsequent genetic counselling are based on family history, segregation analysis (assessing how the variant is inherited within a family, for example, if it is present in a healthy grandfather, then pathogenicity can be excluded) and using databases with genotype–phenotype correlations of DMD variants (Leiden Open Variation Database (LOVD) and the UMD-DMD France Database2,130). Applying the frameshift rule to identified CNVs allows DMD to be distinguished from BMD with ~90% accuracy119.
Management
Multidisciplinary management of DMD
Despite major therapeutic advances over the past 30 years, there is no cure for DMD. Nevertheless, a multidisciplinary medical, surgical and rehabilitative approach targeting the symptoms of DMD can alter the natural course of the disease, improving QOL and longevity131–133. Although DMD primarily affects cardiac and skeletal muscles, there are also a multitude of extra-muscular manifestations and secondary con- sequences of muscle weakness; these manifestations require a coordinated, multidisciplinary, patient-centred approach throughout the different stages of the disease. This approach should occur from diagnosis to end of life and should anticipate the specific needs of the individual according to the stage of the disease, including a transition from paediatric to adult care and advanced care planning, and it should also address broader aspects of health and QOL. In addition to a core team of physicians who have expertise in patients with DMD, nurses, physiotherapists, speech therapists and dieticians are key partners in the prevention and management of symptoms for patients with DMD. In addition, psychologists, occupational therapists and social workers should provide assistance to address psychosocial issues, to improve participation and to support the achievement of life goals.
Best care guidelines have been published for DMD. These guidelines are most often not evidence-based as large-scale randomized controlled trials for this disease are lacking. However, these recommendations are based on the expertise of key opinion leaders on the best available evidence that was collected and analysed with acknowledged methodologies to reach a consensus117,134,135. These recommendations should be seen as guidance for the care and management of DMD but should be tailored to the need and preferences of individual patients and should be adapted according to emerging new evidence in the care of DMD.
Respiratory care.
Improved respiratory care for patients with DMD has considerably increased life expectancy9,136. Guidelines for respiratory care include the assessment of respiratory function annually from diagnosis and every 6 months after loss of ambulation. In addition, the guidelines also include the use of mechanically assisted coughing and the provision of respiratory support via mechanical ventilation in those with hypoventilation137. Symptoms of nocturnal hypoventilation, such as morning headaches, fatigue, anorexia and frequent nocturnal awakenings, should trigger sleep studies to detect and treat this issue134.
Cardiac care.
Cardiac involvement in DMD is characterized by the development of a progressive dilated cardiomyopathy, resulting in congestive heart failure, cardiac insufficiency, conduction abnormalities, ventricular or supraventricular arrhythmia, and risk of sudden early death9,136. The improvement in respiratory care for patients with DMD and the provision of respiratory support has resulted in a shift in causes of death from primarily respiratory failure to acute cardiac events owing to dilated cardiomyopathy, cardiac fibrosis and conduction disturbances, and cardiac insufficiency. Management of the cardiac manifestations of DMD includes early detection of the symptoms of insufficiency and arrhythmias via assessment at diagnosis and every subsequent year. This assessment should include a physical examination, electrocardiography, echocardiography or, when possible, cardiac MRI134,138. After the onset of cardiac symptoms, the frequency of examination should increase at the discretion of the cardiologist. Symptoms of cardiac insufficiency and arrhythmias are treated with angiotensin-converting enzyme (ACE) inhibitors and beta-blockers. The early initiation of ACE inhibitors as a cardioprotective treatment is recommended in patients approaching 10 years of age, as prophylactic treatment with ACE inhibitors has been suggested to delay the onset of cardiac symptoms139–141.
Orthopaedic management.
DMD is characterized by the development of limb muscle contractures (a shortening of the muscles leading to deformity) and scoliosis. In addition, impaired bone metabolism with reduced bone mineralization is a well-known feature of DMD and is negatively affected by corticosteroid therapy and can predispose individuals to compression fractures. The overall aim of orthopaedic management is to optimize or preserve the weakened muscle function and to prevent severe deformities such as contractures or scoliosis. The indication for orthopaedic interventions and surgical procedures should be assessed and planned with the members of the multidisciplinary team, involving physiotherapists, occupational therapists and orthotists.
Orthopaedic management in patients who are ambulant focuses on the prevention of contractions of Achilles tendons, knee and hip flexors, and iliotibial bands with physiotherapy and the use of orthoses to preserve stability and ambulatory capacity. In patients who are non-ambulant, further attention should be paid to adequate sitting posture to improve comfort and prevent (worsening of) scoliosis and contractures in the lower limbs and to the prevention of contractures in the upper limbs and hands, which could impair fine motor activities.
Glucocorticosteroid treatment has reduced the occurrence of severe scoliosis requiring surgery in adolescents with DMD142; however, careful clinical and radiological monitoring of the spine is still indicated in patients receiving glucocorticosteroids as scoliosis can develop at a later age, particularly when corticosteroids are stopped134. Typically, a baseline spine x-ray to detect vertebral fractures is recommended in all patients, with annual follow-up assessments when the patient becomes non-ambulant. If a vertebral compression fracture is found, treatment with intravenous bisphosphonates may be considered134. If scoliosis is present, the clinical and radiological assessment should be repeated ever 6–12 months depending on the curve and skeletal maturity134. These assessments will guide the decision to perform a posterior spinal fusion based on age, velocity of the evolution of the scoliosis, skeletal maturity and general condition of the patients. The perioperative risks of posterior spinal fusion should be limited by involving a multidisciplinary team with experience in neuromuscular disorders and anaesthesiologists informed about the specific risks related to this population such as acute rhabdomyolysis, adrenal crisis owing to chronic glucocorticosteroid treatment and increased risks of cardiorespiratory complications.
Endocrinological management.
Although corticosteroids are associated with improvements in DMD disease course and survival, they are also associated with treatment-related adverse effects that affect multiple systems. Endocrinological management should address impaired growth, bone health, glucose and fat metabolism, and delayed puberty, all of which are compromised in patients with DMD and are further affected by glucocorticosteroids. Hypogonadism and delayed puberty treatment should conform to the standard of care for those with pathological pubertal delay in the adolescent population117. Dietary advice focusing on healthy eating habits, reduction of fat and refined sugars, and adequate intake of calcium and vitamin D should be provided from diagnosis to minimize the risk of overweight. Of note, dietary advice on caloric intake should be adjusted based on the decline in physical activity in patients with DMD and should address the excessive appetite often associated with glucocorticosteroid treatment117.
Gastrointestinal management.
Symptoms of involvement of visceral smooth muscle, such as delayed gastric emptying and intestinal paresis, become more apparent in adults with DMD. In addition, constipation and gastroesophageal reflux disease (GERD) are very frequent and frequent complications of DMD, respectively. Preventative measures and pharmacological interventions should address these gastrointestinal symptoms. Daily treatment with osmotic laxatives or lactulose might be necessary for patients with DMD and constipation and retrograde enemas might be helpful in those with faecal impaction. Treatment of GERD consists of gastric acid suppression using histamine 2 receptor antagonists (such as ranitidine) or proton-pump inhibitors (such as lansoprazole or omeprazole). Dietary approaches to prevent GERD symptoms include eating smaller, more frequent meals and decreasing dietary fat intake117. Oropharyngeal weakness and oesophageal dysmotility may jeopardize safe and adequate food intake and result in undernutrition, which should lead to the use of a percutaneous endoscopic gastrostomy tube to optimize nutritional status and limit the risk of aspiration (breathing food into airways). As with many manifestations of DMD, there is heterogeneity in the timing onset of symptoms of dysphagia (difficulty swallowing) and percutaneous endoscopic gastrostomy tube placement should be dictated by the symptoms. As many patients with DMD survive into adulthood, there is a corresponding increase in the number of patients that require tube feeding. At the first sign of gastric symptoms, proton-pump inhibitors should accompany treatment with glucocorticosteroids to limit the risk of gastric ulcers117.
Urological management.
Men with DMD can develop signs and symptoms of bladder and urinary tract dysfunction, such as small capacity, hyper-reflexive bladder and detrusor sphincter dyssynergia (disturbance of muscle coordination) resulting in urinary urgency, retention and hesitancy of stream. These symptoms require urological management and may be palliated with a pharmacological approach such as oxybutynin, which can decrease urinary tract symptoms and may improve QOL143,144.
The dystrophin isoform Dp71 is highly expressed in the kidneys. Although the exact function of Dp71 is not fully known, its structure suggests a role in the protection of renal epithelial cells143–145. Renal dysfunction has been reported as a frequent complication in patients with advanced DMD, which is aggravated by inadequate fluid intake and the use of diuretics (the latter can be prescribed by cardiologists for the management of cardiac insufficiency associated with oedema); these patients should be referred to a renal specialist145.
Neurodevelopmental and neuropsychological management.
Specific attention should be paid to the management of neurodevelopmental and neuropsychological disorders in patients with DMD34,146. Patients with DMD have a higher incidence of cognitive impairment, attention deficit/hyperactivity disorder, autism spectrum disorder, anxiety and obsessive-compulsive disorder compared with the general population146. A referral for neuropsychological evaluations should be considered at diagnosis of DMD but is essential when concerns about developmental progress arise. Educational support may be required and will be driven by the results of neuropsychological tests, which are increasingly performed as part of routine care. The treatment of attention deficit/hyperactivity disorder or other behavioural or psychiatric problems is based on the symptoms and follows guidelines for the treatment of these disorders in the general population. Further research to improve the knowledge of these manifestations of DMD and to provide guidelines to identify, monitor and treat these manifestations is crucial as they have a major effect on functioning and QOL of both patients and their families.
