ABSTRACT
IL-17 immune responses in cancer are controversial, with both tumor-promoting and tumor-repressing effects observed. To clarify the role of IL-17 signaling in cancer progression, we used syngeneic tumor models from different tissue origins. We found that deficiencies in host IL-17RA or IL-17A/F expression had varying effects on the in vivo growth of different solid tumors including melanoma, sarcoma, lymphoma, and leukemia. In each tumor type, the absence of IL-17 led to changes in the expression of mediators associated with inflammation and metastasis in the tumor microenvironment. Furthermore, IL-17 signaling deficiencies in the hosts resulted in decreased anti-tumor CD8+ T cell immunity and caused tumor-specific changes in several lymphoid cell populations. Our findings were associated with distinct patterns of IL-17A/F cytokine and receptor subunit expression in the injected tumor cell lines. These patterns affected tumor cell responsiveness to IL-17 and downstream intracellular signaling, leading to divergent effects on cancer progression. Additionally, we identified IL-17RC as a critical determinant of the IL-17-mediated response in tumor cells and a potential biomarker for IL-17 signaling effects in tumor progression. Our study offers insight into the molecular mechanisms underlying IL-17 activities in cancer and lays the groundwork for developing personalized immunotherapies.
KEYWORDS: Angiogenesis, CD8+ lymphocytes, IL-17RC, inflammation, interleukin 17 (IL-17), tumor immunology
Introduction
The interleukin 17 (IL-17) family is composed of six cytokines, with IL-17A and IL-17F being the most extensively studied. These cytokines are produced by a range of immune cells and can promote inflammation by inducing cytokines, chemokines, growth factors, metalloproteinases (MMP), and antimicrobial peptides.1 Consequently, they have been associated with both the resolution of infections and the promotion of autoimmune diseases.2 In cancer, IL-17A has been detected in serum and tumor-associated fluids, while IL-17-producing cells have been identified in blood, lymphoid organs, and tumor tissues from various human and experimental cancer types.3,4 However, despite intense research in recent years, the role of IL-17 in cancer is still unclear, with conflicting reports of both tumor-promoting and tumor-repressing effects.5
IL-17 cytokines mediate their effects through surface receptors composed of two or three subunits of the IL-17 receptor (IL-17 R) family.2 IL-17RA is a common subunit and forms a canonical receptor complex with IL-17RC that mediates signaling by IL-17A, IL-17F, and IL-17A/F.6 However, IL-17RA can also complex with IL-17RD to allow signaling by IL-17A but not IL-17F,7,8 triggering unique gene regulation compared to the canonical complex.9 IL-17RC can also form homodimeric complexes for IL-17RA-independent IL-17 signaling.10 These different receptor complexes and particular cytokine/receptor affinities may determine the response to IL-17 cytokines, leading to variations in the impact of this pathway on cancer.
IL-17A has been associated with poor outcomes in human cancers, while Th17 cells have been linked to better prognosis, though this varies depending on the tumor type.11 IL-17A can promote tumor growth by directly affecting cancer cells and indirectly modulating stromal and immune cells in the tumor microenvironment (reviewed in12). For example, IL-17A promotes self-renewal, therapy resistance, and survival in cancer cells of various origins. Additionally, IL-17A induces the expression of pro-angiogenic factors and MMPs in tumor cells and surrounding tissues, leading to angiogenesis, cell invasion, and metastasis. IL-17A can also reshape the tumor immune infiltrates by recruiting myeloid cells that support immunosuppression and tumor progression. Despite the potential role of IL-17A in tumorigenesis, studies have shown that IL-17A and IL-17-producing cells can enhance anti-tumor immunity by recruiting and activating effector immune cell subsets (reviewed in4). For instance, IL-17A recruits dendritic cells and neutrophils, leading to enhanced anti-tumor CD8+ T cell immunity in specific cancer settings. IL-17A also promotes CXCL9 and CXCL10 production, recruiting effector T cells and NK cells and sustaining Th1-type responses. Additionally, IL-17A induced class I and II MHC molecules in Meth-A fibrosarcoma cells, promoting tumor cell elimination. These findings suggest that the role of IL-17A in established tumors varies depending on the tumor-specific context and tumor cell responsiveness to IL-17.5 Therefore, the IL-17/IL-17 R pathway may be a potential immunotherapeutic target for cancer3,12
We investigated the impact of IL-17/IL-17 R signaling on cancer progression using syngeneic tumor models from different tissue origins. Our study analyzed the effects of IL-17A/F:IL-17RA signaling in host cells on tumor growth and immunity, observing contrasting effects on different tumor types. We found that deficiencies in host IL-17RA or IL-17A/F expression resulted in changes in the expression pattern of several mediators within the tumor microenvironment in a tumor-type-specific manner, impacting the anti-tumor CD8+ T cell response and the recruitment of lymphoid cell populations. The tumor cell responsiveness to IL-17 was determined by the expression pattern of cytokines and receptor subunits of the IL-17 family present in the injected cell lines. Notably, we identified IL-17RC as an important determinant of the IL-17-mediated transcriptional response in tumor cells, making it a potential predictive biomarker for the overall effect of IL-17 signaling in tumor progression. These findings provide insights into the molecular mechanisms underlying the divergent activities of IL-17 in cancer and have important implications for developing personalized immunotherapies.
Material and methods
Cell lines
The B16.SIY (melanoma), MC57.SIY (fibrosarcoma), C1498.SIY (leukemia), and EL4.SIY (lymphoma) cell lines were previously modified to express a GFP-SIYRYYGL fusion protein.13–15 These cell lines were kindly provided by Dr. Thomas Gajewski. MC38 cells were provided by Dr. Clothilde Théry. Homogeneous GFP-positive cells were selected by cell sorting and periodically tested for mycoplasma contamination. When required, cells were cultured in triplicate with IL-17A and IL-17F (200 ng/mL) under serum-reduced conditions. For blocking experiments, anti-IL-17RC or control IgG goat (5 μg/mL) was added.16
Murine tumor models
C57BL/6 WT, IL-17RA KO, and IL-17A and IL-17F double knockout (IL-17A/F DKO) mice (both sexes, 6–12 weeks-old) were injected subcutaneously (s.c.) in the right flank with 2 × 106 B16.SIY (melanoma), MC57.SIY (fibrosarcoma), and C1498.SIY (leukemia) cells or 0.5 × 106 EL4.SIY (lymphoma) cells. Experimental protocols with laboratory animals were approved by the IACUC (RD732/18).
Tumor harvest, immunofluorescence, and flow cytometry
Tumors were collected at the specified time point for subsequent analyses. For B16.SIY and EL4.SIY tumors, the harvest time (17–18 days post-injection) was chosen based on previous studies15,17,18 to enable the development of tumor-specific CD8+ T cell responses. Immunofluorescence and flow cytometry studies were performed according to standard procedures described in detail in Supplementary Materials.
