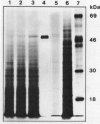
Abstract
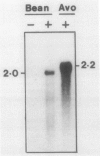
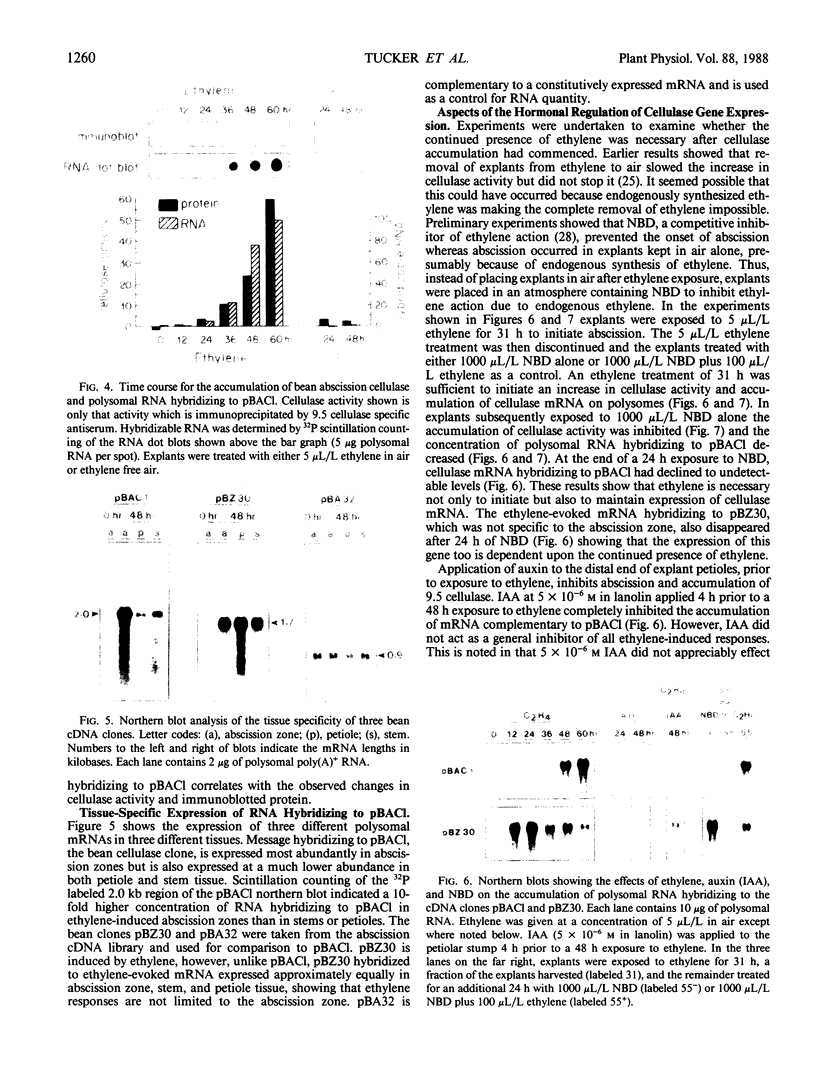
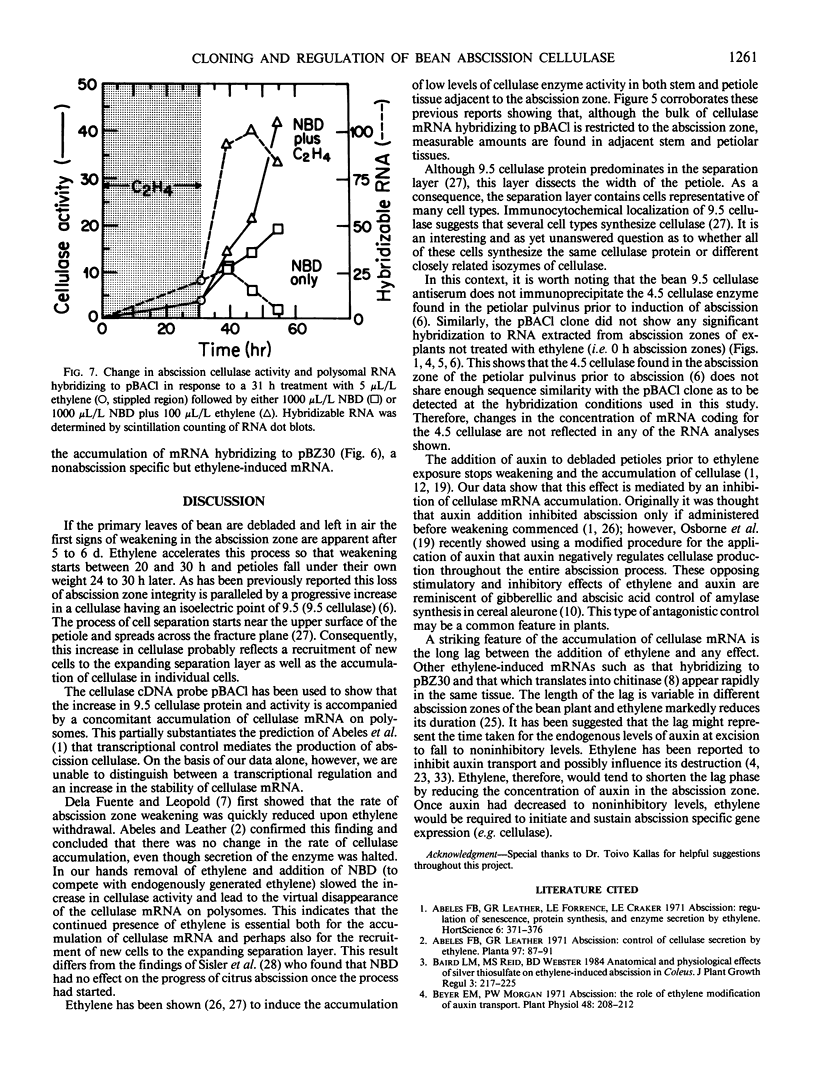
The physiology and anatomy of abscission has been studied in considerable detail; however, information on the regulation of gene expression in abscission has been limited because of a lack of probes for specific genes. We have identified and sequenced a 595 nucleotide bean (Phaseolus vulgaris cv Red Kidney) abscission cellulase cDNA clone (pBACl). The bean cellulase cDNA has extensive nucleic and amino acid sequence identity with the avocado cellulase cDNA pAV363. The 2.0 kilobase bean mRNA complementary to pBACl codes for a polypeptide of approximately 51 kilodalton (shown by hybrid-selection followed by in vitro translation). Bean cellulase antiserum is shown to immunoprecipitate a 51 kilodalton polypeptide from the in vitro translation products of abscission zone poly(A)+ RNA. Ethylene initiates bean leaf abscission and tissue-specific expression of cellulase mRNA. If ethylene treatment of bean explants was discontinued after 31 h and then 2,5-norbornadiene given to inhibit responses resulting from endogenously synthesized ethylene, polysomal cellulase mRNA hybridizing to pBACl decreased. Thus, ethylene is required not only to initiate abscission and cellulase gene expression but also to maintain continued accumulation of cellulase mRNA. Explants treated with auxin 4 hours prior to a 48 hour treatment with ethylene showed no substantial accumulation of RNA hybridizing to pBACl or expression of cellulase activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer E. M., Morgan P. W. Abscission: the role of ethylene modification of auxin transport. Plant Physiol. 