Glucocorticosteroid treatment
The most recent guidelines strongly recommend the use of the glucocorticosteroids prednisone or deflazacort in boys with DMD when their motor development stops or starts to decline and to continue treatment throughout life117. The age of initiating steroids varies between patients but should not be before the patient reaches 2 years of age and is generally around 4–5 years of age. There is no clear indication on which glucocorticosteroid should be used as both have evidence of improving strength and motor function and can delay loss of ambulation and pulmonary function, reducing the need for scoliosis surgery and delaying the onset of cardiomyopathy. Following earlier evidence that prednisone was more often associated with increased weight gain and deflazacort with cataracts147, recent studies have reported a possible better role of daily deflazacort compared with daily prednisolone in delaying loss of ambulation and in increasing survival148,149. This aspect, however, is still controversial150 as a similar delay in loss of ambulation has also been reported in a cohort treated with daily prednisolone in another study151. The delay in loss of ambulation varies according to the treatment regimen; the average delay in those patients taking daily steroids is 2 years, whereas the delay is 1 year in those taking an on/off regimen152. There is no consensus on regimen, as daily treatment is associated with more adverse effects and more recent studies have suggested the possibility of a twice-weekly regimen153. An ongoing study, the FOR DMD study (NCT01603407), is assessing the long-term efficacy and safety of prednisone and deflazacort at different regimens and will hopefully help to address some of these issues.
The exact mechanism by which glucocorticosteroids delay disease progression in DMD are not fully elucidated, as glucocorticoid receptor activation has pleiotropic effects. However, it has been suggested that glucocorticosteroids decrease inflammation and increase total muscle mass and strength in patients with DMD through the stimulation of insulin-like growth factors, decreased cytokine production, decreased lymphocyte reaction, enhanced myoblast proliferation and upregulation of synergistic molecules154 . Furthermore, glucocorticosteroids like prednisone and deflazacort also display mineralocorticoid activity (that is, they activate mineralocorticoid receptors in addition to the glucocorticoid receptors), which may play a role in a wide range of adverse effects associated with this treatment, including hypertension, fluid retention, weight gain and skin atrophy. Whether these adverse effects can be limited using a dissociative steroidal drug with some mineralocorticoid antagonistic activity (vamorolone) is being evaluated in a clinical trial for DMD155.
Clinical trials and approved therapies
Over the past two decades, several therapeutic approaches have focused on the different steps of DMD pathophysiology. These approaches can be broadly divided into those targeting the restoration of dystrophin production and those trying to reduce the secondary consequences of dystrophin deficiency. Several of these approaches are being evaluated in clinical trials and are not available in clinical settings (see Outlook). However, some of these approaches have received regulatory approval in the USA, Europe and Japan1.
Small molecules targeting nonsense mutations.
Two randomized, double-blind, placebo-controlled trials of ataluren156,157, an orally bioavailable small molecule that induces ribosomal read-through of nonsense mutations, failed to achieve the 48 weeks primary endpoint of improved distance walked in the 6-minute walk test (SMWT) compared with patients who received placebo treatment. However, these trials found a clear trend of therapeutic efficacy, specifically a 29-meter increase in the SMWT and an improvement in timed function tests in those who received ataluren compared with placebo, which, together with a favourable safety profile, led to conditional approval by the EMA158. Accordingly, this drug has been used in Europe for over 4 years. Results of the European Drug Registry (STRIDE) confirm the efficacy and safety profile of the pivotal studies159. Another confirmatory placebo-controlled study to assess the functional effects of ataluren is ongoing. This drug has not been approved by the FDA160.
Antisense oligonucleotides for out-of-frame deletions.
The exon-skipping approach is based on the finding that out-of-frame mutations result in severe DMD, whereas in-frame mutations result in milder BMD. The aim of this approach is to restore the reading frame of dystrophin transcripts so that partially functional, BMD-like dystrophins can be produced. Reading frame restoration is achieved with antisense oligonucleotides (ASOs), small pieces of modified RNA that specifically bind to a target exon during pre-mRNA splicing. This binding prevents the inclusion of the exon into mRNA. ASOs are very small (20–30 nucleotides) and can be systemically delivered in humans161.
The ASO approach is mutation specific as different exons need to be skipped depending on the size and location of the mutation. However, as most patients carry a deletion in hotspots, the skipping of certain exons is applicable to larger groups of patients. A proof-of-concept for ASO-mediated exon skipping and dystrophin restoration has been achieved in cell and animal models. However, due to ASO, transcript and protein turnover, repeated treatment is required. Clinical development is most advanced for ASOs targeting exons where the skipping applies to the largest groups, that is, exon 51 (14%), exon 45 (8%), exon 53 (8%) and exon 44 (6%)8.
ASO-mediated exon skipping uses weekly systemic administration of ASO in boys with DMD who harbour eligible out-of-frame deletions to manipulate pre-mRNA by removing one exon adjacent to the deletion to generate an in-frame mutation. Three ASOs that are designed to skip exon 51 (eteplirsen)162,163 or exon 53 (golodirsen164 and viltolarsen165) have shown some evidence of induced dystrophin restoration in small cohorts of patients and have been granted conditional approval by the FDA (eteplirsen, golodirsen and viltolarsen) and the Japanese Ministry of Health, Welfare and Labour (viltolarsen). Confirmatory studies to assess the clinical benefit are under way for exon 51 and 53 skipping (NCT03218995, NCT03532542, NCT03985878, NCT03992430, NCT04060199, NCT04179409 and NCT04337112) as well as for exon 45 skipping (NCT03532542 and NCT04179409).
Quality of life
DMD is a progressive, disabling and life-limiting disorder that will affect the lives of patients and their families. Some QOL studies in patients with DMD have reported a reduced QOL whereas others have found no differences compared with healthy individuals; these differences relate to the instruments used to assess QOL and the fact that health-related QOL and QOL are used interchangeably166.
Reduced physical ability and a greater disease severity are generally assumed to be the main factors determining impaired QOL in individuals with a neuromuscular disorder and this association has been confirmed in some studies that have found a reduced health-related QOL in patients with DMD compared with published normative data167–169. However, QOL is a broader, multidimensional concept relating to one’s fulfilment of individual needs, desires and expectations, encompassing more than mere functional abilities and physical health. Emotional well-being, self-assessed health, social life, family support, perceived control, recreational opportunities and sexual relations have the strongest correlation with life satisfaction170. In addition, some studies have found that impairment and disability do not significantly affect life satisfaction and QOL170,171. Indeed, some studies have found that long-term ventilated adults with DMD report only moderately affected QOL171,172. Despite the negative impact of muscle weakness, loss of function, and pain and fatigue, satisfaction with care and management, social interaction and support, adjusted expectations, and acceptance can help mitigate the negative effects of DMD on QOL173,174. Indeed, patients with DMD adapt to their disabilities and develop new goals, values and expectations175.
Although children and young adults with DMD rate their life as satisfactory, parents and care givers tend to underestimate the QOL of the patient with DMD using their own values176,177. This finding raises concerns about the effect of a subjective assessment of QOL by health-care providers, which could interfere with the decision-making process for life-sustaining interventions in addition to decision-making for care and management in acute situations. This concern has been highlighted recently in relation to the COVID-19 pandemic, where triages in countries experiencing a heavy strain on their health-care system would disadvantage patients with neuromuscular disorders in later disease stages in the access to an intensive care unit178. This underscores the need for a regular assessment of perceived QOL and advance care planning.
A diagnosis of DMD in their children will profoundly affect the life of parents. Feelings of isolation, depression, stress, anxiety, anger and exhaustion are frequently reported by parents of children with DMD179–181. In addition, practical issues, financial difficulties, physical exhaustion and sleep deprivation add to the grief and worries for their child and may put an additional strain on their spousal relationship. Furthermore, mothers who are carriers may have feelings of guilt for passing on the disorder to their child182.
Acceptance and effective coping skills are the most important determinants for QOL175. Improving access to external resources, such as patient or caregiver support groups, and to a multidisciplinary care team with a comprehensive approach of all aspects of the condition as well as participation in social and leisure activities could all support the individual and their family in developing appropriate coping mechanisms.
Outlook
Many therapeutic approaches for DMD are in development and can be split into approaches aiming to restore dystrophin and approaches targeting the downstream effects of loss of dystrophin to preserve muscle tissue for as long as possible. The therapeutic approaches for DMD do not restore muscle tissue and function that is lost; therefore, it is important to treat patients as early as possible. Furthermore, the efficacy of dystrophin-restoring therapies will rely on the quality of muscle, as only muscle will express dystrophin (transcript targeting) or benefit from dystrophin expression (genome editing and gene delivery). It is likely that a combination of approaches will be used in the future to restore dystrophin and maintain muscle quality and to slow down disease progression as much as possible.
Stem cell transplantation
Of the dystrophin restoring strategies, stem cell transplantation has the longest history. The rationale of stem cell transplantation for DMD is appealing as transplanted cells will contain a functional copy of DMD and will contribute to muscle repair upon transplantation. However, in practice, the abundance of muscle (>30% of body mass) and the number of individual skeletal muscles (>700) pose severe challenges to the development of an efficient stem cell therapy approach.
Stem cell transplantation can be either allogenic (which requires immunosuppression in the recipient) or re-transplantation of corrected patient-derived cells. In addition, several types of stem cells can be used for transplantation, including satellite cells (myoblasts), bone marrow-derived cells, pericytes and mesenchymal stem cells. Satellite cells (myoblasts) are muscle stem cells that are quiescent and reside underneath the basal lamina (the layer of connective tissue that surrounds each muscle fibre). These cells are activated upon muscle damage to proliferate, self-renew and repair. Bone marrow-derived cells, pericytes and mesenchymal stem cells have the capacity to enter muscle and contribute to myogenesis and can also be used for transplantation. Of note, all these cell types have only a limited capacity to be expanded in vitro. Induced pluripotent stem cells differentiated into myogenic lineages offer another option that does not face this challenge; however, this approach has a risk for unlimited growth and teratoma formation.
For each type of cell used, delivery is not optimal, as myoblasts do not exit the blood vessels following delivery and, although the other stem cells can enter the muscle, only a very small percentage of transplanted stem cells actually do so. Systemic trials have failed to induce significant dystrophin restoration. Some trials using local high-density myoblast injections found some dystrophin restoration around the needle tracks183,184; however, this approach is only feasible for small, superficial muscles.
As an alternative approach, stem cell transplantation has also been used to improve muscle quality. The rationale is that the transplanted cells produce factors that have a positive effect on skeletal muscle repair and heart pathology. This effect is transient as the transplanted cells will perish. In a trial using cardiosphere-derived cells, treatment is repeated every 3 months. Preliminary results from a phase I/II clinical trial showed treatment was well tolerated and were suggestive of a positive effect on upper limb and heart function185.