Angiogenesis and inflammation related protein array
To evaluate angiogenesis-related proteins, tumor lysates were prepared and quantified using the Bradford protein assay and probed in the Proteome Profiler Mouse Angiogenesis Array Kit (ARY015, R&D). Films were scanned and analyzed using NIH ImageJ software. The Excel spreadsheet and ClustVis tool were used to generate heat maps illustrating relative protein quantities. Enrichment analysis of KEGG pathways was conducted using EnrichR for upregulated and downregulated proteins in each condition.19
Transcript quantification
RNA was extracted from the samples using Tri-Reagent (Sigma). Quality and concentration were assessed by spectrophotometry at 260 nm and by 260/280 ratio. cDNA was synthesized using the M-MLV retrotranscriptase kit (M1701, Promega) and random primers (B070–40, Biodynamics). Real-time qPCR was performed on pooled samples from two to three replicates with TaqMan probes targeting specific transcripts and 18S as endogenous control on the StepOnePlus Real-Time PCR System (Supplementary Table S5). The results were analyzed using the comparative CT (ΔΔCT) method, and fold change of relative expression was represented as heat maps using the Excel spreadsheet and ClustVis tool.
Western blots
Whole cell and nuclear extracts were obtained using RIPA or nuclear extraction buffers containing protease (Complete Mini EDTA-free; Roche) and phosphatase inhibitors (HaltTM Phosphatase Inhibitor Cocktail, Thermo Fisher Scientific). Protein concentration was measured using the Bradford assay and analyzed with SDS-PAGE gel. Membranes were incubated with specific primary and the appropriate fluorophore-coupled secondary antibodies (Supplementary Table S4). Blots were revealed using the Odyssey Infrared Imaging System (LI-COR Biosciences) at 700–800 nm. Densitometric analysis was performed using ImageJ software.
RNA sequencing
Cells were lysed using TCL buffer (Qiagen) containing 1% β-mercaptoethanol, and RNA was extracted using the Single-Cell RNA Purification Kit (Norgen) with RNase-Free DNase Set (Qiagen) treatment. RNA quality was assessed using the Agilent RNA 6000 Pico Kit (RIN > 8), and retrotranscription was performed with the SMART-Seq v4 Ultra Low Input RNA Kit for Sequencing. Illumina-compatible libraries were generated using the NextEra XT Preparation Kit and sequenced using the Illumina Novaseq 6000. The RNA-seq analysis pipeline is detailed in Supplementary Material. The data are available athttps://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA855854.
TCGA data analysis
mRNA expression data from 31 RNA-seq datasets belonging to The Cancer Genome Atlas (TCGA PanCancer Atlas) database were retrieved from cBioportal to investigate IL-17RA, IL-17RC, and IL-17RD gene expression (https://c-Bioportal.org - mRNA Expression, RSEM (Batch normalized) for each gene). Survival analysis was conducted using harmonized RNA-seq data from Skin cutaneous melanoma (SKCM, n = 472) and Acute Myeloid leukemia (LAML, n = 151) and overall survival data was defined as time to death (R TCGAbiolinks package). Patients were stratified into low- and high-expression groups using median gene expression levels. Kaplan–Meier curves were generated, and statistical significance was determined using the Log-rank test with the R survival package.20 A detailed summary of TCGA studies retrieved is provided in Supplementary Materials table S6.
Statistical analysis
Statistical tests (t-test, one-way/two-way ANOVA, and Kruskal-Wallis) were conducted using GraphPad Prism 8.0 software. Significance was defined as p < .05.
Results
Expression defects of IL-17RA and IL-17A/F in murine hosts result in distinct progression among syngeneic tumor models
To investigate the role of host IL-17 signaling in tumor progression, we conducted an extensive array of in vivo, ex vivo, and in vitro experiments (Figure 1a). First, we compared the growth of syngeneic murine models of melanoma (B16.SIY), fibrosarcoma (MC57.SIY), lymphoma (EL4.SIY), and leukemia (C1498.SIY) in wild-type (WT), IL-17RA KO, and IL-17A/F DKO mice. We observed that B16.SIY (melanoma) and MC57.SIY (fibrosarcoma) tumors showed increased volume in IL-17RA KO and IL-17A/F DKO mice (Figure 1b,c). Conversely, EL4.SIY (lymphoma) and C1498.SIY (leukemia) exhibited delayed progression and reduced tumor volume in deficient mice compared to the WT mice (Figure 1d,e). These findings provide insights into the complex and context-dependent effects of IL-17 signaling on tumor progression. Considering their distinct growth behaviors in the absence of IL-17 signaling (Supplemental Figure S1a,b), we selected B16.SIY (melanoma) and EL4.SIY (lymphoma) models for further analysis.
Figure 1.
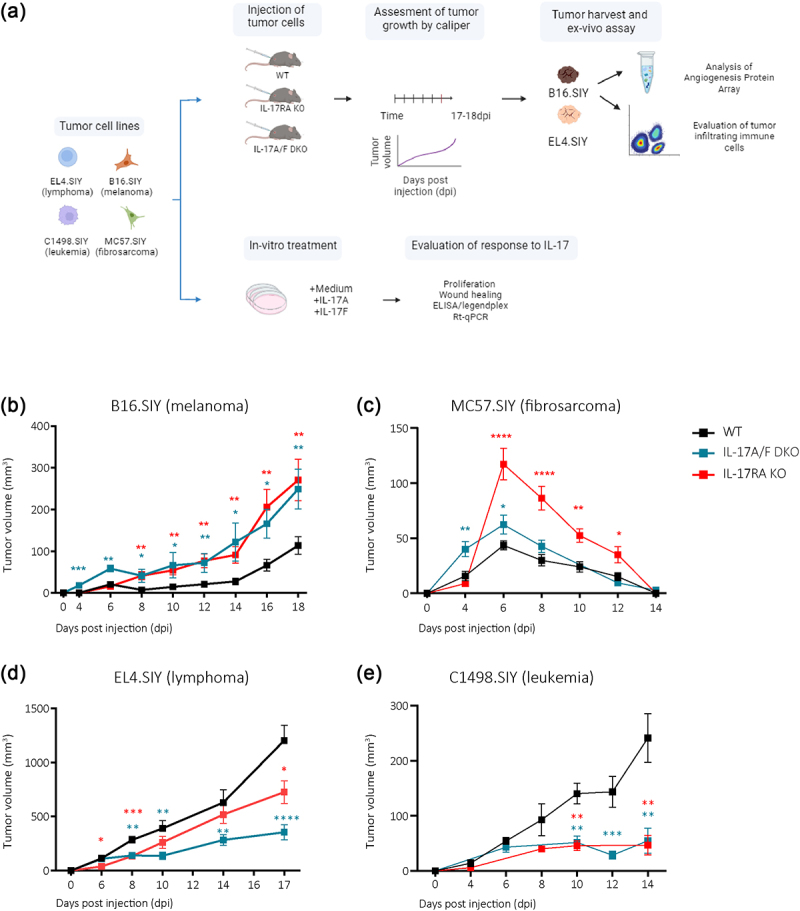
IL-17 signaling defects in hosts result in distinct tumor progression patterns.
(a) Schematic illustration of studies conducted using B16.SIY (melanoma), MC57.SIY (fibrosarcoma), EL4.SIY (lymphoma), or C1498.SIY (leukemia) cells. For in vivo and ex vivo analysis, cells were injected into the right flank of IL-17RA KO, IL-17A/F DKO, and WT mice. (b – e) Curves of the average tumor volume measured on different days post-injection. The red line represents IL-17RA KO mice, blue line represents IL-17 A/F DKO mice, and black line represents WT controls. P-values relative to WT were calculated by multiple t-test. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Host IL-17RA and IL-17A/F expression deficiencies have variable effects on inflammatory and angiogenic mediator expression in different tumors.
