Abstract
Objective
To analyze the clinical data of elderly patients with peritoneal dialysis (PD) and compare patient and technique survival rates between Group 1 (65–74 years old) and Group 2 (≥75 years old).
Methods
This retrospective study enrolled 296 elderly patients (≥65 years old) on maintenance PD who were admitted to the Peritoneal Dialysis Center of the Second Hospital of Soochow University. The patients were categorized by outcome into ongoing PD, changed to hemodialysis, renal recovery dialysis stopped, or death groups. The patients were divided into Group 1 (65–74 years old) and Group 2 (≥75 years old). Patient survival and technique survival rates were calculated by the Kaplan–Meier method. Factors associated with patient survival were analyzed using the Cox regression model.
Results
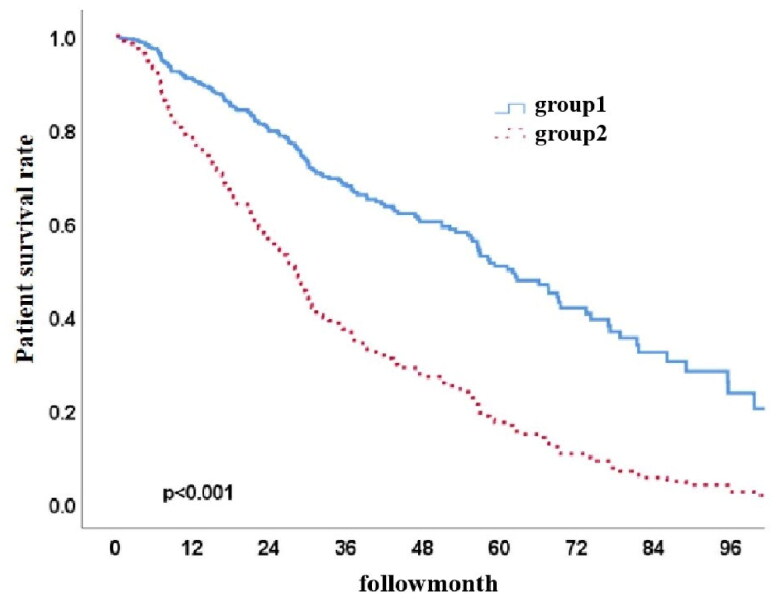
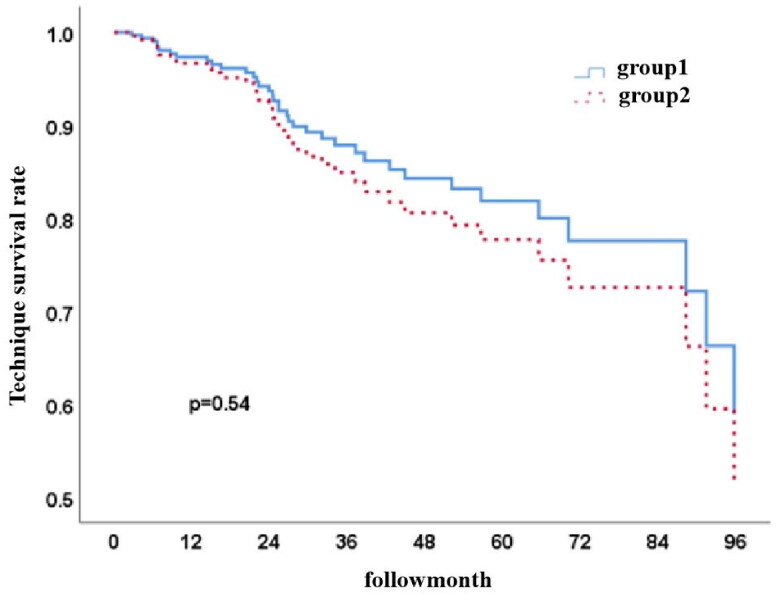
There were 176 (59.5%) subjects in Group 1 and 120 (40.5%) subjects in Group 2. The primary causes of death were cardiovascular events, peritonitis, and other infections. The patient survival rates at 1, 3, and 5 years were 91.2%, 68.0%, and 51.3% in Group 1 and 76.8%, 37.5%, and 17.6% in Group 2 (p < 0.001, HR 0.387, 95% CI 0.282–0.530). There was no statistically significant difference in the technique survival rate between the two groups (p = 0.54).
Conclusion
The elderly PD patients in this cohort mostly died from cardiovascular events, with a higher patient survival rate in Group 1 and similar technique survival in both groups. Older age, lower prealbumin, higher creatinine, not being on activated vitamin D, and high Charlson’s comorbidity index (CCI) score were independent risk factors for death.
Keywords: Peritoneal dialysis, patient survival, technical survival, 65–74 years old versus ≥75 years old
Introduction
The rising elderly population is facing an increasing incidence of kidney failure. Among end-stage kidney disease (ESKD) patients, 39%-50% are elderly worldwide [1–3], resulting in a rising demand for kidney replacement therapy. Currently, hemodialysis and peritoneal dialysis (PD) are the standard kidney replacement therapies for elderly patients, while transplantation is a rarely used option [4]. Compared with hemodialysis, PD eliminates the need for vascular access, protects residual kidney function, has a low hemodynamic impact, costs less, and improves patients’ quality of life [5–7]. However, some elderly patients cannot complete dialysis independently, which limits PD use. A reduction in medical burden and achievement of better social benefits by PD (versus hemodialysis) have led to the PD-First policy [8]. Previous studies have suggested comparable medium- and long-term prognoses of hemodialysis and PD, but some elderly patients have opted for palliative care due to the short life expectancy and associated complications [9]. Thus, there is a debate regarding prognosis analysis [10]. It is highly important to improve the patient survival rate for elderly patients, especially those ≥75 years old. Recent studies have shown that PD has a better patient survival rate than hemodialysis among elderly patients [11,12]. The purpose of this study was to analyze the clinical data of elderly patients on peritoneal dialysis (PD) and compare patient survival and technique survival between Group 1 (65–74 years old) and Group 2 (≥75 years old).