Gene therapy
Gene therapy for DMD aims to restore the missing dystrophin by providing a functional copy of DMD or by repairing DMD. Gene addition therapy uses viral vectors to deliver a cDNA copy of functional DMD to affected tissues. Although most viruses do not have a natural tropism for skeletal muscles and heart, adeno-associated viruses (AAVs) are an exception and can infect these tissues very efficiently. However, AAV only has a limited carrying capacity of ~4.7 kb, whereas the Dp427 muscle isoform of dystrophin is encoded by an ~11.4 kb cDNA. As internally deleted dystrophins can be partly functional (as evidenced in patients with BMD), ‘mini-dystrophin’ and ‘micro-dystrophin’ constructs have been generated. The micro-dystrophin constructs lack all but the most crucial domains (the N-terminal ABD, 4–5 spectrin-like repeats, 2–3 hinge regions and the CR domain). The cDNA for these micro-dystrophins fits in AAV vectors and the expression of these micro-dystrophin constructs improves pathology in mouse and dog models of DMD65.
Clinical trials evaluating the systemic delivery of different versions of micro-dystrophin cDNA using different serotypes of AAV vectors at high doses (1–3 × 1014 vectors/kg) are ongoing186. Preliminary results from these studies have confirmed micro-dystrophin expression in the majority of muscle fibres (>80%) in muscle biopsies at levels of over 60%120,187, although it is not yet clear whether treatment will ameliorate disease progression. However, severe adverse events have been observed in a subset of patients, including transient renal failure (likely due to an innate immune response) and transient elevation of liver enzymes (likely due to a cellular immune response to the vector)65,187. Notably, all treated patients included in these studies were pre-screened to rule out a pre-existing humoral immune response to the viral capsid used and all patients were pre-treated with high doses of steroids to suppress an immune response to the virus. Additionally, AAV administration induces an anti-AAV capsid neutralizing antibody, which precludes retreatment. Furthermore, some patients already have pre-existing AAV neutralizing antibodies, which excludes them from receiving this treatment. Strategies to counter this antibody response, such as plasmapheresis, alternative AAV serotype and immune-modulating drugs, are being explored.
Although the preliminary results are promising, AAVs rarely integrate into the host genome and it is possible that, with muscle turnover, the micro-dystrophin transgene will disappear with time. Whether this happens and how long it takes remain unknown. Furthermore, how functional the micro-dystrophins are in humans and their corresponding therapeutic effects are also unknown.
With the development of genome editing systems, such as CRISPR/Cas9, it is now possible to make targeted modifications in the genome. CRISPR/Cas9 uses guide RNAs that hybridize to specific complementary regions in DNA and guide the Cas9 enzyme to induce a double stranded break at the targeted locations188. These breaks are then repaired by the DNA repair systems. In dividing cells, error-free repair can occur via homologous recombination, thus providing a possibility to correct mutations. By contrast, in non-dividing cells, the error-prone, non-homologous end-joining system is used. As the most affected tissues in DMD are post-mitotic, the focus on genome editing therapy for DMD has been on using non-homologous end joining. Inspired by the exon-skipping approach, guide RNAs are designed such that they restore the reading frame. This can be achieved in multiple ways through, for example, deleting an exon, abolishing a splice site such that the exon is not included in the mRNA or reframing an exon (FIG. 5).
Fig. 5 |. Dystrophin-restoring approaches.
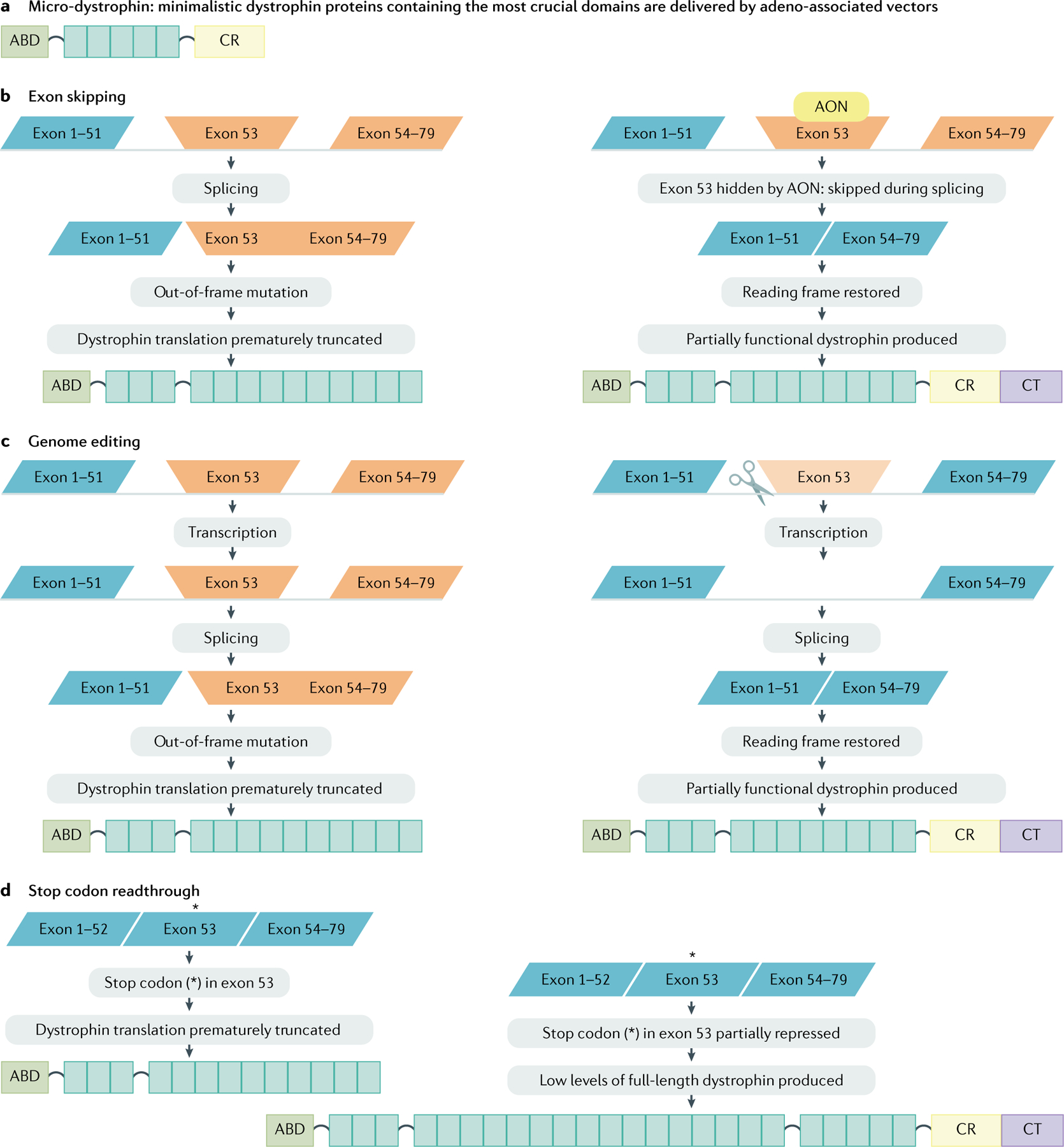
a | Micro-dystrophin gene therapy. To accommodate the limited capacity of adeno-associated viral vectors, cDNA constructs encoding only the most crucial parts of dystrophin have been generated to enable the expression of micro-dystrophins. b | Exon skipping. Antisense oligonucleotides (AONs) are used to target a specific exon during pre-mRNA splicing. The AON hides the targeted exon from the splicing machinery causing it to be skipped, thus restoring the reading frame, allowing the production of a Becker-like dystrophin. c | Genome editing. Guide RNAs are used to direct Cas9 to target regions in the DNA to delete an exon from the gene, thereby restoring the reading frame of mRNA produced from this gene, allowing the production of Becker-like dystrophin. d | Stop codon readthrough. For patients with nonsense mutations (indicated by an asterisk), compounds can suppress the premature stop codon use, facilitating instead the inclusion of an amino acid. Thus, a full-length dystrophin can be produced. ABD, actin-binding domain; CR, cysteine-rich domain; CT, C-terminal domain.
Proof-of-concept of this approach has been achieved, confirming that genome editing can restore dystrophin production in DMD cell and animal models189,190. Some studies further demonstrated efficient editing of muscle stem cells in the mouse model191,192. Genome editing is a mutation-specific approach because different exons need to be deleted for different mutations. Efforts to make this approach less mutation specific are being undertaken such as by deleting exons 45–55 (REF.193) or exons 47–58 (REF.194). However, all the genome-editing work is still in a preclinical phase and multiple challenges have to be overcome to apply this approach systemically in humans, including optimal delivery of the genome-editing components, the risk of off-target editing and the risk of an immune response against Cas9 (REF.195). Alternatively, patient-derived cells could be edited ex vivo and then transplanted back. However, this route is suboptimal owing to the inability to efficiently deliver cells to the musculature.
Rather than delivering a functional copy of the (micro-)dystrophin gene, efforts are also ongoing to deliver cDNA of genes that encode proteins that can improve muscle mass (such as follistatin196) or target disease mechanisms (such as SERCA2a)88. This approach has the advantage that it applies to all muscular dystrophies. However, it will likely have a more limited impact on disease progression as the primary cause of the disease is not addressed.
Exon skipping
As previously mentioned, three ASOs are now approved for DMD, although the approval was based on low levels of dystrophin restoration rather than on a confirmation of functional effects. Drisapersen, another exon 51-skipping ASO with a 2’-O-methyl phosphorothioate modification, has been evaluated in placebo-controlled trials; however, the FDA did not approve this drug owing to a lack of convincing evidence of functional efficacy and due to the occurrence of injection site reactions, proteinuria and, in a subset of patients, thrombocytopenia197.
ASOs are mutation-specific approaches and different dystrophin proteins will be formed after skipping different exons for different mutations. In fact, skipping the same exon can lead to the formation of different dystrophins; for example, the deletion of exons 47–50, 48–50, 49–50 and 52 can be restored by exon 51 skipping. However, the dystrophins produced after exon 51 skipping will vary. Similarly, a deletion of exon 52 can be restored by either exon 51 or exon 53 skipping, resulting in two different dystrophins. Although in-frame dystrophins are partially functional if they have an ABD and a CR-rich domain, their functionality depends on the connections between spectrin-like repeats198; for example, the connection between spectrin-like repeats can be close to normal, normal or disjointed and some disjointed connections impede the binding of proteins such as nNOS199,200.