To investigate the underlying mechanisms of tumor growth in IL-17 defective murine hosts, we evaluated the expression of 53 inflammation and angiogenesis-related proteins in tumor lysates from B16.SIY melanomas and EL4.SIY lymphomas in WT, IL-17RA KO, and IL-17A/F DKO mice using a proteome profiler antibody array. Our analysis identified 27 proteins expressed in all tested conditions (Figure 2a and Supplementary Figure S1c), with 11 to 14 proteins that were significantly over or under-expressed in tumors from deficient IL-17 signaling hosts, compared to WT mice (Figure 2b). Notably, the changes in the expression of VEGF in B16.SIY tumors developed in WT, IL-17RA KO, and IL-17A/F DKO mice had no substantial impact on angiogenesis indicated by consistent vessel density assessed through CD31 staining (Supplementary Figure S1d).
Figure 2.
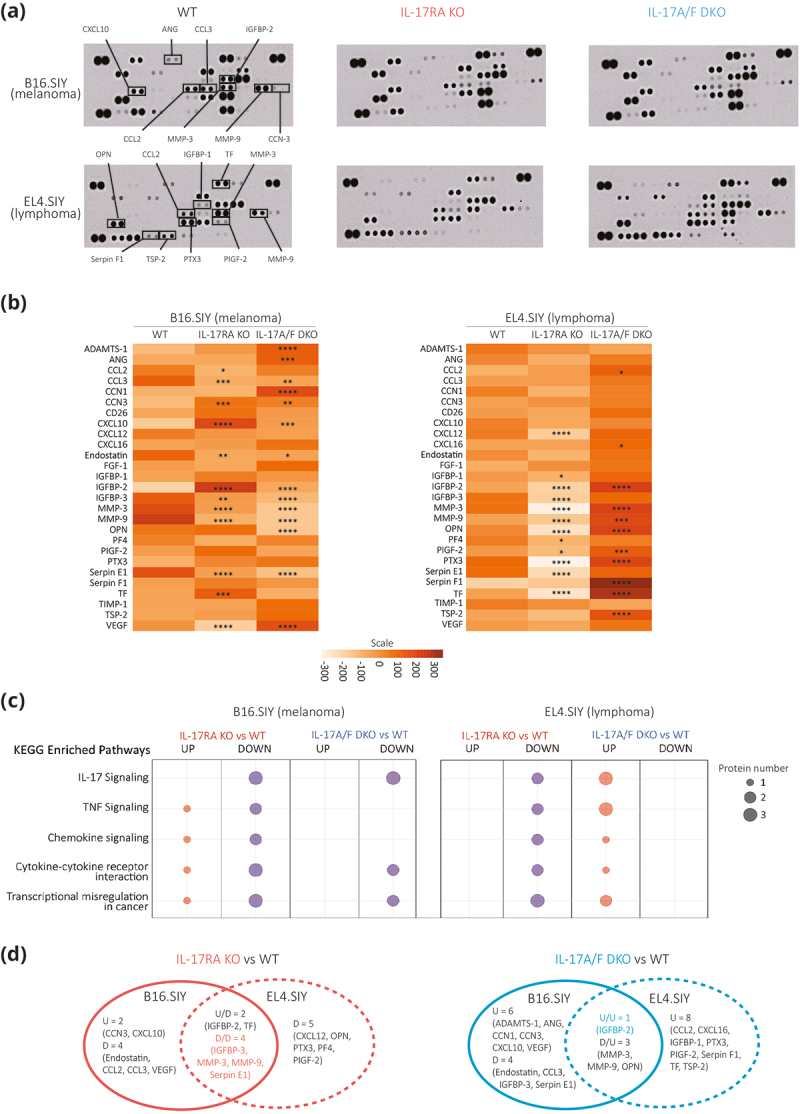
Defective host IL-17 signaling has variable impacts on tumor inflammation and angiogenesis protein expression depending on tumor type.
Mouse Angiogenesis Array Kit was used to assess the levels of tumor-related proteins in the lysates of B16.SIY melanomas and EL4.SIY lymphomas from WT, IL-17RA KO, and IL-17A/F DKO mice. (a) Array membranes after sample probing. Selected proteins of interest are annotated. (b) Heat maps showing the pixel intensity of the 27 proteins found in the lysates of either B16.SIY melanomas (left) or EL4.SIY lymphomas (right) developed in IL-17RA KO and IL-17A/F DKO mice compared to WT mice. (c) Bubble chart of significantly enriched KEGG pathways of the proteins that were over or under expressed in the B16.SIY melanomas and EL4.SIY lymphomas developed in IL-17RA KO or IL-17A/F DKO mice compared to WT mice. (d) Venn diagrams show differentially expressed proteins in B16.SIY versus EL4.SIY tumor lysates developed in IL-17RA KO compared to WT mice and IL-17A/F DKO mice compared to WT mice. P values are calculated with two-way ANOVA followed by Dunnett’s multiple comparison. *p < .05; **p < .01; ***p < .001; ****p < .0001.
Further KEGG pathway enrichment analysis revealed five biologically relevant pathways containing one to three over or under-expressed proteins in the analyzed combinations (Figure 2c). Few shared changes in protein expression were observed in B16.SIY melanomas and EL4.SIY lymphomas from deficient IL-17 signaling hosts, including downregulation of IGFBP-3, MMP-3, MMP-9, and Serpin E1, and up-regulation of IGFBP-2 (Figure 2d). Collectively, these findings indicate that IL-17 signaling can influence the expression of multiple proteins in the tumor microenvironment, varying with the tumor type and, likely, leading to distinct outcomes in tumor progression.
Defective host IL-17/IL-17RA pathway compromises anti-tumor CD8+ T cell immunity irrespective of tumor type and progression
Since immune components may play pro- and anti-tumor roles, we compared the tumor immune infiltrate in B16.SIY melanomas versus EL4.SIY lymphomas from WT hosts. We found that melanoma exhibited a higher frequency of CD45+ cells (Figure 3a). Then, we compared CD45+ tumor infiltrating cell composition with multiparametric flow cytometry followed by unsupervised analysis. We identified 10 cell clusters (P0-P9) (Figure 3b and Supplementary Figure S2a) and significant differences in the frequency of certain infiltrating subpopulations among both tumor models, with B16.SIY presenting an increased infiltration of neutrophil-like cells (P8) and a reduced presence of NK (P7) and CD4+ T cells (P3) compared to EL4.SIY lymphomas (Figure 3c).
Figure 3.