Patients and methods
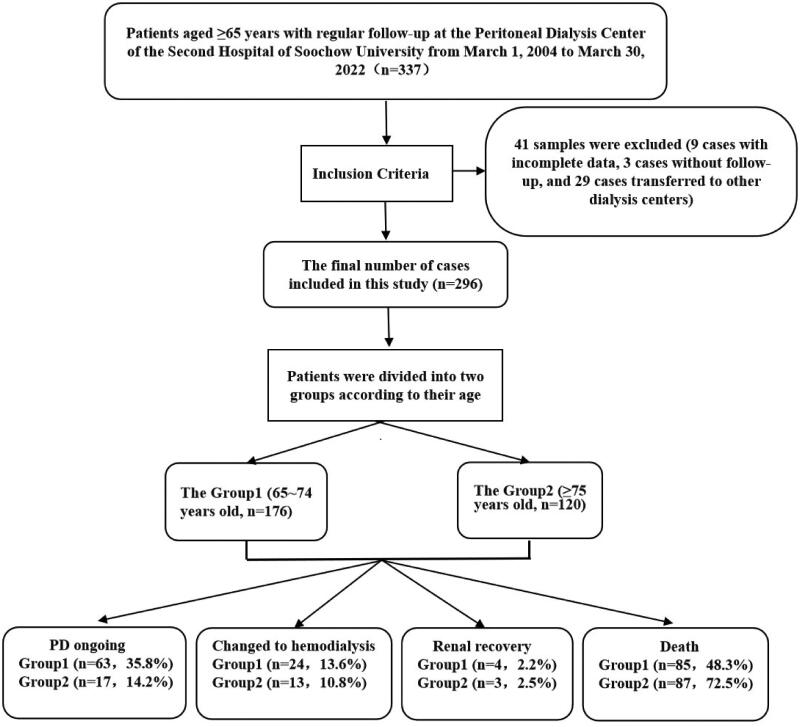
Among 1087 patients regularly followed up at the PD center of the Second Hospital affiliated with Soochow University from 01-March 2004 to 30-March 2022, 337 were aged ≥65 years old. Excluding 9 cases with incomplete data, three patients were lost to follow-up, and 29 patients were transferred to other dialysis centers; 296 patients were recruited as study subjects (Figure 1). The inclusion criteria were as follows: (1) fulfilling the clinical diagnostic criteria for end-stage kidney disease (ESKD), GFR < 10 mL/min/1.73 m2 in patients without diabetes and GFR < 15 mL/min/1.73 m2 in patients with diabetes; (2) ≥65 years old; and (3) ≥3 months of maintenance dialysis. The exclusion criteria were as follows: (1) acute kidney injury; (2) incomplete clinical data; (3) lost visits or transferred to other dialysis centers; (4) <3 months of PD; (5) no regular follow-up (follow-up interval >3 months); and (6) diagnosed with a tumor at baseline. The eligible patients were surgically implanted with a PD catheter (Tenckhoff or swan-neck tube) in the hospital. They underwent PD using Baxter lactate dialysis solution using a protocol designed according to clinical symptoms and the dialysis adequacy index. The study was approved by the Ethics Committee of the Second Hospital of Soochow University (JD-HG-2022-19).
Figure 1.
Flow chart for eligible patients.
Study methodology
This was a retrospective cohort study in which patients were followed up from the start of PD to 31 December 2022. Outcomes (PD ongoing, changed to hemodialysis, renal recovery dialysis stopped, and death), time, and cause of events were recorded. The primary endpoint event at follow-up was death, and the secondary endpoint was technique failure, i.e., transfer to hemodialysis. Patients were divided by age at the initiation of PD into Group 1 (65–74 years) or Group 2 (75 years and older).
Data collection and definitions
The PD patient baseline data collected included sex, age, body mass index, primary disease, complications, 24-h urine volume, medications taken, predialysis biochemical indices, kidney function, dialysis adequacy indices (Kt/v, CCr, rGFR, nPcR) and initial peritoneal transit, where Kt/v = urea clearance index, CCr = total creatinine clearance, rGFR = residual glomerular filtration rate, and nPcR = normalized protein catabolic rate. Initial indices were measured three months after PD began, followed by initial peritoneal transit observed at six months after PD. PD Adequest was used to calculate Kt/V and rGFR = (residual kidney urea clearance + residual kidney creatinine clearance)/2; peritoneal transport was evaluated by the peritoneal equilibration test (PET) and classified by the ratio of dialysate to plasma creatinine concentration (D/P) into high transport (D/p > 0.80), low transport (D/p < 0.50), low average transport (0.5 < D/p < 0.65) and high average transport (0.66 < D/p < 0.80). Disease outcomes included ongoing PD, changed to hemodialysis, renal recovery dialysis stopped, and death. Cardiovascular conditions included heart failure, coronary atherosclerotic heart disease (angina pectoris, myocardial infarction), and arrhythmias.
Statistical analyses
SPSS 24.0 software was used for all statistical analyses, of which normally distributed measurement data are expressed as the mean ± SD, nonnormally distributed measurement data as the median (1/4, 3/4), and count data as cases (%). ANOVA was used for comparing continuous variables in multiple groups, whereas the X2 test was performed for comparison between groups. The patient survival rate and technique survival rate were calculated by the Kaplan–Meier’s method. The technique survival rate of similar factors was assessed for significance by the log-rank test, and variables with p < 0.05 for single-factor analysis were included in multifactorial analysis. Survival statistics are hazard ratios (HRs) and 95% confidence intervals (CIs). p < 0.05 was considered a statistically significant difference.
Results
Baseline characteristics
Among the 1087 patients who started maintenance PD, 296 elderly (≥65 years) patients were included in the study and divided into Group 1 (176, 59.5%) and Group 2 (120, 40.5%). The mean age was 69.0 ± 2.9 years in Group 1 and 79.2 ± 3.7 years in Group 2. There was no significant difference in the proportion of women between the two groups (p = 0.908). Causes of ESKD comprised chronic glomerulonephritis (36.5%), hypertensive nephropathy (33.8%), diabetic nephropathy (19.3%), and other causes. Chronic glomerulonephritis was the most common cause of ESKD in Group 1 (36.9%), while hypertensive nephropathy was the most common cause of ESKD in Group 2 (38.3%). Additionally, hemoglobin, creatinine, parathyroid hormone (PTH), blood phosphorus, body mass index, Kt/V and nPCR significantly differed between the two groups (p < 0.05). Specifically, baseline creatinine, body mass index (BMI), PTH, blood phosphorus, Kt/V, and nPCR were higher in Group 1 than in Group 2, whereas baseline hemoglobin was lower in Group 1 than in Group 2 (p < 0.05). Doses of angiotensin receptor blockers (ARBs), diuretics, and activated vitamin D were significantly higher in Group 1 (p < 0.05). The initial peritoneal transport was dominated by high average transport (34%), followed by low average transport (33%) and high transport (9%) (Table 1).