Techniques to improve ASO efficiency and uptake by skeletal muscle and heart are being explored such as different chemical modifications, arginine-rich peptide conjugates or muscle-homing conjugates. In parallel, multiexon skipping is being explored as an option to treat larger groups of patients. Here, the focus is on exon 45–55 skipping as patients with BMD and deletion of exons 45–55 generally have a very mild phenotype20. In addition, the delivery of antisense genes using AAV vectors is being explored. In these studies, the U7 small nuclear ribonucleoprotein (snRNP) gene is modified such that the RNA sequence that normally binds to histone RNA binds to target exons. This approach is a combination of gene therapy and exon skipping and has shown promising results in animal models201. A clinical trial to assess exon 2 skipping is ongoing in patients with DMD with exon 2 duplications based on promising preclinical results202.
Nonsense mutation readthrough
In addition to ataluren, which has been approved by the EMA, gentamicin has stop codon readthrough capacity and has been evaluated as a treatment for DMD203. However, due to its safety profile, long-term treatment is not an option. Various other chemical compounds have been screened to identify a readthrough drug with a better safety profile.
Utrophin upregulation
Utrophin — a ubiquitous protein that is enriched at neuromuscular junctions — shares a high level of homology with dystrophin and can recruit most of the proteins involved in the DAPC. In developing muscles, utrophin is expressed in the same regions of muscle fibres as dystrophin but utrophin production is downregulated in muscle when dystrophin expression commences. Utrophin is often upregulated in patients with DMD and animal models that lack dystrophin and further upregulation of utrophin in mouse models ameliorated the pathology of DMD204,205. High-throughput screening identified ezutromid as a potential compound to upregulate utrophin expression in muscle; however, this compound showed very limited bioavailability and no therapeutic effect was observed in a placebo-controlled trial in patients with DMD206. Based on these data, the clinical development of ezutromid has been abolished. However, ezutromid was found to upregulate utrophin by acting as an aryl hydrocarbon receptor antagonist and other aryl hydrocarbon receptor antagonists were also found to upregulate utrophin207; accordingly, other compounds may be able to upregulate utrophin.
Approaches to preserve muscle
Therapeutic approaches that target the secondary consequences of dystrophin loss are under development for DMD. For example, as the chronic use of glucocorticosteroids is accompanied by many adverse effects, alternative steroids that have a better safety profile are being explored such as vamorolone208,209. Vamorolone seems to be well tolerated in patients and adverse effects are seen at doses that exceed those used for prednisone and deflazacort210. This compound is now being tested in placebo-controlled trials. Edasalonexent (an NF-κB inhibitor and, therefore, a potent inhibitor of inflammation) is also in clinical development for DMD. However, although it was well tolerated211, no efficacy was observed in a double-blind, randomized placebo-controlled trial and development of this drug was stopped.
Compounds to improve muscle mass have also been evaluated for the treatment of DMD. One such example is myostatin (a growth factor of the TGFβ family that inhibits muscle growth); as animals and humans lacking myostatin are extremely muscular212,213, the inhibition of myostatin was explored as a way to compensate for the loss of muscle tissue in patients with DMD. Soluble myostatin receptors (ActIIB) resulted in improved muscle mass in healthy volunteers; however, longer-term treatment in patients with DMD was not feasible owing to adverse effects, including spontaneous bleeding214, which was likely due to interference with activin signalling. A more specific approach involved myostatin antibodies; however, results from two large placebo-controlled trials with two different myostatin antibodies215 did not find a therapeutic effect. In hindsight, this finding is not unexpected as myostatin levels are very low in patients with DMD, so further inhibition may not be possible.
Approaches to improve mitochondrial function in DMD have also been studied. These compounds are expected to reduce oxidative stress and fibrosis. One compound targeting mitochondrial function, idebenone, demonstrated a reduced decline in respiratory function in patients with DMD not using corticosteroids in a placebo-controlled trial216; however, studies in patients using corticosteroids were prematurely terminated as no therapeutic effects were found in the interim analysis (NCT02814019 and NCT03603288). In addition, histone deacetylase inhibition has been explored as a method to improve regeneration and reduce inflammation in patients with DMD. Givinostat was tested in a small open-label study, where treatment reduced fibrosis in all patients based on muscle biopsies taken before and after the clinical trial217. A phase III placebo-controlled trial is ongoing to assess the functional effects of givinostat treatment in patients with DMD (NCT02851797).
Acknowledgements
The laboratory of A.A.-R. is part of the Duchenne Center Netherlands. Duchenne muscular dystrophy (DMD) research in the laboratory of A.A.-R. is currently supported by Duchenne Parent Project, Duchenne UK, Prinses Beatrix Spierfonds, Spieren voor Spieren and the EU Horizon 2020 project BIND. DMD research in the laboratory of D.D. is currently supported by the National Institutes of Health (NS90634, AR70517, AR69085), Jackson Freel DMD Research Fund, Jesse’s Journey: The Foundation for Gene and Cell Therapy, Parent Project Muscular Dystrophy (USA), Hope for Javier, Michael’s Cause, Ryan’s Quest, Pietro’s Fight, Duchenne UK, Ryan’s Rally, Team Joseph, Charley’s Fund, Cure Duchenne, and Jett Foundation. DMD research in the laboratory of N.G. is currently supported by Duchenne Parent Project, Rondou Fonds and Kan-Go! Fonds. We thank NIH NeuroBioBank for providing patient muscle tissue for histological studies. We thank N. Wasala (University of Missouri) and K. Zhang (University of Missouri) for their help in the preparation of Fig. 2.
Footnotes
Competing interests
D.D. is a member of the scientific advisory board for Solid Biosciences and an equity holder of Solid Biosciences. D.D. is an inventor on patents licensed to various companies. D.D. has served as an ad hoc consultant for 4DMT, Decibel Therapeutics, Evox, Primary Insight, Vida Ventures, Global Guidepoint and GLG consultancy in the past 3 years. The lab of D.D. has received research support from Solid Biosciences and Edgewise Therapeutics in the past 3 years. N.G. has received compensation as member of scientific boards or as speaker at symposia from Sarepta, Pfizer, Italpharmaco and PTC Therapeutics. S.T. has patents on sequences for exon skip by antisense nucleic acids as a member of NCNP together with Nippon Shinyaku. As principal inventor of these patents, S.T. is entitled to a share of royalties. S.T. discloses being an ad hoc consultant for Ono Pharmaceutical, Daiichisankyo, Asahikasei Pharma, Teijin Pharma, AGADA Biosciences, and Wave and being a member of the scientific advisory boards of Nippon Shinyaku, Taiho Pharma and Sarepta therapeutics. S.T. received speaker honoraria from Japan Health Science Foundation and Astellas Pharma and has also received research supports from Taiho Pharma, Daiichisankyo, Nippon Shinyaku, Takeda Pharmaceutical and the Noguchi Institute in the past 3 years. E.M. is a Principal Investigator in clinical trials and advisory board member for Sarepta, Santhera, PTC, Roche, Italfarmaco, NS Pharma and Pfizer. A.A.-R. discloses being employed by Leiden University Medical Center (LUMC), which has patents on exon-skipping technology, some of which has been licensed to BioMarin and subsequently sublicensed to Sarepta. As co-inventor of some of these patents, A.A.-R. is entitled to a share of royalties. A.A.-R. further discloses being an ad hoc consultant for PTC Therapeutics, Sarepta Therapeutics, CRISPR Therapeutics, Summit PLC, Alpha Anomeric, BioMarin Pharmaceuticals Inc., Eisai, Astra Zeneca, Santhera, Audentes, Global Guidepoint and GLG consultancy, Grunenthal, Wave, and BioClinica, having been a member of the Duchenne Network Steering Committee (BioMarin), and being a member of the scientific advisory boards of ProQR, Hybridize Therapeutics, Silence Therapeutics, Sarepta Therapeutics and Philae Pharmaceuticals. Remuneration for these activities is paid to LUMC. LUMC also received speaker honoraria from PTC Therapeutics and BioMarin Pharmaceuticals and funding for contract research from Italpharmaco and Alpha Anomeric. Project funding is received from Sarepta Therapeutics.
Peer review information
Nature Reviews Disease Primers thanks J. Novak, who co-reviewed with T. Partridge, P. Clemens, H. Gordish-Dressman, M. Ryan, J. Tremblay, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
References
- 1. Mercuri E, Bonnemann CG & Muntoni F Muscular dystrophies. Lancet 394, 2025–2038 (2019). Comprehensive overview of the clinical and genetic aspects of muscular dystrophies.
- 2.Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, Van Ommen GJB & Den Dunnen JT Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 34, 135–144 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Monaco AP, Bertelson CJ, Liechti-Gallati S, Moser H & Kunkel LM An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics 2, 90–95 (1988). [DOI] [PubMed] [Google Scholar]
- 4.García-Rodríguez R et al. Premature termination codons in the DMD gene cause reduced local mRNA synthesis. Proc. Natl Acad. Sci. USA 117, 16456–16464 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mendell JR et al. Evidence-based path to newborn screening for Duchenne muscular dystrophy. Ann. Neurol 71, 304–313 (2012). [DOI] [PubMed] [Google Scholar]
- 6.Ryder S et al. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: an evidence review. Orphanet J. Rare Dis 12, 79 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mah JK et al. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord 24, 482–491 (2014). Systematic study into the global epidemiology of DMD and BMD.