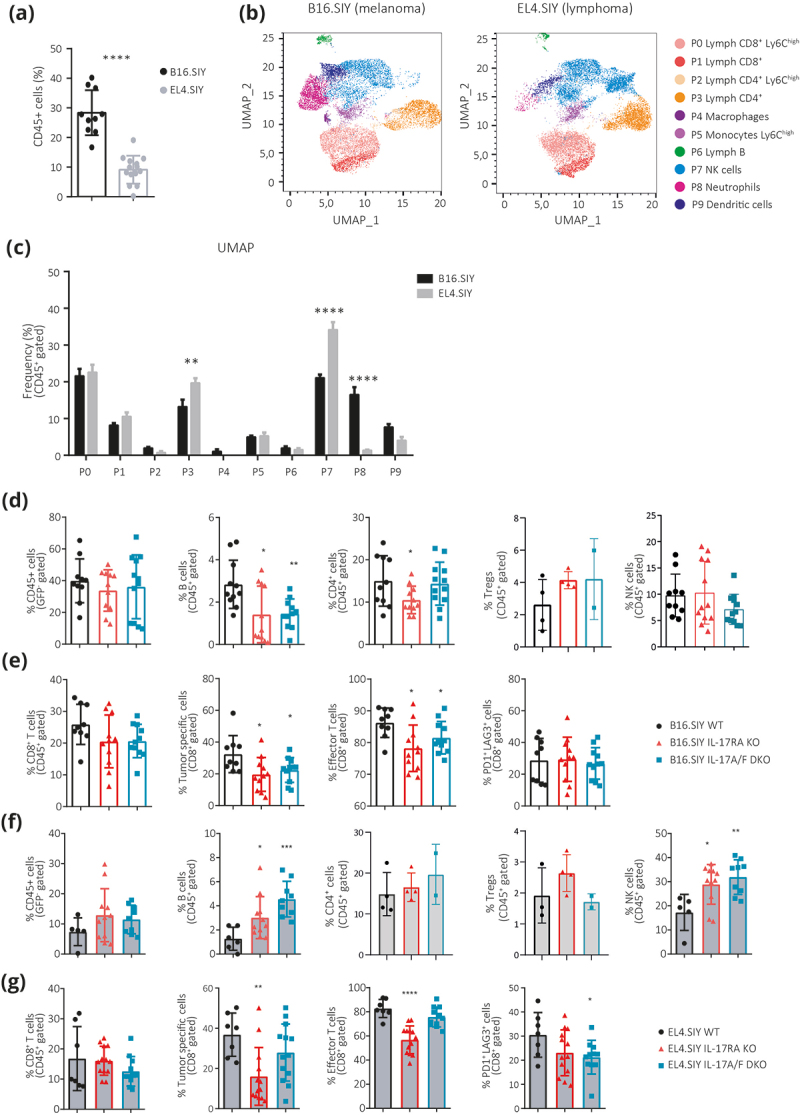
Defective host IL-17 signaling affects tumor-specific CD8+ T cell immunity and alters tumor-infiltrating subpopulations in a tumor type-dependent manner.
(a) Percentage of CD45+ cells within total live cells in B16.SIY melanomas and EL4.SIY lymphomas from WT mice collected at d18pi and d17pi, respectively. (b) UMAP projection of CD45+ cells evaluated in (a). Clusters (P0-P9) identified by FlowSOM are overlaid in the indicated colors. (c) Frequency of each cluster defined in (b) within B16.SIY melanoma (black) and EL4.SIY lymphomas (gray). (d – g) Frequency of the indicated immune subpopulations in B16.SIY melanomas and EL4.SIY lymphomas collected from WT, IL-17RA KO, and IL-17A/F DKO mice. P-values calculated by two-tailed Student’s t-test. *p < .05; **p < .01; ***p < .001; ****p < .0001.
We then investigated the impact of IL-17 signaling on immune cell infiltration in B16.SIY melanomas and EL4.SIY lymphomas (Supplementary Figure S2b). For B16.SIY melanomas, deficiencies in host IL-17RA and IL-17A/F expression did not alter CD45+ cell frequencies (Figure 3d) and had minor effects on myeloid cell subsets, including a reduction in monocytes and M-MDSC, while neutrophils, G-MDSC, and macrophages remained constant (Supplementary Figure S2c). Notably, lymphoid populations exhibited more pronounced changes in the absence of IL-17 signaling, with a decrease in B cells and CD4+ T cells, while Treg cell and NK cell frequencies were conserved (Figure 3d). Furthermore, deficient IL-17RA and IL-17A/F signaling resulted in decreased frequencies of tumor-specific and effector CD44highCD62Llow CD8+ T cells, while tumor-exhausted-like (PD1+Lag3+) CD8+ T cells were unaffected (Figure 3e). In EL4.SIY lymphomas, host IL-17RA and IL-17A/F deficiencies did not impact total CD45+ cell infiltration (Figure 3f). Subtle shifts occurred in myeloid cells, with a reduction in monocytes and an increase in M-MDSC and macrophages, while neutrophils and G-MDSC remained unchanged (Supplementary Figure S2d). Lymphoid compartment alterations showed increased B cell and NK cell frequencies, with no changes in CD4+ T cells and Tregs (Figure 3f). Although total CD8+ T cell infiltration in EL4.SIY lymphomas was not significantly different across strains, IL-17RA KO mice displayed reduced tumor-specific and effector CD8+ T cell infiltration, whereas exhausted-like CD8+ T cell frequencies were preserved (Figure 3g). Conversely, IL-17A/F DKO mice demonstrated consistent tumor-specific and effector CD8+ T cell percentages, alongside reduced exhausted CD8+ T cell frequencies. These differences may arise from complete IL-17 signaling impairment in IL-17RA KO mice, contrasting with partial compensation in IL-17A/F DKO mice, where the EL4.SIY lymphoma might counterbalance the absence of endogenous IL-17.
Collectively, our results indicate that host IL-17 signaling has varying effects on immune cell infiltration depending on the tumor type, and impaired IL-17 signaling decrease tumor-specific CD8+ T cell immunity without necessarily enhancing tumor progression.
Tumor cell lines express IL-17 cytokines and receptors in distinct patterns associated with specific gene signatures
Subsequently, we analyzed the expression of cytokines and receptor subunits of the IL-17A/IL-17RA pathway in tumor cells to investigate how it may dictate specific IL-17 effects. We found that only EL4.SIY (lymphoma) and C1498.SIY (leukemia) cells expressed Il17a transcripts, while Il17f mRNA was present in all four cell lines tested (Figure 4a). Additionally, Il17ra and Il17rd transcripts were detected in all cell lines, while Il17rc was limited to MC57.SIY (fibrosarcoma) and B16.SIY (melanoma) cells (Figure 4b). At the protein level, we validated these results and found that IL-17A as well as IL-17F production was limited to EL4.SIY (lymphoma) and C1498.SIY (leukemia) cells, despite Il17f mRNA being present in all evaluated cell lines (Figure 4c). Consistent with the transcriptional data, we observed the expression of IL-17RA and IL-17RD proteins in all cell lines, while IL-17RC was restricted to MC57.SIY and B16.SIY cells (Figures 4d,e).
Figure 4.