Table 1.
Baseline characteristics of PD patients.
| Indicators | overall | Group1 | Group2 | P |
|---|---|---|---|---|
| N = 296 | Age 65–74 y, N = 176 | age ≥ 75 y, N = 120 | ||
| Age (years) | 73.2 ± 6.0 | 69.0 ± 2.9 | 79.2 ± 3.7 | <0.001 |
| Female (cases, %) | 132(44.6) | 78(44.3) | 54(45.0) | 0.908 |
| Body mass index(kg/m2) | 22.17 ± 3.15 | 22.58 ± 3.10 | 21.58 ± 3.13 | 0.007 |
| Primary disease | 0.513 | |||
| Chronic glomerulonephritis | 108(36.5) | 65(36.9) | 43(35.8) | |
| Hypertensive kidney damage | 100(33.8) | 54(30.7) | 46(38.3) | |
| Diabetic Nephropathy | 57(19.3) | 35(19.9) | 22(18.3) | |
| Combined diabetes | 79(26.7) | 50(28.4) | 29(24.2) | 0.418 |
| Hemoglobin (g/L) | 95.15 ± 22.28 | 92.89 ± 20.8 | 98.45 ± 23.99 | 0.035 |
| Serum creatinine (μmol/L) | 600.50(472.5, 762.5) | 627.50(484.8, 818.3) | 573.0(450.8, 717.8) | 0.031 |
| BUN (mmol/L) | 24.45(19.53, 30.30) | 24.90(19.6, 30.4) | 24.26(18.6, 29.9) | 0.361 |
| Uric acid (μmol/L) | 493.22 ± 135.73 | 493.53 ± 140.26 | 492.77 ± 129.55 | 0.963 |
| Pre-albumin(mg/dl) | 0.35 ± 2.19 | 0.45 ± 2.83 | 0.21 ± 0.07 | 0.364 |
| Alkaline phosphatase (mmol/L) | 107.69 ± 299.35 | 92.77 ± 75.04 | 129.69 ± 462.31 | 0.302 |
| Calcium (mmol/L) | 2.05 ± 0.25 | 2.03 ± 0.24 | 2.06 ± 0.27 | 0.305 |
| Phosphorus (mmol/L) | 1.67 ± 0.46 | 1.73 ± 0.46 | 1.59 ± 0.43 | 0.008 |
| Cholesterol (mmol/L) | 4.34 ± 1.31 | 4.27 ± 1.23 | 4.43 ± 1.42 | 0.307 |
| LDL(mmol/L) | 2.56 ± 1.18 | 2.53 ± 1.18 | 2.61 ± 1.17 | 0.566 |
| HDL(mmol/L) | 1.15 ± 0.57 | 1.15 ± 0.65 | 1.14 ± 0.42 | 0.85 |
| Triglycerides (mmol/L) | 1.50 ± 0.93 | 1.53 ± 0.98 | 1.45 ± 0.85 | 0.488 |
| Serum albumin (g/L) | 32.47 ± 6.03 | 32.38 ± 6.42 | 32.60 ± 5.43 | 0.756 |
| CRP(mg/L) | 17.14 ± 24.27 | 16.33 ± 25.19 | 18.33 ± 22.89 | 0.498 |
| TCO2 (mmol/L) | 20.86 ± 13.48 | 21.37 ± 14.11 | 20.15 ± 12.57 | 0.456 |
| Potassium(mmol/L) | 4.46 ± 0.87 | 4.47 ± 0.81 | 4.44 ± 0.96 | 0.734 |
| Sodium(mmol/L) | 140.34 ± 9.43 | 140.03 ± 11.52 | 140.79 ± 5.31 | 0.502 |
| Chloride(mmol/L) | 104.71 ± 6.62 | 104.86 ± 6.67 | 104.51 ± 6.59 | 0.667 |
| iPTH (pg/L) | 202.7(105.7, 306.6) | 220.0(105.8, 357.0) | 178.90(104.0, 265.0) | 0.024 |
| Taking medication | ||||
| Calcium antagonists | 234(82.1) | 143(84.6) | 91(78.4) | 0.182 |
| Alpha-blockers | 47(16.4) | 30(17.6) | 17(14.7) | 0.503 |
| Beta-blockers | 93(32.5) | 56(32.9) | 37(31.9) | 0.853 |
| αβ-blockers | 55(19.2) | 34(20.0) | 37(31.9) | 0.689 |
| ACEI | 13(4.5) | 8(4.7) | 5(4.3) | 0.875 |
| ARB | 132(46.2) | 87(51.2) | 45(38.8) | 0.039 |
| Diuretics | 172(60.1) | 112(65.9) | 60(51.7) | 0.016 |
| Activated vitamin-D | 76(26.6) | 53(31.2) | 23(19.8) | 0.033 |
| Calcium tablets | 146(51.0) | 94(55.3) | 52(44.8) | 0.082 |
| Statins | 33(11.6) | 20(11.2) | 13(11.8) | 0.871 |
| Initial dialysis adequacy index | ||||
| Kt/V | 1.77(1.52, 2.11) | 1.81(1.57, 2.23) | 1.74(1.45, 2.00) | 0.039 |
| Ccr | 62.87(48.91, 80.43) | 65.03(52.47, 82.14) | 61.30(43.67, 74.11) | 0.161 |
| eGFR | 4.43(2.59,6.53) | 4.45(3.16,6.54) | 4.26(2.14,6.55) | 0.308 |
| nPCR | 5.19 (4.14, 6.67) | 5.75 (4.57, 7.26) | 4.68 (3.75, 5.75) | <0.001 |
| Initial peritoneal function | 0.732 | |||
| High Transit | 25(8.4) | 13(7.4) | 12(10.0) | |
| High average transit | 91(30.7) | 56(31.8) | 35(29.2) | |
| Low average transit | 88(29.7) | 51(29.0) | 37(30.8) | |
| Low transit | 67(22.6) | 43(24.4) | 24(20.0) | |
| Abdominal dialysis age | 36.22 ± 26.37 | 37.47 ± 29.94 | 26.25 ± 20.14 | 0.002 |
| percentage of helper PD | 195(65.9) | 107(60.8) | 88(73.3) | 0.438 |
| Cardiovascular disease | 0.328 | |||
| Myocardial infarction | 9(3.1) | 5(2.8) | 4(3.0) | |
| Heart failure | 84(28.4) | 52(29.5) | 32(26.7) | |
| Stroke | 39(13.2%) | 22(12.5) | 17(14.1) | |
| CCI | 3.46 ± 1.52 | 3.49 ± 1.64 | 3.40 ± 1.31 | 0.628 |
iPTH, parathyroid hormon; BUN, blood urea nitrogen; eGFR, evaluated glomerular filtration rate; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; Kt/V: urea clearance index; CCr: total creatinine clearance, ml/min; CCI, Charlson’s comorbidity index; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; CRP, C-reactive protein; p < 0.05 indicates a significant difference.