- 8.Bladen CL et al. The TREAT-NMD Duchenne muscular dystrophy registries: conception, design, and utilization by industry and academia. Hum. Mutat 34, 1449–1457 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Kieny P et al. Evolution of life expectancy of patients with Duchenne muscular dystrophy at AFM Yolaine de Kepper centre between 1981 and 2011. Ann. Phys. Rehabil. Med 56, 443–454 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Ferrier P, Bamatter F & Klein D Muscular dystrophy (Duchenne) in a girl with Turner’s syndrome. J. Med. Genet 2, 38–46 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chelly J et al. De novo DNA microdeletion in a girl with Turner syndrome and Duchenne muscular dystrophy. Hum. Genet 74, 193–196 (1986). [DOI] [PubMed] [Google Scholar]
- 12.Satre V, Monnier N, Devillard F, Amblard F & Lunardi J Prenatal diagnosis of DMD in a female foetus affected by Turner syndrome. Prenat. Diagn 24, 913–917 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Takeshita E et al. Duchenne muscular dystrophy in a female with compound heterozygous contiguous exon deletions. Neuromuscul. Disord 27, 569–573 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Ishizaki M, Kobayashi M, Adachi K, Matsumura T & Kimura E Female dystrophinopathy: review of current literature. Neuromuscul. Disord 28, 572–581 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Holloway SM et al. Life expectancy and death from cardiomyopathy amongst carriers of Duchenne and Becker muscular dystrophy in Scotland. Heart 94, 633–636 (2008). [DOI] [PubMed] [Google Scholar]
- 16. Bladen CL et al. The TREAT-NMD DMD global database: analysis of more than 7000 Duchenne muscular dystrophy mutations. Hum. Mutat 36, 395–402 (2015). Systematic analysis of different mutation types and locations of mutations in patients around the world.
- 17.Magri F et al. Genotype and phenotype characterization in a large dystrophinopathic cohort with extended follow-up. J. Neurol 258, 1610–1623 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Garcia S et al. Identification of de novo mutations of Duchenne/Becker muscular dystrophies in southern Spain. Int. J. Med. Sci 11, 988–993 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kesari A et al. Integrated DNA, cDNA, and protein studies in Becker muscular dystrophy show high exception to the reading frame rule. Hum. Mutat 29, 728–737 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Nakamura A et al. Comparison of the phenotypes of patients harboring in-frame deletions starting at exon 45 in the Duchenne muscular dystrophy gene indicates potential for the development of exon skipping therapy. J. Hum. Genet 62, 459–463 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Nakamura A et al. Deletion of exons 3–9 encompassing a mutational hot spot in the DMD gene presents an asymptomatic phenotype, indicating a target region for multiexon skipping therapy. J. Hum. Genet 61, 663–667 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Nevin NC, Hughes AE, Calwell M & Lim JH Duchenne muscular dystrophy in a female with a translocation involving Xp21. J. Med. Genet 23, 171–173 (1986). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen WJ et al. Molecular analysis of the dystrophin gene in 407 Chinese patients with Duchenne/Becker muscular dystrophy by the combination of multiplex ligation-dependent probe amplification and Sanger sequencing. Clin. Chim. Acta 423, 35–38 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Yu H, Chen YC, Liu GL & Wu ZY A de novo mutation in dystrophin causing muscular dystrophy in a female patient. Chin. Med. J 130, 2273–2278 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caskey CT, Nussbaum RL, Cohan LC & Pollack L Sporadic occurrence of Duchenne muscular dystrophy: evidence for new mutation. Clin. Genet 18, 329–341 (1980). [DOI] [PubMed] [Google Scholar]
- 26.Haldane JB The rate of spontaneous mutation of a human gene. 1935. J. Genet 83, 235–244 (2004). [DOI] [PubMed] [Google Scholar]
- 27.Helderman-van den Enden AT et al. Recurrence risk due to germ line mosaicism: Duchenne and Becker muscular dystrophy. Clin. Genet 75, 465–472 (2009). [DOI] [PubMed] [Google Scholar]
- 28.Dreyfus JC, Schapira G & Schapira F Serum enzymes in the physiopathology of muscle. Ann. N. Y. Acad. Sci 75, 235–249 (1958). [DOI] [PubMed] [Google Scholar]
- 29.Moser H Duchenne muscular dystrophy: pathogenetic aspects and genetic prevention. Hum. Genet 66, 17–40 (1984). [DOI] [PubMed] [Google Scholar]
- 30.Koenig M et al. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50, 509–517 (1987). [DOI] [PubMed] [Google Scholar]
- 31. Hoffman EP, Brown RH Jr. & Kunkel LM Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51, 919–928 (1987). This paper reports the discovery of the dystrophin protein and its absence in DMD.
- 32.Chelly J et al. Dystrophin gene transcribed from different promoters in neuronal and glial cells. Nature 344, 64–65 (1990). [DOI] [PubMed] [Google Scholar]
- 33.Górecki DC et al. Expression of four alternative dystrophin transcripts in brain regions regulated by different promoters. Hum. Mol. Genet 1, 505–510 (1992). [DOI] [PubMed] [Google Scholar]
- 34.Doorenweerd N et al. Timing and localization of human dystrophin isoform expression provide insights into the cognitive phenotype of Duchenne muscular dystrophy. Sci. Rep 7, 12575 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Souza VN et al. A novel dystrophin isoform is required for normal retinal electrophysiology. Hum. Mol. Genet 4, 837–842 (1995). [DOI] [PubMed] [Google Scholar]
- 36.Lidov HG, Selig S & Kunkel LM Dp140: a novel 140 kDa CNS transcript from the dystrophin locus. Hum. Mol. Genet 4, 329–335 (1995). [DOI] [PubMed] [Google Scholar]
- 37.Byers TJ, Lidov HG & Kunkel LM An alternative dystrophin transcript specific to peripheral nerve. Nat. Genet 4, 77–81 (1993). [DOI] [PubMed] [Google Scholar]
- 38.Hugnot JP et al. Distal transcript of the dystrophin gene initiated from an alternative first exon and encoding a 75-kDa protein widely distributed in nonmuscle tissues. Proc. Natl Acad. Sci. USA 89, 7506–7510 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinsley JM, Blake DJ & Davies KE Apo-dystrophin-3: a 2.2kb transcript from the DMD locus encoding the dystrophin glycoprotein binding site. Hum. Mol. Genet 2, 521–524 (1993). [DOI] [PubMed] [Google Scholar]
- 40.Gao QQ & McNally EM The dystrophin complex: structure, function, and implications for therapy. Compr. Physiol 5, 1223–1239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ervasti JM & Sonnemann KJ Biology of the striated muscle dystrophin-glycoprotein complex. Int. Rev. Cytol 265, 191–225 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Zhao J et al. Dystrophin contains multiple independent membrane-binding domains. Hum. Mol. Genet 25, 3647–3653 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amann KJ, Renley BA & Ervasti JM A cluster of basic repeats in the dystrophin rod domain binds F-actin through an electrostatic interaction. J. Biol. Chem 273, 28419–28423 (1998). [DOI] [PubMed] [Google Scholar]
- 44.Prins KW et al. Dystrophin is a microtubule-associated protein. J. Cell Biol 186, 363–369 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nelson DM et al. Rapid, redox-mediated mechanical susceptibility of the cortical microtubule lattice in skeletal muscle. Redox Biol 37, 101730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stone MR, O’Neill A, Catino D & Bloch RJ Specific interaction of the actin-binding domain of dystrophin with intermediate filaments containing keratin 19. Mol. Biol. Cell 16, 4280–4293 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhosle RC, Michele DE, Campbell KP, Li Z & Robson RM Interactions of intermediate filament protein synemin with dystrophin and utrophin. Biochem. Biophys. Res. Commun 346, 768–777 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Huang X et al. Structure of a WW domain containing fragment of dystrophin in complex with beta-dystroglycan. Nat. Struct. Biol 7, 634–638 (2000). [DOI] [PubMed] [Google Scholar]
- 49.Ayalon G, Davis JQ, Scotland PB & Bennett V An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan. Cell 135, 1189–1200 (2008). [DOI] [PubMed] [Google Scholar]
- 50.Rezniczek GA et al. Plectin 1f scaffolding at the sarcolemma of dystrophic (mdx) muscle fibers through multiple interactions with beta-dystroglycan. J. Cell Biol 176, 965–977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yamashita K et al. The 8th and 9th tandem spectrin-like repeats of utrophin cooperatively form a functional unit to interact with polarity-regulating kinase PAR-1b. Biochem. Biophys. Res. Commun 391, 812–817 (2010). [DOI] [PubMed] [Google Scholar]
- 52. Lai Y et al. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest 119, 624–635 (2009). This paper showed, for the first time, the direct binding of dystrophin to nNOS.
- 53.Anderson JT, Rogers RP & Jarrett HW Ca2+-calmodulin binds to the carboxyl-terminal domain of dystrophin. J. Biol. Chem 271, 6605–6610 (1996). [DOI] [PubMed] [Google Scholar]
- 54.Reynolds JG, McCalmon SA, Donaghey JA & Naya FJ Deregulated protein kinase A signaling and myospryn expression in muscular dystrophy. J. Biol. Chem 283, 8070–8074 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Constantin B Dystrophin complex functions as a scaffold for signalling proteins. Biochim. Biophys. Acta 1838, 635–642 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Allen DG, Whitehead NP & Froehner SC Absence of dystrophin disrupts skeletal muscle signaling: roles of Ca2+, reactive oxygen species, and nitric oxide in the development of muscular dystrophy. Physiol. Rev 96, 253–305 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai Y, Zhao J, Yue Y & Duan D α2 and α3 helices of dystrophin R16 and R17 frame a microdomain in the alpha1 helix of dystrophin R17 for neuronal NOS binding. Proc. Natl Acad. Sci. USA 110, 525–530 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang NC et al. The dystrophin glycoprotein complex regulates the epigenetic activation of muscle stem cell commitment. Cell Stem Cell 22, 755–768. e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lumeng C et al. Interactions between β2-syntrophin and a family of microtubule-associated serine/threonine kinases. Nat. Neurosci 2, 611–617 (1999). [DOI] [PubMed] [Google Scholar]
- 60.Sarparanta J Biology of myospryn: what’s known? J. Muscle Res. Cell Motil 29, 177–180 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Sugita S et al. A stoichiometric complex of neurexins and dystroglycan in brain. J. Cell Biol 154, 435–445 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mokri B & Engel AG Duchenne dystrophy: electron microscopic findings pointing to a basic or early abnormality in the plasma membrane of the muscle fiber. Neurology 25, 1111–1120 (1975). [DOI] [PubMed] [Google Scholar]
- 63.Aartsma-Rus A & van Putten M Assessing functional performance in the mdx mouse model. J. Vis. Exp 85, 51303 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stedman HH et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature 352, 536–539 (1991). [DOI] [PubMed] [Google Scholar]
- 65. Duan D Systemic AAV micro-dystrophin gene therapy for Duchenne muscular dystrophy. Mol. Ther 26, 2337–2356 (2018). Comprehensive overview of microdystrophin gene therapy development, its opportunities and challenges.