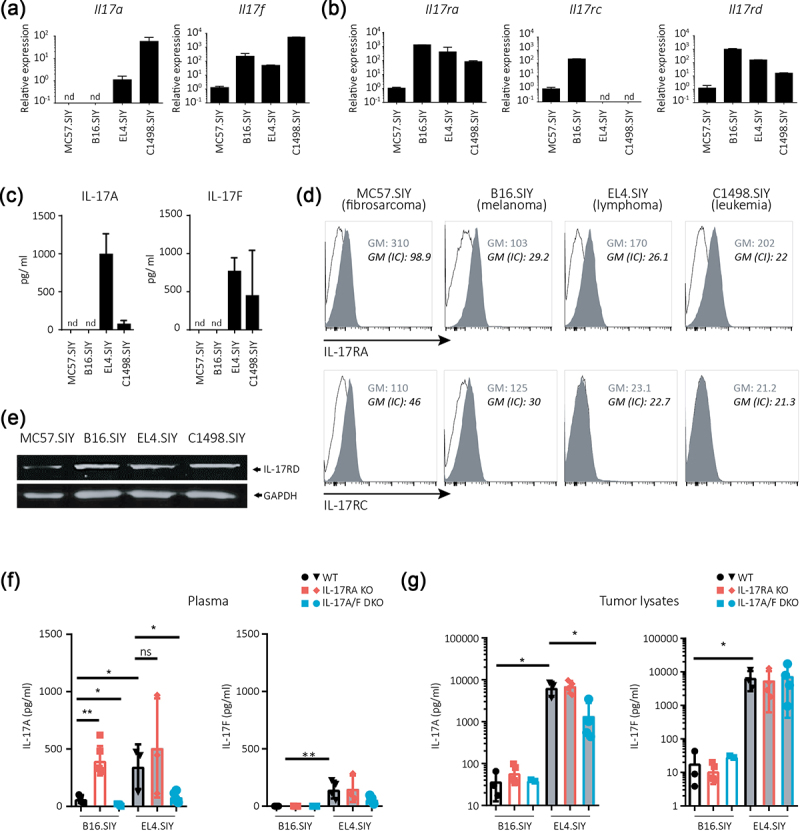
Expression profiles of IL-17A/F:RA pathway cytokines and receptors differ among cell lines and tumors of varying tissue origins.
(a,b) Relative amounts of Il17a and Il17f (a) and Il17ra, Il17rc, and Il17rd (b) transcripts in the indicated tumor cell lines. Transcript amounts determined by RT-qPCR were normalized to 18S and MC57.SIY transcript levels. (c) IL-17A and IL-17F concentrations in the culture supernatants of different tumor cells. (d) Representative histograms of IL-17RA and IL-17RC expressions in different tumor cells. Isotype control (IC) staining is shown in black. Geometric mean (GM) of fluorescence intensity for IL-17Rs and IC stainings are displayed. (e) IL-17RD expression determined by western blot in different tumor cells. (f,g) Concentrations of IL-17A and IL-17F in plasma (f) and tumor lysates (g) of B16.SIY (melanoma) and EL4.SIY (lymphoma) bearing WT, IL-17RA KO, and IL-17A/F DKO mice. In A-C, n = 3 replicates per group. nd: non-detectable. In f and g, p value calculated with one-way ANOVA. *p < .05; **p < .01; ***p < .001; ****p < .0001.
We also measured systemic and local IL-17 cytokine concentrations in WT, IL-17RA KO, and IL-17A/F DKO mice bearing B16.SIY melanomas and EL4.SIY lymphomas. Systemic levels of IL-17A were elevated in B16.SIY-bearing mice, but not in EL4.SIY-bearing mice lacking IL-17RA compared to their WT counterparts (Figure 4f). IL-17A/F DKO injected with B16.SIY (melanoma) or EL4.SIY (lymphoma) cells showed reduced plasma IL-17A concentrations compared to WT. Plasma IL-17F levels were undetectable in B16.SIY-bearing mice and low in all strains bearing EL4.SIY lymphomas. Local levels of IL-17A and IL-17F in tumor lysates were similar in all B16.SIY-bearing mice (Figure 4g). EL4.SIY lymphomas exhibited higher IL-17A and IL-17F concentrations than B16.SIY melanomas, but IL-17A level was lower in tumors from IL-17A/F DKO mice.
Afterwards, RNA sequencing was performed to evaluate the gene signature associated with the IL-17 pathway in EL4.SIY (lymphoma) and B16.SIY (melanoma) cells. Analysis of the differentially expressed genes showed substantial differences in gene expression between cell lines and confirmed the particular expression of molecules from the IL-17 pathway in tumor cells. Thus, Il17rc gene expression was higher in B16.SIY than in EL4.SIY (lymphoma) cells, while Il17a and Il17f transcripts were more abundant in EL4.SIY (lymphoma) cells (Supplementary Figure S3a). Moreover, examination of the 49 genes of the “IL-17 pathway” retrieved from the KEGG database revealed that 28 genes showed contrasting expression between B16.SIY (melanoma) and EL4.SIY (lymphoma) cells (Supplementary Figure S3b).
Altogether, these results underscore that cells from different tissue origins show different patterns of expression of cytokines and receptors of the IL-17A/IL-17RA pathway, paralleled with activation of particular IL-17 related gene signatures.
IL-17A stimulation elicits distinct in vitro responses in tumor cell lines
We investigated the effect of IL-17 stimulation on the four tumor cell lines by assessing changes in proliferation, migration, and secretion of cytokines and chemokines. Stimulation with IL-17A and IL-17F did not affect the cell count of three of the tumor cell lines, while MC57.SIY (fibrosarcoma) cells showed a reduction in cell numbers when exposed to IL-17A (Supplementary Figure S4a). Additionally, neither cytokine affected the migration capacity of adherent cell lines (Supplementary Figure S4b). However, the stimulation of B16.SIY (melanoma) and MC57.SIY (fibrosarcoma) cells with IL-17A and IL-17F induced the production of CXCL1, which was not observed in EL4.SIY (lymphoma) and C1498.SIY (leukemia) (Figure 5a). Furthermore, there was no significant inducible effect on the secretion of IL-1β, IL-6, TNF, or CXCL2 in any of the tumor cell lines (Supplementary Figure S4c).
Figure 5.
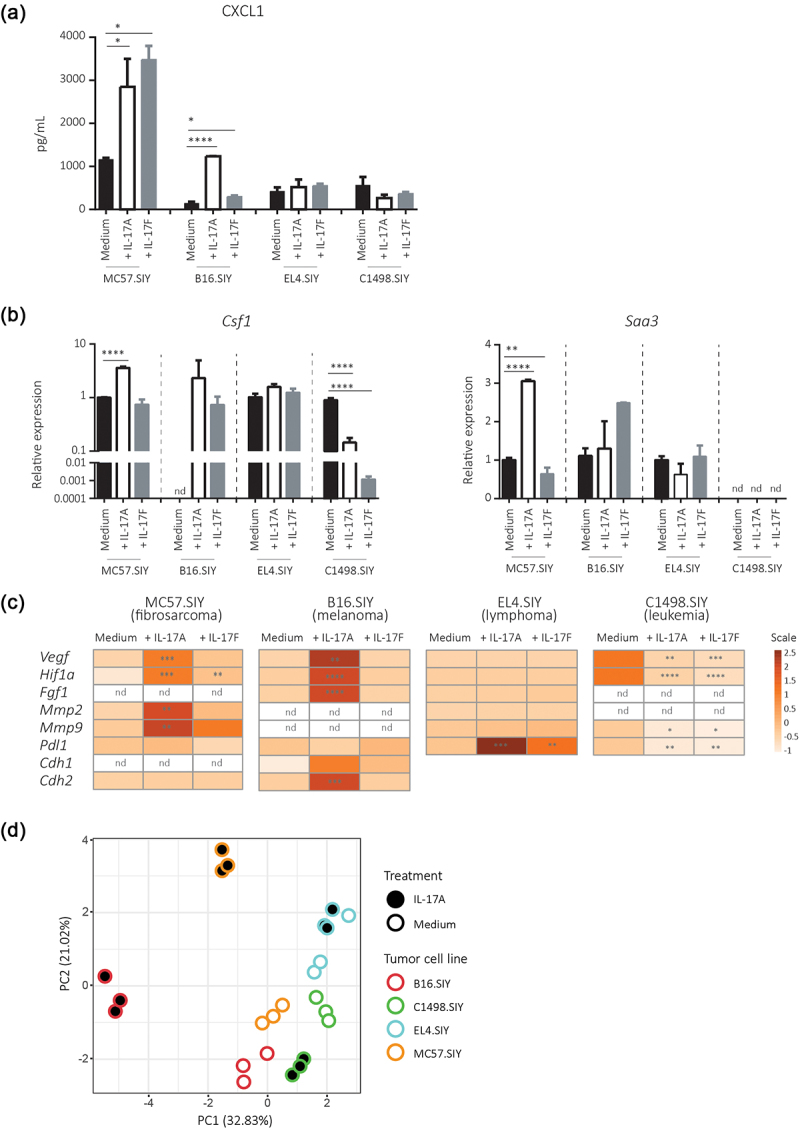
IL-17A and IL-17F distinctly modulates the expression of target and tumor progression genes in different tumor types in vitro.