Occurrence of peritonitis
One hundred fifteen (39%, 115/296) elderly PD patients developed PD-associated peritonitis and 24 of them (20.9%, 24/115) died or transferred to hemodialysis instead. Among them, 54.8% (63/115) experienced peritonitis once, 20.9% (24/115) experienced peritonitis twice, and 24.3% (28/115) experienced peritonitis three or more times with a total of 215 episodes of peritonitis. The results of bacterial culture in peritoneal dialysis fluid indicated that gram-positive cocci accounted for 42.3% (91/215), and gram-negative bacilli accounted for 30.7% (66/215). There was no significant difference in the incidence of peritonitis between Group 1 (36.3 patients/month) and Group 2 (34.5 patients/month) (p > 0.05).
Outcomes and causes
This study enrolled 296 elderly PD patients. There were 176 subjects in Group 1, including those on maintenance PD (63 cases, 35.8%), who changed to hemodialysis (24 cases, 13.6%), who achieved renal recovery (4 cases, 2.2%), and who died (85 cases, 48.3%). There were 120 subjects in Group 2, including those who maintained PD (17 cases, 14.2%), changed to hemodialysis (13 cases, 10.8%), achieved renal recovery (3 cases, 2.5%), and died (87 cases, 72.5%) (Figure 1). Cardiovascular events occurred in 40 cases (13.5%), peritonitis in 24 cases (8.1%), infection in 23 cases (7.8%), stroke in 19 cases (6.4%) and multiorgan failure in 21 cases (7.1%). Specifically, cardiovascular events (24 cases), multiorgan failure (12 cases), and peritonitis (6 cases) were dominant in Group 2, while peritonitis (18 cases), cardiovascular events (16 cases), and stroke (13 cases) were the primary outcomes in Group 1. In this study, 115 (39%) elderly PD patients developed PD-related peritonitis, of whom 20.9% died or were switched to hemodialysis treatment, the latter due to peritonitis (9/37) and inadequate dialysis (9/37); inadequate dialysis (7/15) was the main reason in Group 2, and peritonitis (5/22) or poorly functioning PD tubing (5/22) was the main reason in Group 1 (Table 2).
Table 2.
Analysis of outcomes and causes in the overall study, group1 and group2.
| Indicators | Overall | Group1 | Group2 |
|---|---|---|---|
| N = 296 | Age 65 – 74 y, N = 176 | Age ≥ 75y, N = 120 | |
| Outcomes | |||
| Continuation of peritoneal dialysis (cases, %) | 80 (27) | 63 (48) | 17 (14) |
| Deaths (cases, %) | 172 (58) | 85 (36) | 87 (73) |
| Conversion to hemodialysis (cases, %) | 37 (13) | 24 (14) | 13 (11) |
| Recovery of renal function (cases, %) | 7 (2) | 4 (2) | 3 (2) |
| Reason for transfer | |||
| Survival (cases, %) | 87 (29.4) | 67 (38.1) | 20 (16.7) |
| Cardiovascular events (cases, %) | 40 (13.5) | 16 (9.1) | 24 (20.0) |
| Peritonitis (cases, %) | 24 (8.1) | 18 (10.2) | 6 (5.0) |
| Infections (cases, %) | 23 (7.8) | 11 (6.3) | 12 (10.0) |
| Stroke (cases, %) | 19 (6.4) | 13 (7.4) | 6 (5.0) |
| Gastrointestinal bleeding (cases, %) | 4 (1.4) | 1 (0.6) | 3 (2.5) |
| Multi-organ failure (cases, %) | 21 (7.1) | 9 (5.1) | 12 (10.0) |
| PD catheter malfunction (cases, %) | 5 (1.7) | 5 (2.8) | 0 (0) |
| Uremic encephalopathy (cases, %) | 5 (1.7) | 4 (2.3) | 1 (0.8) |
| Inadequate dialysis (cases, %) | 9 (3.0) | 2 (1.1) | 7 (5.8) |
| Malignant tumors (cases, %) | 9 (3.0) | 6 (3.4) | 3 (2.5) |
| Malnutrition (cases, %) | 14 (5.7) | 6 (3.4) | 8 (6.7) |
| Other (cases, %) | 36 (29.4) | 18 (10.2) | 18 (15.0) |
| Reasons for transfer to hemodialysis | n = 37 | n = 22 | n = 15 |
| I nadequate dialysis (cases, %) | 9 (24.3) | 2 (9) | 7 (46.7) |
| Peritonitis (cases, %) | 9 (24.3) | 5 (22.7) | 4 (26.7) |
| PD Catheter malfunction (cases, %) | 5 (13.5) | 5 (22.7) | 0 (0) |
| Other (cases, %) | 14 (37.8) | 10(45.5) | 4 (26.7) |
Patient survival and technique survival rates
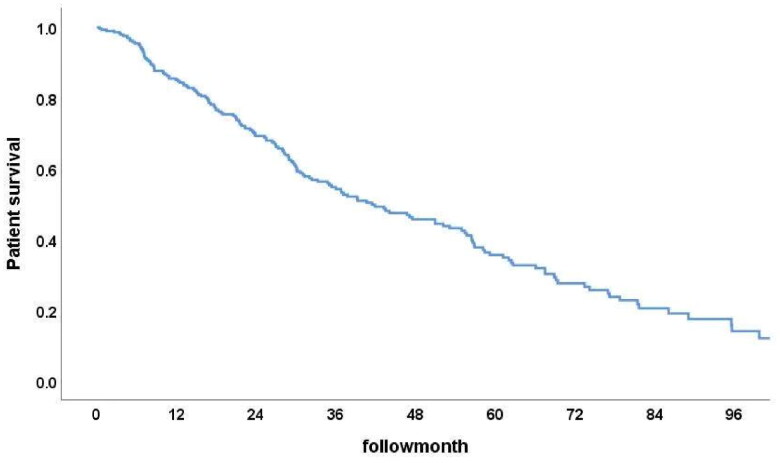
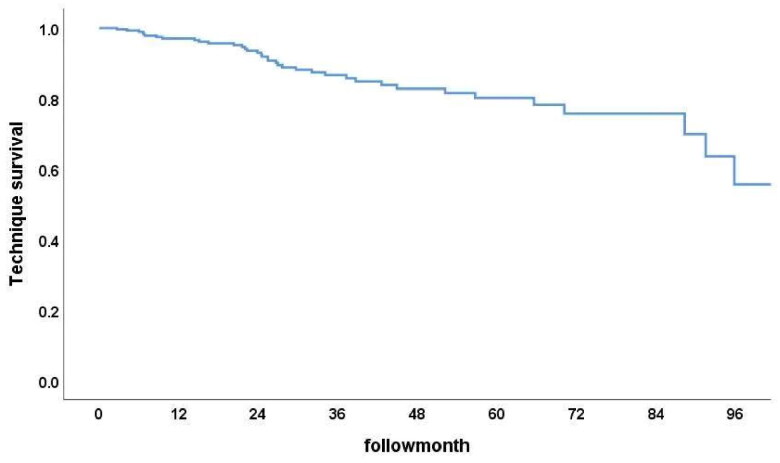