1971 Aug;48(2):208–212. doi: 10.1104/pp.48.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dela Fuente R. K., Leopold A. C. Kinetics of abscission in the bean petiole explant. Plant Physiol. 1969 Feb;44(2):251–254. doi: 10.1104/pp.44.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Jackson A. O., Larkins B. A. Influence of Ionic Strength, pH, and Chelation of Divalent Metals on Isolation of Polyribosomes from Tobacco Leaves. Plant Physiol. 1976 Jan;57(1):5–10. doi: 10.1104/pp.57.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Osborne D. J. Ethylene, the natural regulator of leaf abscission. Nature. 1970 Mar 14;225(5237):1019–1022. doi: 10.1038/2251019a0. [DOI] [PubMed] [Google Scholar]
- Kushad M. M., Poovaiah B. W. Deferral of senescence and abscission by chemical inhibition of ethylene synthesis and action in bean explants. Plant Physiol. 1984 Oct;76(2):293–296. doi: 10.1104/pp.76.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Lewis L. N., Varner J. E. Synthesis of Cellulase during Abscission of Phaseolus vulgaris Leaf Explants. Plant Physiol. 1970 Aug;46(2):194–199. doi: 10.1104/pp.46.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M. L., Laties G. G. Interrelationship of Gene Expression, Polysome Prevalence, and Respiration during Ripening of Ethylene and/or Cyanide-Treated Avocado Fruit. Plant Physiol. 1984 Feb;74(2):307–315. doi: 10.1104/pp.74.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]