IL-17A or IL-17F (200 ng/mL) were added to cultured B16.SIY (melanoma), MC57.SIY (fibrosarcoma), EL4.SIY (lymphoma), and C1498.SIY (leukemia) cells in serum-reduced conditions. (a) Concentration of CXCL1 measured in 48 h culture supernatants. (B) Relative amounts of Csf1 and Saa3 mRNA determined after 24 h of stimulation by RT-qPCR. (c) Heat maps summarizing the relative levels of Hif1a, Vegf, Mmp2, Mmp9, Fgf, Pdl1, E-cadherin (Cdh1), and N-cadherin (Cdh2) transcripts after 24 h of stimulation. Transcript amounts were normalized to 18S and medium transcript levels. P value calculated with One-way ANOVA. *p < .05; **p < .01; ***p < .001; ****p < .0001. (d) Scatter plot of tumor cell lines along the two principal components (54% of variance) obtained after principal component analysis of tumor cell lines according to the variables depicted in Figure 5a–5 and Supplementary Figure S4.
Then, we examined the transcriptional response of genes associated with an IL-17 gene signature.2 IL-17A stimulation increased Csf1 and/or Saa3 mRNA levels in B16.SIY (melanoma) and MC57.SIY (fibrosarcoma) cells, whereas IL-17F stimulation increased Csf1 in B16.SIY cells. Conversely, EL4.SIY (lymphoma) and C1498.SIY (leukemia) cells did not respond to or show reduced levels of these transcripts when stimulated with IL-17A and IL-17F (Figure 5b). IL-17A and/or IL-17F stimulation induced the transcription of Vegf, Hif1a, Mmp2, Mmp9, Cdh1, and/or Cdh2 in B16.SIY (melanoma) and MC57.SIY cells but had no effect or inhibited these genes in EL4.SIY (lymphoma) and C1498.SIY (leukemia) cells (Figure 5c). However, Pdl1 gene response was different; it was unchanged in B16.SIY (melanoma) and MC57.SIY (fibrosarcoma) cells but was induced in EL4.SIY (lymphoma) cells and inhibited in C1498.SIY (leukemia) cells upon stimulation with IL-17A and IL-17F. Our results suggest that IL-17A and IL-17F elicit different patterns of response in tumor cell lines likely based on their specific IL-17 R subunit expression profiles. Using an unsupervised approach, we conducted principal component analysis (PCA) on the responses to IL-17A stimulation to identify tumor cell lines exhibiting similar responses (Figure 5d). As anticipated, tumor cell lines in the medium condition clustered relatively close. Notably, both B16.SIY and MC57.SIY, following IL-17A treatment, exhibited a similar deviation from the medium condition along PC1 and PC2. In contrast, the response of EL4.SIY and C1498.SIY cells to IL-17 was more nuanced and diverse. These data reinforce the concept that IL-17A and IL-17F trigger distinct response patterns in tumor cell lines, likely influenced by their unique IL-17 R subunit expression profiles.
IL-17A induces cell-type specific responses with activation of canonical and non-canonical signaling pathways
To further explore the distinct response patterns to IL-17, we investigated the intracellular signaling pathway and gene expression changes in B16.SIY (melanoma) and EL4.SIY (lymphoma) cells upon IL-17 stimulation. In B16.SIY (melanoma) cells, IL-17A induced a weak and prolonged phosphorylation of the p65 NF-κB subunit and increased translocation of p65 to the nucleus, whereas EL4.SIY (lymphoma) cells did not respond significantly (Figure 6a). The basal phosphorylation of ERK and p38 in both cell types was unaffected by IL-17A (Supplementary Figure S5a). We then examined the differential gene expression program triggered by IL-17A in B16.SIY (melanoma) versus EL4.SIY (lymphoma) cells, evaluating their transcriptional profiles using RNA sequencing. We found 374 differentially expressed genes (DEGs) in B16.SIY (melanoma) cells and 540 DEGs in EL4.SIY (lymphoma) cells stimulated with IL-17A compared to non-stimulated cells. Only 12 genes were commonly modulated in both cell lines (Supplementary Figure S5b,c), while the DEGs unique to each cell line belonged to different gene categories (Figure 6b). Ingenuity Pathway Analysis (IPA) showed that IL-17A stimulation in B16.SIY (melanoma) cells modulated genes associated with top canonical pathways such as “Role of Cytokines in Mediating Communication between Immune Cells,” “Th1 and Th2 Activation Pathway,” and “IL-17 signaling” and associated to Inflammation (Figure 6c and Supplementary Figure S5d). Meanwhile, EL4.SIY (lymphoma) cells exhibited gene modulation in pathways related to cellular growth and cell-to-cell interactions like “Axonal Guidance Signaling” and “Synaptogenesis Signaling Pathway” and were associated with “Cancer.” Moreover, GSEA revealed that chemokine activity and ribosomal function were enriched in IL-17A-stimulated B16.SIY (melanoma) cells, while extracellular matrix constitution and cation channel activation were enriched in IL-17A-stimulated EL4.SIY (lymphoma) cells (Figure 6d). These results indicated that IL-17A induces significant transcriptional changes in both cell lines, but with distinct outcomes.
Figure 6.