- 66.Bradley WG, Hudgson P, Larson PF, Papapetropoulos TA & Jenkison M Structural changes in the early stages of Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 35, 451–455 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pearson CM Histopathological features of muscle in the preclinical stages of muscular dystrophy. Brain 85, 109–120 (1962). [DOI] [PubMed] [Google Scholar]
- 68.Brenman JE, Chao DS, Xia H, Aldape K & Bredt DS Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell 82, 743–752 (1995). [DOI] [PubMed] [Google Scholar]
- 69.Sander M et al. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl Acad. Sci. USA 97, 13818–13823 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patel A et al. Dystrophin R16/17-syntrophin PDZ fusion protein restores sarcolemmal nNOSmu. Skelet. Muscle 8, 36 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kodippili K et al. Dual AAV gene therapy for Duchenne muscular dystrophy with a 7-kb mini-dystrophin gene in the canine model. Hum. Gene Ther 29, 299–311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prosser BL, Ward CW & Lederer WJ X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445 (2011). [DOI] [PubMed] [Google Scholar]
- 73.Khairallah RJ et al. Microtubules underlie dysfunction in Duchenne muscular dystrophy. Sci. Signal 5, ra56 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li D, Yue Y, Lai Y, Hakim CH & Duan D Nitrosative stress elicited by nNOSmu delocalization inhibits muscle force in dystrophin-null mice. J. Pathol 223, 88–98 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim JH, Kwak HB, Thompson LV & Lawler JM Contribution of oxidative stress to pathology in diaphragm and limb muscles with Duchenne muscular dystrophy. J. Muscle Res. Cell Motil 34, 1–13 (2013). [DOI] [PubMed] [Google Scholar]
- 76.Grounds MD et al. Biomarkers for Duchenne muscular dystrophy: myonecrosis, inflammation and oxidative stress. Dis. Models Mech 13, dmm.043638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rando TA Role of nitric oxide in the pathogenesis of muscular dystrophies: a “two hit” hypothesis of the cause of muscle necrosis. Microsc. Res. Tech 55, 223–235 (2001). [DOI] [PubMed] [Google Scholar]
- 78.Dudley RW et al. Dynamic responses of the glutathione system to acute oxidative stress in dystrophic mouse (mdx) muscles. Am. J. Physiol. Regul. Integr. Comp. Physiol 291, R704–710 (2006). [DOI] [PubMed] [Google Scholar]
- 79.Petrillo S et al. Oxidative stress in Duchenne muscular dystrophy: focus on the NRF2 redox pathway. Hum. Mol. Genet 26, 2781–2790 (2017). [DOI] [PubMed] [Google Scholar]
- 80.Turner PR, Westwood T, Regen CM & Steinhardt RA Increased protein degradation results from elevated free calcium levels found in muscle from mdx mice. Nature 335, 735–738 (1988). [DOI] [PubMed] [Google Scholar]
- 81.Millay DP et al. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med 14, 442–447 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Phillips MF & Quinlivan R Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst. Rev 4, CD004571 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kyrychenko S et al. Hierarchical accumulation of RyR post-translational modifications drives disease progression in dystrophic cardiomyopathy. Cardiovasc. Res 97, 666–675 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bellinger AM et al. Hypernitrosylated ryanodine receptor calcium release channels are leaky in dystrophic muscle. Nat. Med 15, 325–330 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kushnir A, Wajsberg B & Marks AR Ryanodine receptor dysfunction in human disorders. Biochim. Biophys. Acta Mol. Cell Res 1865, 1687–1697 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Capogrosso RF et al. Ryanodine channel complex stabilizer compound S48168/ARM210 as a disease modifier in dystrophin-deficient mdx mice: proof-of-concept study and independent validation of efficacy. FASEB J 32, 1025–1043 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Voit A et al. Reducing sarcolipin expression mitigates Duchenne muscular dystrophy and associated cardiomyopathy in mice. Nat. Commun 8, 1068 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wasala NB et al. Single SERCA2a therapy ameliorated dilated cardiomyopathy for 18 months in a mouse model of Duchenne muscular dystrophy. Mol. Ther 28, 845–854 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dumont NA et al. Dystrophin expression in muscle stem cells regulates their polarity and asymmetric division. Nat. Med 21, 1455–1463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bello L & Pegoraro E The “usual suspects”: genes for inflammation, fibrosis, regeneration, and muscle strength modify Duchenne muscular dystrophy. J. Clin. Med 8, 649 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cappellari O, Mantuano P & De Luca A “The Social Network” and muscular dystrophies: the lesson learnt about the niche environment as a target for therapeutic strategies. Cells 9, 1659 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Grounds MD Two-tiered hypotheses for Duchenne muscular dystrophy. Cell. Mol. Life Sci 65, 1621–1625 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sandri M, Coletto L, Grumati P & Bonaldo P Misregulation of autophagy and protein degradation systems in myopathies and muscular dystrophies. J. Cell Sci 126, 5325–5333 (2013). [DOI] [PubMed] [Google Scholar]
- 94.De Palma C et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis 3, e418 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pal R et al. Src-dependent impairment of autophagy by oxidative stress in a mouse model of Duchenne muscular dystrophy. Nat. Commun 5, 4425 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xiong Y, Zhou Y & Jarrett HW Dystrophin glycoprotein complex-associated Gbetagamma subunits activate phosphatidylinositol-3-kinase/Akt signaling in skeletal muscle in a laminin-dependent manner. J. Cell. Physiol 219, 402–414 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.De Palma C, Perrotta C, Pellegrino P, Clementi E & Cervia D Skeletal muscle homeostasis in Duchenne muscular dystrophy: modulating autophagy as a promising therapeutic strategy. Front. Aging Neurosci 6, 188 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Evans NP, Misyak SA, Robertson JL, Bassaganya-Riera J & Grange RW Immune-mediated mechanisms potentially regulate the disease time-course of Duchenne muscular dystrophy and provide targets for therapeutic intervention. PM R 1, 755–768 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tidball JG, Welc SS & Wehling-Henricks M Immunobiology of inherited muscular dystrophies. Compr. Physiol 8, 1313–1356 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rosenberg AS et al. Immune-mediated pathology in Duchenne muscular dystrophy. Sci. Transl. Med 7, 299rv294 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bello L et al. Functional changes in Becker muscular dystrophy: implications for clinical trials in dystrophinopathies. Sci. Rep 6, 32439 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wasala L et al. Cardiac specific expression of ∆H2-R15 mini-dystrophin normalized all ECG abnormalities and the end-diastolic volume in a 23-m-old mouse model of Duchenne dilated cardiomyopathy. Hum. Gene Ther 29, 737–748 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Vo AH & McNally EM Modifier genes and their effect on Duchenne muscular dystrophy. Curr. Opin. Neurol 28, 528–534 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ricotti V et al. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol 58, 77–84 (2016). [DOI] [PubMed] [Google Scholar]
- 105.Bello L et al. Association study of exon variants in the NF-κB and TGFβ pathways identifies CD40 as a modifier of Duchenne muscular dystrophy. Am. J. Hum. Genet 99, 1163–1171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Spitali P et al. TCTEX1D1 is a genetic modifier of disease progression in Duchenne muscular dystrophy. Eur. J. Hum. Genet 28, 815–825 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Klietsch R, Ervasti JM, Arnold W, Campbell KP & Jorgensen AO Dystrophin-glycoprotein complex and laminin colocalize to the sarcolemma and transverse tubules of cardiac muscle. Circ. Res 72, 349–360 (1993). [DOI] [PubMed] [Google Scholar]
- 108.Meng H, Leddy JJ, Frank J, Holland P & Tuana BS The association of cardiac dystrophin with myofibrils/Z-disc regions in cardiac muscle suggests a novel role in the contractile apparatus. J. Biol. Chem 271, 12364–12371 (1996). [DOI] [PubMed] [Google Scholar]
- 109.Johnson EK et al. Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS ONE 7, e43515 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Thangarajh M et al. Relationships between DMD mutations and neurodevelopment in dystrophinopathy. Neurology 93, e1597–e1604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Doorenweerd N et al. Reduced cerebral gray matter and altered white matter in boys with Duchenne muscular dystrophy. Ann. Neurol 76, 403–411 (2014). [DOI] [PubMed] [Google Scholar]
- 112.Pilgram GS, Potikanond S, Baines RA, Fradkin LG & Noordermeer JN The roles of the dystrophin-associated glycoprotein complex at the synapse. Mol. Neurobiol 41, 1–21 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huard J, Côté PY, Parent A, Bouchard JP & Tremblay JP Dystrophin-like immunoreactivity in monkey and human brain areas involved in learning and motor functions. Neurosci. Lett 141, 181–186 (1992). [DOI] [PubMed] [Google Scholar]
- 114.Chieffo D et al. Early neurodevelopmental findings predict school age cognitive abilities in Duchenne muscular dystrophy: a longitudinal study. PLoS ONE 10, e0133214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cohen EJ, Quarta E, Fulgenzi G & Minciacchi D Acetylcholine, GABA and neuronal networks: a working hypothesis for compensations in the dystrophic brain. Brain Res. Bull 110, 1–13 (2015). [DOI] [PubMed] [Google Scholar]
- 116. Doorenweerd N Combining genetics, neuropsychology and neuroimaging to improve understanding of brain involvement in Duchenne muscular dystrophy — a narrative review. Neuromuscul. Disord 30, 437–442 (2020). Comprehensive and clear review on brain involvement of dystrophin.
- 117. Birnkrant DJ et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol 17, 251–267 (2018). Part 1 of a three-part standard-of-care document for DMD.
- 118.Aartsma-Rus A et al. Evidence-based consensus and systematic review on reducing the time to diagnosis of Duchenne muscular dystrophy. J. Pediatr 204, 305–313.e314 (2019). [DOI] [PubMed] [Google Scholar]
- 119. Aartsma-Rus A, Ginjaar IB & Bushby K The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet 53, 145–151 (2016). Educational paper on how different mutations cause DMD and how they can be detected with diagnostic techniques.