Among the 296 PD patients, the 1-year, 3-year, and 5-year patient survival rates were 85%, 54.8%, and 36.6% (Figure 2), while the technique survival rates were 97.2%, 87.1%, and 80.0%, respectively (Figure 3). Furthermore, survival rates at 1, 3, and 5 years were 91.2%, 68.0%, and 51.3% in Group 1 and 76.8%, 37.5%, and 17.6% in Group 2, respectively. The cumulative patient survival rate was significantly higher in Group 1 than in Group 2 (p < 0.001), with a similar trend for 5-year technique survival rates at 82% and 78% in the two groups, respectively (p = 0.54) (Figures 4 and 5).
Figure 2.
Patient survival rate.
Figure 3.
Technique survival rate among surviving subjects.
Figure 4.
Patient survival curves for both groups (p < 0.001, HR 0.387, 95% CI 0.282–0.530).
Figure 5.
Technique survival curves for both groups (p = 0.54, HR 0.792, 95% CI 0.396–1.584).
Independent risk factors for death in PD patients
Univariate Cox regression analysis of age, sex, BMI, primary disease, predialysis medication, initial peritoneal transport, presence of comorbid diabetes and peritonitis, hemoglobin, iPTH, blood biochemical electrolytes and medications revealed that age (p < 0.001), baseline prealbumin (p = 0.028), creatinine (p = 0.018), iPTH (p = 0.031), lack of activated vitamin D supplements (p < 0.001), cardiovascular disease at baseline (p = 0.001), and Charlson’s comorbidity index (CCI) (p < 0.001) were risk factors for patient mortality (Table 3). Multivariate Cox regression analysis of factors with p < 0.05 indicated that age (p < 0.001, HR 1.062, 95% CI 1.035–1.101), baseline prealbumin (p = 0.002, HR 0.031, 95% CI 0.003–0.276), creatinine (p = 0.025, HR 1.001, 95% CI 1.000–1.003), lack of activated vitamin D supplements (p = 0.021, HR 1.618, 95% CI 1.076–2.631), and CCI (p = 0.004, HR 1.210, 95% CI 1.093–1.312) were independent factors for death in elderly PD patients (Table 4).
Table 3.
Risk factors for death in elderly patients with peritoneal dialysis (univari-ate analysis).
| Indicators | P | HR | 95.0% CI of Exp(B) |
|
|---|---|---|---|---|
| Lowerlimit | Upper limit | |||
| Age(years) | <0.001 | 1.067 | 1.027 | 1.108 |
| Body mass index(kg/m2) | 0.125 | 0.951 | 0.891 | 1.014 |
| iPTH(pg/L) | 0.031 | 1.001 | 1.000 | 1.003 |
| Hemoglobin(g/L) | 0.127 | 1.007 | 0.998 | 1.015 |
| Serum creatinine(umol/L) | 0.018 | 1.001 | 1.000 | 1.002 |
| BUN (mmol/L) | 0.983 | 1.000 | 0.969 | 1.032 |
| Blood uric acid(umol/L) | 0.906 | 1.000 | 0.998 | 1.002 |
| Pre-albumin(mg/dl) | 0.028 | 0.030 | 0.001 | 0.690 |
| Alkaline phosphatase(U/L) | 0.425 | 0.999 | 0.997 | 1.001 |
| Calcium(mmol/L) | 0.535 | 1.344 | 0.528 | 3.419 |
| Phosphorus(mmol/L) | 0.581 | 0.835 | 0.440 | 1.583 |
| Cholesterol(mmol/L) | 0.571 | 1.131 | 0.739 | 1.729 |
| LDL(mmol/L) | 0.728 | 1.081 | 0.696 | 1.680 |
| HDL(mmol/L) | 0.056 | 0.544 | 0.292 | 1.015 |
| Triglycerides(mmol/L) | 0.761 | 0.957 | 0.724 | 1.267 |
| Serum Albumin(g/L) | 0.217 | 0.975 | 0.938 | 1.015 |
| CRP(mg/L) | 0.270 | 1.006 | 0.995 | 1.017 |
| TCO2(mmol/L) | 0.513 | 0.996 | 0.983 | 1.009 |
| Potassium(mmol/L) | 0.971 | 0.996 | 0.781 | 1.269 |
| Sodium(mmol/L) | 0.820 | 1.006 | 0.954 | 1.062 |
| Chloride(mmol/L) | 0.281 | 1.024 | 0.981 | 1.070 |
| Kt/V | 0.316 | 0.811 | 0.538 | 1.222 |
| Total Ccr | 0.981 | 1.000 | 0.994 | 1.006 |
| eGFR(ml/min/1.73m2) | 0.670 | 1.015 | 0.949 | 1.085 |
| Not taking diuretics | 0.197 | 1.282 | 0.879 | 1.868 |
| Not taking activated vitamin D | 0.000 | 2.351 | 1.459 | 3.787 |
| Cardiovascular disease | 0.001 | 1.718 | 1.233 | 2.394 |
| CCI | <0.001 | 1.252 | 1.133 | 1.385 |
iPTH, parathyroid hormon; BUN, blood urea nitrogen; eGFR, evaluated glomerular filtration rate; Kt/V: urea clearance index; CCr: total creatinine clearance, ml/min; CCI, Charlson’s comorbidity index; HDL, High-density lipoprotein; LDL, Low-density lipoprotein; CRP, C-reactive protein; p < 0.05 indicates a significant difference.