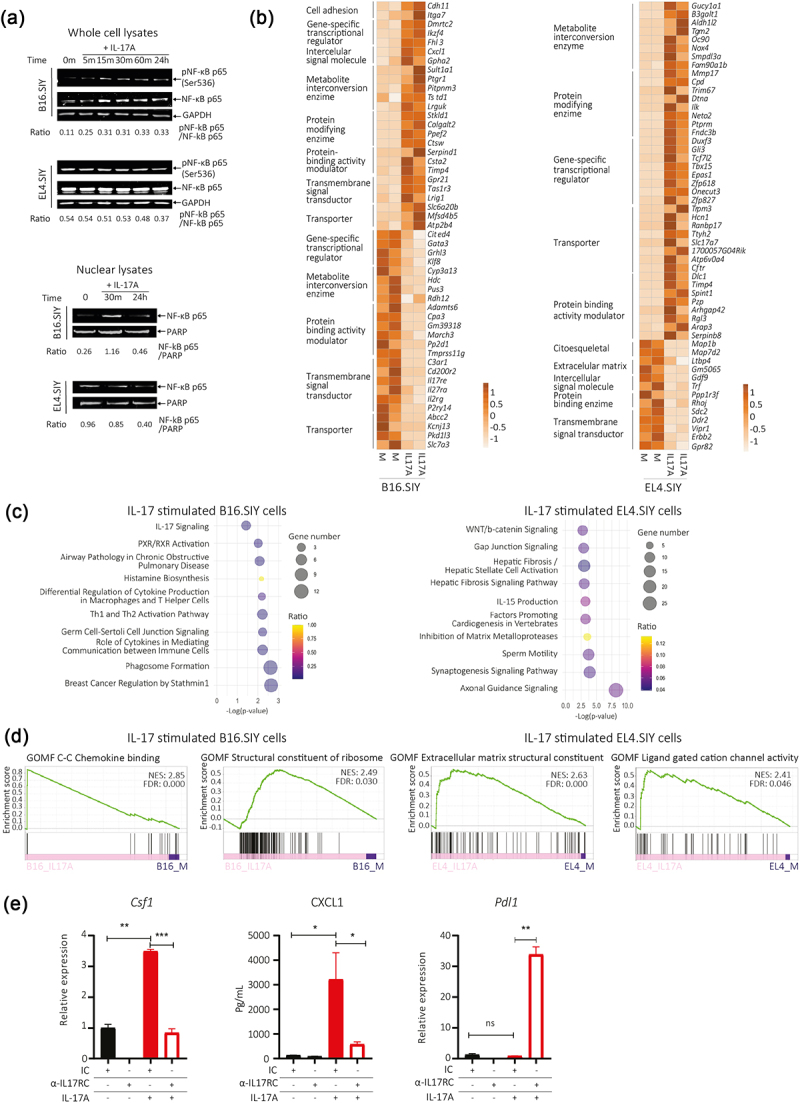
IL-17A induces cell-type specific signaling pathways and transcriptional responses influenced by IL-17RC expression.
(a) Expression of NF-κB p65 subunit in whole cell and nuclear lysates evaluated by western blot in B16.SIY (melanoma) and EL4.SIY (lymphoma) cells stimulated with IL-17A. (b – d) Analysis of RNA-seq performed in B16.SIY (melanoma) and EL4.SIY (lymphoma) cells stimulated or not with IL-17A (100 ng/mL) for 24 h. (b) Heat maps show top 50 genes modulated by IL-17 in B16.SIY (melanoma) or EL4.SIY (lymphoma) cells, grouped by biological function. (c) Bubble chart displays top enriched canonical pathways after IPA of DEGs induced in B16.SIY (melanoma) and EL4.SIY (lymphoma) cells by IL-17A stimulation. (d) GSEA plots show two top enriched pathways identified in the whole transcriptome of B16.SIY (melanoma) and EL4.SIY (lymphoma) stimulated cells, using the M5 (gene ontology sets) mouse collection from MSigDB. NES: normalized enrichment scores. FDR: false discovery rate. (e) Bar graph shows relative Csf1 and/or Pdl1 mRNA levels in cell lysates and CXCL1 concentration in culture supernatants of B16.SIY (melanoma) cultured with or without IL-17A and a blocking IL-17RC antibody or isotype control for 24 h under serum-reduced conditions. The results are shown as mean ± SD of three replicates for each condition, and the p-values were calculated by two-tailed unpaired t-test. ns indicates non-significant.
IL-17RC blockade alters B16.SIY cell response to IL-17.
Our findings suggested that IL-17RC expression may be a critical determinant of IL-17 effects in tumor cells. To investigate this further, B16.SIY (melanoma) cells were stimulated with IL-17A in the presence of a blocking IL-17RC antibody. IL-17RC blockade prevented Cfs1 upregulation and reduced CXCL1 secretion, while increasing Pdl1 transcript levels (Figure 6e). Comparable results were observed in MC38 cells, another IL-17-responsive cell line.21 Upon IL-17 stimulation, MC38 cells exhibited increased CXCL1 production without significant changes in Csf1 and Pdl1 transcript levels. Blockade of IL-17RC led to reduced CXCL1 induction, conserved Csf1 and increased Pdl1 transcript amounts (Supplementary Figure S5e). Altogether, these results highlight the essential role of IL-17RC signaling in activating classical IL-17A target genes and proteins. Notably, blocking IL-17RC did not completely eliminate the response to IL-17A, but rather switched it to a different set of genes, possibly due to signaling through IL-17RA and IL-17RD.
IL-17 R subunit expression profiles in human cancers are associated with varying clinical outcomes
Finally, we analyzed IL-17 R subunit expression in human tumors using data from TCGA, observing heterogeneity across different cancer types (Supplementary Figure S6a). To investigate the relevance of IL-17 signaling in human tumor progression, we compared the expression of IL-17 R subunits in patient samples from SKCM and LAML, two cancer types that shared tissue origins with B16.SIY (melanoma) and EL4.SIY (lymphoma) cells. SKCM samples had lower Il17ra but higher Il17rc and Il17rd expression compared to LAML samples (Figure 7a). In the SKCM cohort, higher IL-17RA expression was associated with longer survival (Figure 7b), while this correlation was not observed in the LAML cohort (Figure 7c). Finally, Il17rc or Il17rd expression levels in SKCM and LAML were not associated with differences in survival (Supplementary Figure S6b). These findings suggest that IL-17RA signaling is protective in tumors expressing high levels of IL-17RC (and IL-17RD), consistent with our experimental cancer models.
Figure 7.