- 120.Verhaart IEC & Aartsma-Rus A Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol 15, 373–386 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Janssen B, Hartmann C, Scholz V, Jauch A & Zschocke J MLPA analysis for the detection of deletions, duplications and complex rearrangements in the dystrophin gene: potential and pitfalls. Neurogenetics 6, 29–35 (2005). [DOI] [PubMed] [Google Scholar]
- 122.Chamberlain JS et al. Diagnosis of Duchenne and Becker muscular dystrophies by polymerase chain reaction. A multicenter study. JAMA 267, 2609–2615 (1992). [DOI] [PubMed] [Google Scholar]
- 123.Aartsma-Rus A et al. Report of a TREAT-NMD/World Duchenne organisation meeting on dystrophin quantification methodology. J. Neuromuscul. Dis 6, 147–159 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Scheuerbrandt G Screening for Duchenne muscular dystrophy in Germany, 1977–2011: a personal story. Muscle Nerve 57, 185–188 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Moat SJ et al. Characterization of a blood spot creatine kinase skeletal muscle isoform immunoassay for high-throughput newborn screening of Duchenne muscular dystrophy. Clin. Chem 63, 908–914 (2017). [DOI] [PubMed] [Google Scholar]
- 126.Gatheridge MA et al. Identifying non-Duchenne muscular dystrophy-positive and false negative results in prior Duchenne muscular dystrophy newborn screening programs: a review. JAMA Neurol 73, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 127.Wood MF et al. Parental attitudes toward newborn screening for Duchenne/Becker muscular dystrophy and spinal muscular atrophy. Muscle Nerve 49, 822–828 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Bianchi DW & Chiu RWK Sequencing of circulating cell-free DNA during pregnancy. N. Engl. J. Med 379, 464–473 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brison N et al. Maternal copy-number variations in the DMD gene as secondary findings in noninvasive prenatal screening. Genet. Med 21, 2774–2780 (2019). [DOI] [PubMed] [Google Scholar]
- 130.Tuffery-Giraud S et al. Genotype-phenotype analysis in 2,405 patients with a dystrophinopathy using the UMD-DMD database: a model of nationwide knowledgebase. Hum. Mutat 30, 934–945 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Eagle M et al. Managing Duchenne muscular dystrophy–the additive effect of spinal surgery and home nocturnal ventilation in improving survival. Neuromuscul. Disord 17, 470–475 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Moxley RT 3rd, Pandya S, Ciafaloni E, Fox DJ & Campbell K Change in natural history of Duchenne muscular dystrophy with long-term corticosteroid treatment: implications for management. J. Child Neurol 25, 1116–1129 (2010). [DOI] [PubMed] [Google Scholar]
- 133.Saito T et al. Study of Duchenne muscular dystrophy long-term survivors aged 40 years and older living in specialized institutions in Japan. Neuromuscul. Disord 27, 107–114 (2017). [DOI] [PubMed] [Google Scholar]
- 134. Birnkrant DJ et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol 17, 347–361 (2018). Part 2 of a three-part standard-of-care document for DMD.
- 135. Birnkrant DJ et al. Diagnosis and management of Duchenne muscular dystrophy, part 3: primary care, emergency management, psychosocial care, and transitions of care across the lifespan. Lancet Neurol 17, 445–455 (2018). Part 3 of a three-part standard-of-care document for DMD.
- 136.Passamano L et al. Improvement of survival in Duchenne muscular dystrophy: retrospective analysis of 835 patients. Acta Myol 31, 121–125 (2012). [PMC free article] [PubMed] [Google Scholar]
- 137.Finder JD et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am. J. Respir. Crit. Care Med 170, 456–465 (2004). [DOI] [PubMed] [Google Scholar]
- 138.Buddhe S et al. Cardiac management of the patient with Duchenne muscular dystrophy. Pediatrics 142 (Suppl. 2), S72–S81 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McNally EM et al. Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation 131, 1590–1598 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Duboc D et al. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J. Am. Coll. Cardiol 45, 855–857 (2005). [DOI] [PubMed] [Google Scholar]
- 141.Duboc D et al. Perindopril preventive treatment on mortality in Duchenne muscular dystrophy: 10 years’ follow-up. Am. Heart J 154, 596–602 (2007). [DOI] [PubMed] [Google Scholar]
- 142.Yilmaz O, Karaduman A & Topaloğlu H Prednisolone therapy in Duchenne muscular dystrophy prolongs ambulation and prevents scoliosis. Eur. J. Neurol 11, 541–544 (2004). [DOI] [PubMed] [Google Scholar]
- 143.Bertrand LA, Askeland EJ, Mathews KD, Erickson BA & Cooper CS Prevalence and bother of patient-reported lower urinary tract symptoms in the muscular dystrophies. J. Pediatr. Urol 12, 398. e391–398.e4 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Haenggi T, Schaub MC & Fritschy JM Molecular heterogeneity of the dystrophin-associated protein complex in the mouse kidney nephron: differential alterations in the absence of utrophin and dystrophin. Cell Tissue Res 319, 299–313 (2005). [DOI] [PubMed] [Google Scholar]
- 145.Matsumura T, Saito T, Fujimura H & Sakoda S Renal dysfunction is a frequent complication in patients with advanced stage of Duchenne muscular dystrophy [Japanese]. Rinsho Shinkeigaku 52, 211–217 (2012). [DOI] [PubMed] [Google Scholar]
- 146.Banihani R et al. Cognitive and neurobehavioral profile in boys with Duchenne muscular dystrophy. J. Child Neurol 30, 1472–1482 (2015). [DOI] [PubMed] [Google Scholar]
- 147.Biggar WD et al. Deflazacort in Duchenne muscular dystrophy: a comparison of two different protocols. Neuromuscul. Disord 14, 476–482 (2004). [DOI] [PubMed] [Google Scholar]
- 148.McDonald CM et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: a prospective cohort study. Lancet 391, 451–461 (2018). [DOI] [PubMed] [Google Scholar]
- 149.McDonald CM et al. Deflazacort vs prednisone treatment for Duchenne muscular dystrophy: a meta-analysis of disease progression rates in recent multicenter clinical trials. Muscle Nerve 61, 26–35 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bylo M, Farewell R, Coppenrath VA & Yogaratnam D A review of deflazacort for patients with Duchenne muscular dystrophy. Ann. Pharmacother 54, 788–794 (2020). [DOI] [PubMed] [Google Scholar]
- 151.Ricotti V et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J. Neurol. Neurosurg. Psychiatry 84, 698–705 (2013). [DOI] [PubMed] [Google Scholar]
- 152.Matthews E, Brassington R, Kuntzer T, Jichi F & Manzur AY Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev 5, CD003725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Connolly AM et al. Twice-weekly glucocorticosteroids in infants and young boys with Duchenne muscular dystrophy. Muscle Nerve 59, 650–657 (2019). [DOI] [PubMed] [Google Scholar]
- 154.Angelini C & Peterle E Old and new therapeutic developments in steroid treatment in Duchenne muscular dystrophy. Acta Myol 31, 9–15 (2012). [PMC free article] [PubMed] [Google Scholar]
- 155.Jaisser F & Farman N Emerging roles of the mineralocorticoid receptor in pathology: toward new paradigms in clinical pharmacology. Pharmacol. Rev 68, 49–75 (2016). [DOI] [PubMed] [Google Scholar]
- 156.Bushby K et al. Ataluren treatment of patients with nonsense mutation dystrophinopathy. Muscle Nerve 50, 477–487 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.McDonald CM et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 390, 1489–1498 (2017). [DOI] [PubMed] [Google Scholar]
- 158.Haas M et al. European medicines agency review of ataluren for the treatment of ambulant patients aged 5 years and older with Duchenne muscular dystrophy resulting from a nonsense mutation in the dystrophin gene. Neuromuscul. Disord 25, 5–13 (2015). [DOI] [PubMed] [Google Scholar]
- 159.Mercuri E et al. Safety and effectiveness of ataluren: comparison of results from the STRIDE registry and CINRG DMD Natural History Study. J. Comp. Eff. Res 9, 341–360 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Aartsma-Rus A & Goemans N A sequel to the eteplirsen saga: eteplirsen is approved in the United States but was not approved in Europe. Nucleic Acid Ther 29, 13–15 (2019). [DOI] [PubMed] [Google Scholar]
- 161.Niks EH & Aartsma-Rus A Exon skipping: a first in class strategy for Duchenne muscular dystrophy. Expert. Opin. Biol. Ther 17, 225–236 (2017). [DOI] [PubMed] [Google Scholar]
- 162.Alfano LN et al. Long-term treatment with eteplirsen in nonambulatory patients with Duchenne muscular dystrophy. Medicine 98, e15858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Mendell JR et al. Longitudinal effect of eteplirsen versus historical control on ambulation in Duchenne muscular dystrophy. Ann. Neurol 79, 257–271 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Frank DE et al. Increased dystrophin production with golodirsen in patients with Duchenne muscular dystrophy. Neurology 94, e2270–e2282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Roshmi RR & Yokota T Viltolarsen for the treatment of Duchenne muscular dystrophy. Drugs Today 55, 627–639 (2019). [DOI] [PubMed] [Google Scholar]
- 166.Uttley L, Carlton J, Woods HB & Brazier J A review of quality of life themes in Duchenne muscular dystrophy for patients and carers. Health Qual. Life Outcomes 16, 237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pangalila RF et al. Prevalence of fatigue, pain, and affective disorders in adults with duchenne muscular dystrophy and their associations with quality of life. Arch. Phys. Med. Rehabil 96, 1242–1247 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Schara U, Geers B, Schmid J & Elsenbruch S Health-related quality of life in patients with Duchenne muscular dystrophy. Neuromuscul. Disord 21, 652 (2011). [Google Scholar]
- 169.Elsenbruch S, Schmid J, Lutz S, Geers B & Schara U Self-reported quality of life and depressive symptoms in children, adolescents, and adults with Duchenne muscular dystrophy: a cross-sectional survey study. Neuropediatrics 44, 257–264 (2013). [DOI] [PubMed] [Google Scholar]
- 170.Abresch RT, Seyden NK & Wineinger MA Quality of life. Issues for persons with neuromuscular diseases. Phys. Med. Rehabil. Clin. N. Am 9, 233–248 (1998). [PubMed] [Google Scholar]