Table 4.
Risk factors for death in elderly patients with PD (multi-factorial Cox regression analysis).
| P | HR | 95.0% CI of Exp(B) |
||
|---|---|---|---|---|
| Lower limit | Upper limit | |||
| Age(year) | <0.001 | 1.062 | 1.035 | 1.101 |
| Pre-albumin(mg/dl) | 0.002 | 0.031 | 0.003 | 0.276 |
| Serum creatinine(umol/L) | 0.025 | 1.001 | 1.000 | 1.003 |
| Not taking activated vitamin D | 0.021 | 1.618 | 1.076 | 2.631 |
| CCI | 0.004 | 1.210 | 1.093 | 1.312 |
CI, confidence interval; HR, hazards ratio. CCI, Charlson’s comorbidity index; p < 0.05 indicates a significant difference.
Discussion
There is a requirement for kidney replacement therapies due to the rising ESKD in the elderly population. A study by Wachterman et al. reported that >50% of ESKD patients would prefer treatment modalities that alleviate pain and discomfort over those that increase survival [13]. Individualized dialysis modalities to improve quality of life is important for older patients [14]. One UK study showed that PD had less impact on the daily life of elderly patients and is, therefore, more suitable for them[4]. Consequently, a higher percentage of elderly patients chose PD: 35% of patients over 85 years old chose PD in France[15]; 40.5% of patients over 65 years old in Canada[16]; 80% of patients approximately 62 years old in Hong Kong[17]; 42.1% at the Abdominal Dialysis Center of Peking Union Medical College Hospital[18] and 31% in our center. Sandrine et al. predicted a further increase in the proportion of elderly patients undergoing PD treatment in the future[19]. While the occurrence of PD-associated peritonitis is one of the reasons why PD is not a priority for many patients or nephrologists, increasingly sophisticated PD techniques have led to a significant reduction in the incidence of peritonitis, according to a 32-year prospective study [20], suggesting a temporal trend of decreasing peritonitis incidence. Another 5-year study also found a decrease in the incidence of peritonitis from 1/16 patient-month (0.75/year risk) to 1/29 patient-month (0.41/year risk)[21]. A Spanish multicenter study observed no significant effect on technique failure or mortality, despite a higher incidence of peritonitis in older (>65 years) patients[22]. Some studies from developed countries have revealed diabetic nephropathy as the most common primary disease in elderly PD patients[16, 23,24]. At the same time, diabetes mellitus and glomerulonephritis were reported as the primary conditions in PD patients in a Hong Kong study[25]. The present study found that chronic glomerulonephritis was the primary disease in elderly patients with PD and that diabetic nephropathy accounted for only 19.3%, less than that reported by developed countries. This may be due to (1) poor medical care that failed to detect and diagnose CKD combined with diabetes on time, thereby underestimating diabetic nephropathy; (2) ethnic and regional differences; and (3) bias in retrospective analyses concerning the assessment of primary disease.
The 1-, 3- and 5-year patient survival rates of elderly PD patients in Group 2 were similar to those reported in the Canadian Organ Replacement Registry (76.8%, 37.5%, and 17.6% vs. 76.6%, 36.7%, and 17.8%, respectively) and lower than those of the Toronto study (85%, 67%, and 42%)[16]. The results for Group 1 were higher than those of studies conducted in Canada and Toronto and at Sun Yat-Sen University (91.2%, 68.0%, 51.3% vs. 83.9%, 52.6%, 32.2% in Canada; vs. 84%, 56%, 38% in Toronto; vs. 81%, 59%, 39% in Sun Yat-Sen University)[26]. In contrast, there was no significant difference in technique survival rates among these studies, consistent with the findings of our study. A retrospective analysis in Hong Kong found no significant differences in 2- and 5-year patient survival and technique survival rates between elderly PD patients over 65 and under 65[26]. A US study found that the technique failure rate in patients over 65 was not higher[27], but a Spanish study showed no difference in technique survival rates between elderly and young patients[22]. These studies suggest that age is not an independent risk factor for technique failure in PD patients, implying that the different patient survival rates between Group 1 and Group 2 were mainly due to age. In contrast, technique failure did not increase with age. Thus, PD is a suitable kidney replacement therapy option for ≥75-year-old patients. The lack of change in technique failure with age may be due to the following: (1) the scale effect at the dialysis center; (2) application of adjuvant PD - approximately 50% of elderly patients use adjuvant PD, such as home- or nursing home-supported PD, and fewer elderly patients forgo PD for medical reasons; or (3) palliative care for some patients, avoiding a switch to hemodialysis without adverse effects on cardiovascular stability, achieving both prolonged life and improved quality of life. While various factors were found to affect the patient survival rate of elderly ESKD patients, such as age, lower serum albumin, total cholesterol, higher serum calcium, creatinine levels, and comorbidities[16, 18, 28], the present study suggested that age, baseline blood prealbumin, creatinine and lack of activated vitamin D supplements are independent factors for death in PD patients, consistent with the findings of other studies.
Activated vitamin D deficiency is prevalent in ESKD patients, and inadequate activated vitamin D levels lead to secondary hyperparathyroidism, an essential indicator of end-stage kidney disease mineral and bone metabolism disorders (CKD-MBD). A study by Hannad et al. demonstrated that activated vitamin D levels were more significantly reduced in the PD group than in the hemodialysis group and that activated vitamin D supplementation reduced mortality and morbidity[29]. Makariou et al. showed that activated vitamin D deficiency was associated with PD peritonitis and peritoneal fibrosis[30]. An experimental animal study in the Netherlands found that activated vitamin D supplementation reduced the incidence of PD-related peritonitis[31]. The AUS study suggested that activated vitamin D supplementation reduces cardiovascular disease risk [32]. This study found that not taking activated vitamin D (p = 0.017, HR 1.353, 95% CI 0.963–1.899) had an independent influence on mortality in PD patients, with older PD patients taking activated vitamin D having a longer mean survival time. However, excessively activated vitamin D supplements increase risk of hypercalcemia and hyperphosphatemia, promoted metastatic calcification, and thus increased mortality and morbidity. Therefore, more studies are needed to explore the optimal safe dose of activated vitamin D in ESKD patients[33].
