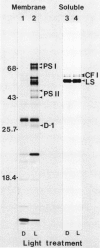
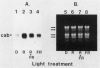
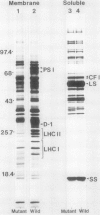
Abstract
The accumulation of radiolabeled plastid-encoded chlorophyll a-apoproteins is light dependent and is controlled at a posttranscriptional level. Illumination of dark-grown barley (Hordeum vulgare L.) with a brief pulse of red light induced the accumulation of radiolabeled chlorophyll a-apoproteins in subsequent protein synthesis assays. The induction of radiolabeled chlorophyll a-apoprotein accumulation was not affected by pretreatment of leaves with cycloheximide. Fluence response studies showed that a red light photoreceptor controls the accumulation of radiolabeled chlorophyll a-apoproteins with a threshold fluence of approximately 50 to 100 microeinsteins per square meter. While red light initiated chlorophyll a-apoprotein accumulation, this process was not reversed by a far red light treatment given immediately after the pulse of red light. The light pulse which initiated the accumulation of radiolabeled chlorophyll a-apoproteins also induced the rapid conversion of protochlorophyllide to chlorophyll a. A chlorophyll-deficient mutant, xan-f10, which is blocked in chlorophyll biosynthesis prior to protochlorophyllide formation, failed to accumulate radiolabeled chlorophyll a-apoproteins in the light even though transcripts for these apoproteins were present. A second mutant, xan-j64, which accumulates chlorophyllide in the light but only low levels of chlorophyll a, also showed reduced accumulation of radiolabeled chlorophyll a-apoproteins upon illumination. These results suggest that the light-induced conversion of protochlorophyllide to chlorophyll a is necessary for accumulation of the plastid-encoded chlorophyll a-apoproteins and one red light photoreceptor controlling this response is the protochlorophyllide holochrome.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apel K. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). Phytochrome-induced decrease of translatable mRNA coding for the NADPH: protochlorophyllide oxidoreductase. Eur J Biochem. 1981 Nov;120(1):89–93. doi: 10.1111/j.1432-1033.1981.tb05673.x. [DOI] [PubMed] [Google Scholar]
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Kuhlemeier C., Nagy F., Chua N. H. Organ-specific and light-induced expression of plant genes. Science. 1986 May 30;232(4754):1106–1112. doi: 10.1126/science.232.4754.1106. [DOI] [PubMed] [Google Scholar]
- Gounaris K., Barber J., Harwood J. L. The thylakoid membranes of higher plant chloroplasts. Biochem J. 1986 Jul 15;237(2):313–326. doi: 10.1042/bj2370313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz B. A., Thompson W. F., Briggs W. R. Phytochrome Regulation of Greening in Pisum: Chlorophyll Accumulation and Abundance of mRNA for the Light-Harvesting Chlorophyll a/b Binding Proteins. Plant Physiol. 1988 Jan;86(1):299–305. doi: 10.1104/pp.86.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Roberts L. L., Briggs W. R., Thompson W. F. Phytochrome Control of Specific mRNA levels in Developing Pea Buds : Kinetics of Accumulation, Reciprocity, and Escape Kinetics of the Low Fluence Response. Plant Physiol. 1986 Aug;81(4):1033–1038. doi: 10.1104/pp.81.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Thompson W. F., Briggs W. R. Different Red Light Requirements for Phytochrome-Induced Accumulation of cab RNA and rbcS RNA. Science. 1984 Dec 21;226(4681):1447–1449. doi: 10.1126/science.226.4681.1447. [DOI] [PubMed] [Google Scholar]
- Klein R. R., Mason H. S., Mullet J. E. Light-regulated translation of chloroplast proteins. I. Transcripts of psaA-psaB, psbA, and rbcL are associated with polysomes in dark-grown and illuminated barley seedlings. J Cell Biol. 1988 Feb;106(2):289–301. doi: 10.1083/jcb.106.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Control of gene expression during higher plant chloroplast biogenesis. Protein synthesis and transcript levels of psbA, psaA-psaB, and rbcL in dark-grown and illuminated barley seedlings. J Biol Chem. 1987 Mar 25;262(9):4341–4348. [PubMed] [Google Scholar]
- Klein R. R., Mullet J. E. Regulation of chloroplast-encoded chlorophyll-binding protein translation during higher plant chloroplast biogenesis. J Biol Chem. 1986 Aug 25;261(24):11138–11145. [PubMed] [Google Scholar]
- Mullet J. E., Klein R. R., Grossman A. R. Optimization of protein synthesis in isolated higher plant chloroplasts. Identification of paused translation intermediates. Eur J Biochem. 1986 Mar 3;155(2):331–338. doi: 10.1111/j.1432-1033.1986.tb09495.x. [DOI] [PubMed] [Google Scholar]
- Mösinger E., Batschauer A., Apel K., Schäfer E., Briggs W. R. Phytochrome regulation of greening in barley : effects on mRNA abundance and on transcriptional activity of isolated nuclei. Plant Physiol. 1988 Mar;86(3):706–710. doi: 10.1104/pp.86.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel H. J., Apel K. The protochlorophyllide holochrome of barley (Hordeum vulgare L.). The effect of light on the NADPH:protochlorophyllide oxidoreductase. Eur J Biochem. 1981 Nov;120(1):95–103. doi: 10.1111/j.1432-1033.1981.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Schiff J. A. A green safelight for the study of chloroplast development and other photomorphogenetic. Methods Enzymol. 1972;24:321–322. doi: 10.1016/0076-6879(72)24079-4. [DOI] [PubMed] [Google Scholar]
- Zhu Y. S., Kung S. D., Bogorad L. Phytochrome control of levels of mRNA complementary to plastid and nuclear genes of maize. Plant Physiol. 1985 Oct;79(2):371–376. doi: 10.1104/pp.79.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]