- 171.Bach JR, Campagnolo DI & Hoeman S Life satisfaction of individuals with Duchenne muscular dystrophy using long-term mechanical ventilatory support. Am. J. Phys. Med. Rehabil 70, 129–135 (1991). [DOI] [PubMed] [Google Scholar]
- 172.Crescimanno G, Greco F, D’Alia R, Messina L & Marrone O Quality of life in long term ventilated adult patients with Duchenne muscular dystrophy. Neuromuscul. Disord 29, 569–575 (2019). [DOI] [PubMed] [Google Scholar]
- 173.Pangalila R Quality of life in Duchenne muscular dystrophy: the disability paradox. Dev. Med. Child Neurol 58, 435–436 (2016). [DOI] [PubMed] [Google Scholar]
- 174.Albrecht GL & Devlieger PJ The disability paradox: high quality of life against all odds. Soc. Sci. Med 48, 977–988 (1999). [DOI] [PubMed] [Google Scholar]
- 175.Rose MR et al. Role of disease severity, illness perceptions, and mood on quality of life in muscle disease. Muscle Nerve 46, 351–359 (2012). [DOI] [PubMed] [Google Scholar]
- 176.Zamani G et al. The quality of life in boys with Duchenne muscular dystrophy. Neuromuscul. Disord 26, 423–427 (2016). [DOI] [PubMed] [Google Scholar]
- 177.Lim Y, Velozo C & Bendixen RM The level of agreement between child self-reports and parent proxy-reports of health-related quality of life in boys with Duchenne muscular dystrophy. Qual. Life Res 23, 1945–1952 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Leclerc T et al. Prioritisation of ICU treatments for critically ill patients in a COVID-19 pandemic with scarce resources. Anaesth. Crit. Care Pain Med 39, 333–339 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.de Moura MC et al. Is functional dependence of Duchenne muscular dystrophy patients determinant of the quality of life and burden of their caregivers? Arq. Neuropsiquiatr 73, 52–57 (2015). [DOI] [PubMed] [Google Scholar]
- 180.Yilmaz O, Yildirim SA, Oksuz C, Atay S & Turan E Mothers’ depression and health-related quality of life in neuromuscular diseases: role of functional independence level of the children. Pediatr. Int 52, 648–652 (2010). [DOI] [PubMed] [Google Scholar]
- 181.Barlow JH & Ellard DR The psychosocial well-being of children with chronic disease, their parents and siblings: an overview of the research evidence base. Child Care Health Dev 32, 19–31 (2006). [DOI] [PubMed] [Google Scholar]
- 182.Kay E & Kingston H Feelings associated with being a carrier and characteristics of reproductive decision making in women known to be carriers of X-linked conditions. J. Health Psychol 7, 169–181 (2002). [DOI] [PubMed] [Google Scholar]
- 183.Skuk D et al. Dystrophin expression in muscles of duchenne muscular dystrophy patients after high-density injections of normal myogenic cells. J. Neuropathol. Exp. Neurol 65, 371–386 (2006). [DOI] [PubMed] [Google Scholar]
- 184.Skuk D et al. First test of a “high-density injection” protocol for myogenic cell transplantation throughout large volumes of muscles in a Duchenne muscular dystrophy patient: eighteen months follow-up. Neuromuscul. Disord 17, 38–46 (2007). [DOI] [PubMed] [Google Scholar]
- 185.Taylor M et al. Cardiac and skeletal muscle effects in the randomized HOPE-Duchenne trial. Neurology 92, e866–e878 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Duan D Micro-dystrophin gene therapy goes systemic in Duchenne muscular dystrophy patients. Hum. Gene Ther 29, 733–736 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mendell JR et al. Assessment of systemic delivery of rAAVrh74.MHCK7.micro-dystrophin in children with duchenne muscular dystrophy: a nonrandomized controlled trial. JAMA Neurol 77, 1122–1131 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang D, Zhang F & Gao G CRISPR-based therapeutic genome editing: strategies and in vivo delivery by AAV vectors. Cell 181, 136–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Chemello F, Bassel-Duby R & Olson EN Correction of muscular dystrophies by CRISPR gene editing. J. Clin. Invest 130, 2766–2776 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Nelson CE, Robinson-Hamm JN & Gersbach CA Genome engineering: a new approach to gene therapy for neuromuscular disorders. Nat. Rev. Neurol 13, 647–661 (2017). [DOI] [PubMed] [Google Scholar]
- 191.Nance ME et al. AAV9 edits muscle stem cells in normal and dystrophic adult mice. Mol. Ther 27, 1568–1585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kwon JB et al. In vivo gene editing of muscle stem cells with adeno-associated viral vectors in a mouse model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev 19, 320–329 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ousterout DG et al. Multiplex CRISPR/Cas9-based genome editing for correction of dystrophin mutations that cause Duchenne muscular dystrophy. Nat. Commun 6, 6244 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Duchêne BL et al. CRISPR-induced deletion with SaCas9 restores dystrophin expression in dystrophic models in vitro and in vivo. Mol. Ther 26, 2604–2616 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Wasala NB, Hakim CH, Yang NN & Duan D Questions answered and unanswered by the first CRISPR editing study in the canine model of Duchenne muscular dystrophy. Hum. Gene Ther 30, 535–543 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mendell JR et al. A phase I/IIa follistatin gene therapy trial for Becker muscular dystrophy. Mol. Ther 23, 192–201 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Goemans N et al. A randomized placebo-controlled phase 3 trial of an antisense oligonucleotide, drisapersen, in Duchenne muscular dystrophy. Neuromuscul. Disord 28, 4–15 (2018). [DOI] [PubMed] [Google Scholar]
- 198.Iyombe-Engembe JP et al. Efficient restoration of the dystrophin gene reading frame and protein structure in DMD myoblasts using the CinDel method. Mol. Ther. Nucleic Acids 5, e283 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Nicolas A et al. Assessment of the structural and functional impact of in-frame mutations of the DMD gene, using the tools included in the eDystrophin online database. Orphanet J. Rare Dis 7, 45 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Delalande O et al. Dystrophin’s central domain forms a complex filament that becomes disorganized by in-frame deletions. J. Biol. Chem 293, 6637–6646 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Goyenvalle A et al. Rescue of dystrophic muscle through U7 snRNA-mediated exon skipping. Science 306, 1796–1799 (2004). [DOI] [PubMed] [Google Scholar]
- 202.Wein N et al. Translation from a DMD exon 5 IRES results in a functional dystrophin isoform that attenuates dystrophinopathy in humans and mice. Nat. Med 20, 992–1000 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Malik V et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol 67, 771–780 (2010). [DOI] [PubMed] [Google Scholar]
- 204.Hirst RC, McCullagh KJ & Davies KE Utrophin upregulation in Duchenne muscular dystrophy. Acta Myol 24, 209–216 (2005). [PubMed] [Google Scholar]
- 205.Miura P & Jasmin BJ Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: how close are we? Trends Mol. Med 12, 122–129 (2006). [DOI] [PubMed] [Google Scholar]
- 206.Muntoni F et al. A phase 1b trial to assess the pharmacokinetics of ezutromid in pediatric Duchenne muscular dystrophy patients on a balanced diet. Clin. Pharmacol. Drug Dev 8, 922–933 (2019). [DOI] [PubMed] [Google Scholar]
- 207.Wilkinson IVL et al. Chemical proteomics and phenotypic profiling identifies the aryl hydrocarbon receptor as a molecular target of the utrophin modulator ezutromid. Angew. Chem 59, 2420–2428 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Heier CR et al. VBP15, a novel anti-inflammatory and membrane-stabilizer, improves muscular dystrophy without side effects. EMBO Mol. Med 5, 1569–1585 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Reeves EKM, Hoffman EP, Nagaraju K, Damsker JM & McCall JM VBP15: preclinical characterization of a novel anti-inflammatory delta 9,11 steroid. Bioorg. Med. Chem 21, 2241–2249 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hoffman EP et al. Vamorolone trial in Duchenne muscular dystrophy shows dose-related improvement of muscle function. Neurology 93, e1312–e1323 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Finanger E et al. Phase 1 study of edasalonexent (CAT-1004), an oral NF-kappaB inhibitor, in pediatric patients with Duchenne muscular dystrophy. J. Neuromuscul. Dis 6, 43–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Shelton GD & Engvall E Gross muscle hypertrophy in whippet dogs is caused by a mutation in the myostatin gene. Neuromuscul. Disord 17, 721–722 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Aiello D, Patel K & Lasagna E The myostatin gene: an overview of mechanisms of action and its relevance to livestock animals. Anim. Genet 49, 505–519 (2018). [DOI] [PubMed] [Google Scholar]
- 214.Campbell C et al. Myostatin inhibitor ACE-031 treatment of ambulatory boys with Duchenne muscular dystrophy: results of a randomized, placebo-controlled clinical trial. Muscle Nerve 55, 458–464 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Wagner KR et al. Randomized phase 2 trial and open-label extension of domagrozumab in Duchenne muscular dystrophy. Neuromuscul. Disord 30, 492–502 (2020). [DOI] [PubMed] [Google Scholar]
- 216.Buyse GM et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): a double-blind randomised placebo-controlled phase 3 trial. Lancet 385, 1748–1757 (2015). [DOI] [PubMed] [Google Scholar]
- 217.Bettica P et al. Histological effects of givinostat in boys with Duchenne muscular dystrophy. Neuromuscul. Disord 26, 643–649 (2016). [DOI] [PubMed] [Google Scholar]
- 218.Petrof BJ Molecular pathophysiology of myofiber injury in deficiencies of the dystrophin-glycoprotein complex. Am. J. Phys. Med. Rehabil 81 (Suppl. 11), S162–S174 (2002). [DOI] [PubMed] [Google Scholar]
- 219.Clerk A, Strong PN & Sewry CA Characterisation of dystrophin during development of human skeletal muscle. Development 114, 395–402 (1992). [DOI] [PubMed] [Google Scholar]
- 220.Mokhtarian A, Lefaucheur JP, Even PC & Sebille A Hindlimb immobilization applied to 21-day-old mdx mice prevents the occurrence of muscle degeneration. J. Appl. Physiol 86, 924–931 (1999). [DOI] [PubMed] [Google Scholar]