In our study, CCI was an independent risk factor for mortality in PD patients. This result is inconsistent with previous studies. Age and comorbidities have been proven to significantly impact the risk of death in elderly PD patients[34,35]. Comorbidities can reflect the severity of PD, which may lead to frequent hospitalization and death. In East Asia, studies have shown that diabetes and cardiovascular disease are risk factors for poor outcomes in elderly PD patients[35,36]. A French study also confirmed that CCI is a risk factor for mortality in elderly PD patients[37]. Recently, F Fabbian et al. reported that CCI is associated with in-hospital mortality in PD patients in Italy[38]. In conclusion, CCI has been well established as an independent risk factor for PD patients.
This study was a single-center retrospective cohort study with a limited sample size, and subsequent prospective studies with multicenter and large samples are needed to improve the long-term prognosis of elderly PD patients in China. Furthermore, our study did not collect frailty scores, which have been proven to be a risk factor for worse outcomes in PD subjects.
In conclusion, normal baseline prealbumin was found to be a protective factor against death in elderly PD patients (p = 0.028, HR = 0.03). Age, creatinine, PTH, no activated vitamin D intake, and CCI were risk factors (all p < 0.05, HR > 1). Multifactor Cox regression analysis of factors with p < 0.05 suggested that baseline preserum albumin was a protective factor against death in elderly PD patients (p = 0.002, HR = 0.031); older age, higher creatinine, and not being on vitamin D and CCI were also risk factors (p < 0.05, HR > 1).
Acknowledgments
This work was supported by grants from the Key Talent’s Subsidy Project in Science and Education, Suzhou, Jiangsu Province, China (KJXW2020017). The Department of Nephrology of the Second Affiliated Hospital of Soochow University funded this study. We thank all staff for their help.
Funding Statement
This work was supported by grants from the Key Talent’s Subsidy Project in Science and Education, Suzhou, Jiangsu Province, China (KJXW2020017).
Authors’ contributions
LSJ and ZW performed the study design. SF, YL, and YW collected the patient data. LYG and XFC were significant contributors to writing the manuscript. KS contributed to the data analysis. HYS contributed to the manuscript review. All the authors have read and approved the final manuscript.
Disclosure statement
Luyan Gao and Xuefeng Chen contributed equally to this article. Linsen Jiang and Zhi Wang are the corresponding authors. We certify that all authors have no financial or other conflicts of interest connected with the submitted article.
Ethical approval
The Second Affiliated Hospital Ethics Committee of Soochow University approved the study protocol. The ethics committee’s approval number is JD-HG-2022-19. This study was conducted under the tenets of the Declaration of Helsinki.
References
- 1.Moist LM, Fenton S, Kim JS, et al. Canadian organ replacement register (CORR): reflecting the past and embracing the future. Can J Kidney Health Dis. 2014;1:1. doi: 10.1186/s40697-014-0026-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donna LL, Niculae C, Paul R, et al. Clinical outcomes, quality of life, and costs in the North thames dialysis study of elderly people on dialysis: a prospective cohort study. The Lancet. 2000;356(9241):1543–9. [DOI] [PubMed] [Google Scholar]
- 3.Wang F, Yang C, Long J, et al. Executive summary for the 2015 annual data report of the China kidney disease network (CK-NET). Kidney Int. 2019;95(3):501–505. doi: 10.1016/j.kint.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Brown EA, Johansson L, Farrington K, et al. Broadening options for long-term dialysis in the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25(11):3755–3763. doi: 10.1093/ndt/gfq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perl J, Wald R, McFarlane P, et al. Hemodialysis vascular access modifies the association between dialysis modality and survival.[J]. Journal of the American Society of Nephrology: JASN. 2011;22(6):1113–1121. doi: 10.1681/ASN.2010111155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masud A, Costanzo E, Zuckerman R, et al. The complications of vascular access in hemodialysis. Semin Thromb Hemost. 2018;44(1):57–59. doi: 10.1055/s-0037-1606180. [DOI] [PubMed] [Google Scholar]
- 7.Juergensen E, Wuerth D, Finkelstein SH, et al. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol. 2006;1(6):1191–1196. doi: 10.2215/CJN.01220406. [DOI] [PubMed] [Google Scholar]
- 8.Li PK, Chow KM.. How to have a successful peritoneal dialysis program. Perit Dial Int. 2003;23(2_suppl):183–187. doi: 10.1177/089686080302302s38. [DOI] [PubMed] [Google Scholar]
- 9.Collins AJ, Hao W, Xia H, et al. Mortality risks of peritoneal dialysis and hemodialysis. Am J Kidney Dis. 1999;34(6):1065–1074. doi: 10.1016/S0272-6386(99)70012-0. [DOI] [PubMed] [Google Scholar]
- 10.Buemi M, Lacquaniti A, Bolignano D, et al. Dialysis and the elderly: an underestimated problem. Kidney Blood Press Res. 2008;31(5):330–336. doi: 10.1159/000164277. [DOI] [PubMed] [Google Scholar]
- 11.Zazzeroni L, Pasquinelli G, Nanni E, et al. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and Meta-Analysis. Kidney Blood Press Res. 2017;42(4):717–727. doi: 10.1159/000484115. [DOI] [PubMed] [Google Scholar]
- 12.Al Wakeel J, Al Harbi A, Bayoumi M, et al. Quality of life in hemodialysis and peritoneal dialysis patients in Saudi Arabia. Ann Saudi Med. 2012;32(6):570–574. doi: 10.5144/0256-4947.2012.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wachterman MW, Marcantonio ER, Davis RB, et al. Relationship between the prognostic expectations of seriously ill patients undergoing hemodialysis and their nephrologists. JAMA Intern Med. 2013;173(13):1206–1214. doi: 10.1001/jamainternmed.2013.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo E, Moody H, Hamilton T, et al. Choice of treatment improves quality of life. A study on patients undergoing dialysis. Arch Intern Med. 1997;157(12):1352–1356. doi: 10.1001/archinte.1997.00440330088011. [DOI] [PubMed] [Google Scholar]
- 15.Couchoud C, Moranne O, Frimat L, et al. Associations between comorbidities, treatment choice and outcome in the elderly with end-stage renal disease. Nephrol Dial Transplant. 2007;22(11):3246–3254. doi: 10.1093/ndt/gfm400. [DOI] [PubMed] [Google Scholar]
- 16.Yang X, Fang W, Kothari J, et al. Clinical outcomes of elderly patients undergoing chronic peritoneal dialysis: experiences from one center and a review of the literature. Int Urol Nephrol. 2007;39(4):1295–1302. doi: 10.1007/s11255-007-9279-6. [DOI] [PubMed] [Google Scholar]
- 17.Li PK, Szeto C.. Success of the peritoneal dialysis programme in Hong Kong. Nephrol Dial Transplant. 2008;23(5):1475–1478. doi: 10.1093/ndt/gfn068. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Wang H, Wang Y, et al. Long term survival of elderly peritoneal dialysis patients. Chinese Journal of Nephropathy. 2017;33(01):1–7. [Google Scholar]
- 19.Sandrine G, Nicolas M, François C, et al. Prognostic survival factors in elderly renal failure patients treated with peritoneal dialysis: a Nine-Year retrospective study. Peritoneal Dialysis International. 2010;30(2):218–226. [DOI] [PubMed] [Google Scholar]
- 20.van Esch S, Krediet RT, Struijk DG.. 32 Years’ experience of peritoneal Dialysis-Related peritonitis in a university hospital. Perit Dial Int. 2014;34(2):162–170. doi: 10.3747/pdi.2013.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuire AL, Carson CF, Inglis TJJ, et al. Effects of a statewide protocol for the management of peritoneal Dialysis-Related peritonitis on microbial profiles and antimicrobial susceptibilities: a retrospective Five-Year review. Perit Dial Int. 2015;35(7):722–728. doi: 10.3747/pdi.2014.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Portolés J, Vega A, Lacoba E, et al. Is peritoneal dialysis suitable technique CKD patients over 65 years? A prospective multicenter study. Nefrología. 2021;41(5):529–538. doi: 10.1016/j.nefro.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Williams ME. Diabetic CKD/ESRD 2010: a progress report? Seminars in Dialysis. 2010;23(2):129–133. doi: 10.1111/j.1525-139X.2009.00698.x. [DOI] [PubMed] [Google Scholar]
- 24.Iseki K. Predictors of diabetic end-stage renal disease in Japan. Nephrology. 2005;10(s2):S2–S6. doi: 10.1111/j.1440-1797.2005.00447.x. [DOI] [PubMed] [Google Scholar]
- 25.Li PK, Law MC, Chow KM, et al. Good patient and technique survival in elderly patients on continuous ambulatory peritoneal dialysis. Perit Dial Int. 2007;27(2_suppl):196–201. doi: 10.1177/089686080702702s34. [DOI] [PubMed] [Google Scholar]
- 26.Joshi U, Guo Q, Yi C, et al. Clinical outcome in elderly patients on chronic peritoneal dialysis: a retrospective study from a single center in China. Perit Dial Int. 2014;34(3):299–307. doi: 10.3747/pdi.2012.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fredric AF, Michael S, Christina WC, et al. Initiatives in peritoneal dialysis: where do We go from here? Peritoneal Dialysis International. 1991;11(3):274–278. [PubMed] [Google Scholar]
- 28.Sipahioglu MH, Aybal A, Unal A, et al. Patient and technique survival and factors affecting mortality on peritoneal dialysis in Turkey: 12 years’ experience in a single center. Perit Dial Int. 2008;28(3):238–245. doi: 10.1177/089686080802800309. [DOI] [PubMed] [Google Scholar]
- 29.Hanna K, Fassett RG, Gill E, et al. Serum 25-hydroxy vitamin D concentrations are more deficient/insufficient in peritoneal dialysis than haemodialysis patients in a sunny climate. J Hum Nutr Diet. 2015;28(3):209–218. doi: 10.1111/jhn.12234. [DOI] [PubMed] [Google Scholar]
- 30.Makariou S, Liberopoulos EN, Elisaf M, et al. Novel roles of vitamin D in disease: what is new in 2011? Eur J Intern Med. 2011;22(4):355–362. doi: 10.1016/j.ejim.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Stavenuiter AWD, Farhat K, Vila Cuenca M, et al. Protective effects of paricalcitol on peritoneal remodeling during peritoneal dialysis. Biomed Res Int. 2015;2015:1–12. doi: 10.1155/2015/468574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watson KE, Abrolat M, L Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation (New York, N.Y.). 1997;96(6):1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 33.Fortier C, Mac-Way F, De Serres SA, et al. Active vitamin D and accelerated progression of aortic stiffness in hemodialysis patients: a longitudinal observational study. Am J Hypertens. 2014;27(11):1346–1354. doi: 10.1093/ajh/hpu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castrale C, Evans D, Verger C, et al. Peritoneal dialysis in elderly patients: report from the french peritoneal dialysis registry (RDPLF). Nephrol Dial Transplant. 2010;25(1):255–262. doi: 10.1093/ndt/gfp375. [DOI] [PubMed] [Google Scholar]
- 35.Abe M, Hamano T, Hoshino J, et al. Predictors of outcomes in patients on peritoneal dialysis: a 2-year nationwide cohort study. Sci Rep. 2019;9(1):3967. doi: 10.1038/s41598-019-40692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fang W, Qian J, Lin A, et al. Comparison of peritoneal dialysis practice patterns and outcomes between a Canadian and a chinese Centre. Nephrol Dial Transplant. 2008;23(12):4021–4028. doi: 10.1093/ndt/gfn372. [DOI] [PubMed] [Google Scholar]
- 37.Genestier S, Meyer N, Chantrel F, et al. Prognostic survival factors in elderly renal failure patients treated with peritoneal dialysis: a nine-year retrospective study. Perit Dial Int. 2010;30(2):218–226. doi: 10.3747/pdi.2009.00043. [DOI] [PubMed] [Google Scholar]
- 38.Fabbian 1, A De Giorgi F, Ferrara F, Alfano G, et al. Comorbidity and in-hospital mortality in peritoneal dialysis patients: data of the emilia romagna region of Italy. Eur Rev Med Pharmacol Sci. 2023;27(14):6867–6875. [DOI] [PubMed] [Google Scholar]