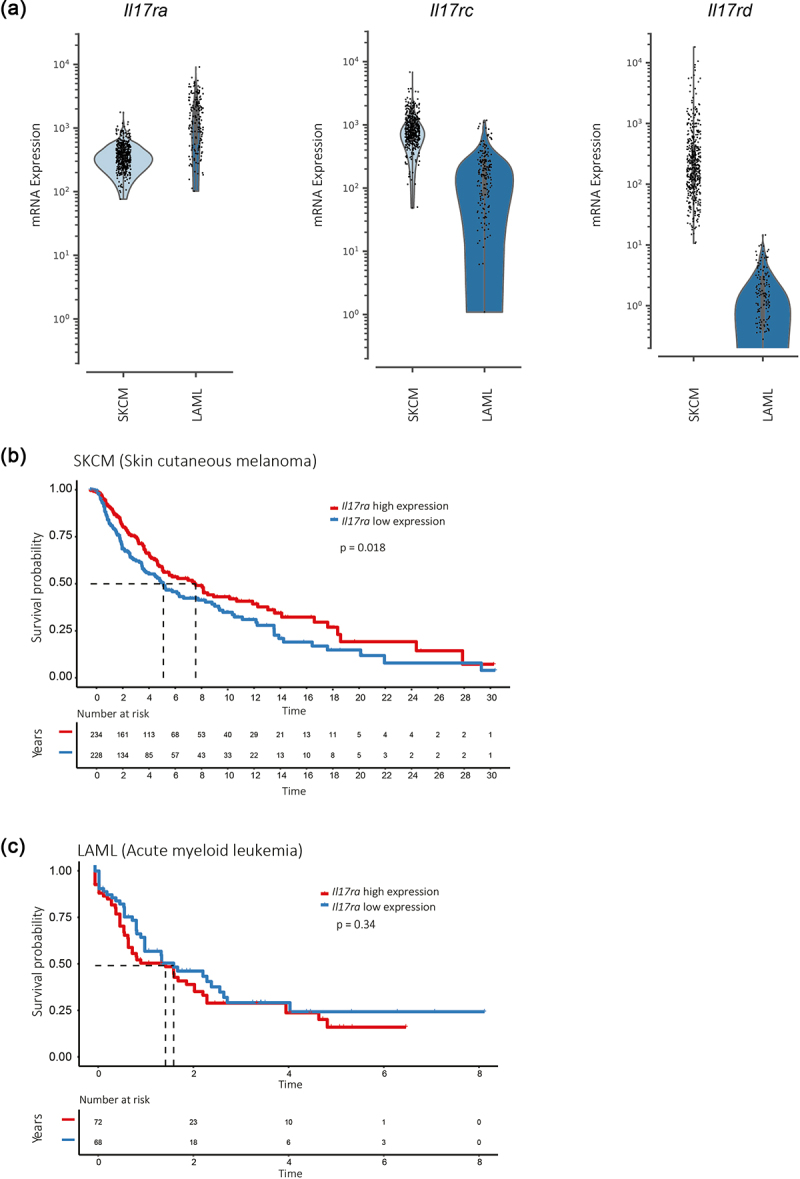
High IL-17RA expression correlates with increased survival probability in patients with skin cutaneous melanoma (SKCM) but not in patients with leukemia (LAML).
(a) mRNA levels of Il17ra, Il17rc, and Il17rd in tumor samples from SKCM and LAML patients. (b,c) Kaplan Meier curves of survival probability in patient cohorts with SKCM (b) and LAML (c) tumors based on high (red) and low (blue) expressions of the IL-17RA subunit. Data were obtained from the TCGA portal and analyzed with R package “survival”.
Discussion
IL-17 can have different impacts on cancer progression, making its role as a therapeutic target controversial.5,12 We found that mesenchymal/epithelial tumors like melanoma and fibrosarcoma exhibited increased progression in hosts with deficient IL-17 signaling. Our findings align with some studies that associated IL-17 with better control of murine melanoma,22–24 while others show that IL-17 promoted B16 (melanoma) tumor growth.25–27 We propose that the immunogenicity of the tumor may be the key factor in reconciling these contrasting observations. In fact, the studies that found a pro-tumor role for IL-17 used parental B16 (melanoma) cells, which generate tumors with relatively poor T cell infiltration and may have reduced immunogenicity.28,29 In contrast, tumors generated by the B16.SIY (melanoma) cell variant used in our work have a slower growth rate and may be more responsive to IL-17-mediated immune responses.14,17 Thus, in implanted tumor models, the expression of foreign antigens can increase immunogenicity and influence anti-tumor responses, potentially tipping the balance of IL-17 function toward tumor elimination.23,30–32 Also, IL-17 would stimulate the anti-cancer response and tumor elimination in immunocompetent mice, but promote angiogenesis and tumor growth in immunocompromised mice.32 A definitive understanding of the relevance of immunogenicity in the impact of IL-17 signaling on tumor growth may require a comprehensive comparison of cell lines with differing immunogenic profiles, such as the B16 variants (F1, F10, variants transfected with OVA, SIY, etc.). Although such a comparison is beyond the scope of the current manuscript, we determined that IL-17 signaling also facilitated the control of the MC57.SIY fibrosarcoma, previously linked to robust CD8+ T cell responses.13,33 This finding reinforces the fact that the presence of tumor-specific immune cells within the tumor microenvironment may be a critical requirement for IL-17 anti-tumor functions.
Conversely, deficiencies in the expression of IL-17RA and IL-17A/F in the host resulted in reduced growth of hematopoietic tumors such as the EL4.SIY lymphoma and the C1498.SIY leukemia. The IL-17A/F:RA pathway was shown to promote immunosuppressive tumor microenvironments and angiogenesis through stromal cell activation in EL4 tumors.34,35 Thus, the dominant IL-17 effector mechanisms in these settings may exacerbate pro-angiogenic and pro-tumorigenic pathways via direct signaling in the tumor microenvironment. In this direction, our proteomic data analysis revealed that host IL-17 signaling had either activating or inhibiting effects on proteins associated with cytokine and chemokine signaling, as well as transcriptional regulation in cancer, with significant differences observed between B16.SIY melanomas and EL4.SIY lymphomas. Although exploratory, our findings suggest that IL-17 signaling has a profound, tumor-specific impact on the tumor microenvironment. It is noteworthy that alterations in the expression of specific mediators may not consistently align with the anticipated biological effects. For instance, variations in VEGF or CXCL10 expression within B16.SIY melanomas from IL-17 signaling-deficient mice did not align with changes in vascular density or cellular infiltration, respectively. These discrepancies could be attributed to the intricate influence of IL-17 signaling on the expression of various mediators within the tumor microenvironment, potentially counteracting the impact of individual changes. In-depth proteomic investigations may be required to establish connections between the modulation of specific angiogenic and inflammatory proteins and the outcomes associated with deficient IL-17 signaling in hosts.
IL-17A has been reported to promote a cytotoxic microenvironment by developing and recruiting anti-tumor myeloid cells, antigen-presenting cells, and activated effector T cells, which restrict tumor growth.5,36 Also, this cytokine pathway may sustain NK cell and CD8+ T cell responses against infections.37–39 Conversely, IL-17 could facilitate a tumor-permissive environment by recruiting an immunosuppressive myeloid compartment and inducing regulatory molecules like PD-L1.34,40 We found that IL-17RA or IL-17A/F deficiency in murine hosts modulated lymphoid subsets rather than monocyte and neutrophil tumor infiltration. Thus, IL-17 signaling was critical for the infiltration of tumor-specific and effector CD8+ T cells in B16.SIY melanoma but it did not influence exhausted-like PD1 and LAG3 co-expressing CD8+ T cells. These findings contrast with previous research showing that, in the absence of CD4+ T cell help, Tc17 cells favored the generation of terminally exhausted CD8+ T cells, linking IL-17 to poorer tumor growth control in a murine model of metastatic melanoma.41 Depletion of CD4+ T cells may emerge as a source of divergence, as it is a widely reported approach to induce a high percentage of exhausted CD8+ T cells in models of chronic LCMV infections,42 and to improve anti-tumor response in certain cancer models likely by targeting regulatory T cells.43,44 Notably, reduced tumor-specific CD8+ T cells were also detected in EL4.SIY lymphomas developed in IL-17RA KO hosts. Altogether, our data suggest that the positive effect of IL-17 on CD8+ T cell immunity may be a general mechanism operating not only in infections38,39 but also in cancer, independent of the tumor type and not necessarily linked to better tumor progression.
Deficiencies in host IL-17 signaling also influenced the infiltration of NK and B cells in B16.SIY melanomas and EL4.SIY lymphomas with divergent outcomes. In agreement with our results with the EL4.SIY model, IL-17 signaling was reported to limit NK activity and promote MC38 and B16F10 tumor growth.45 However, this cytokine pathway does not affect NK cell infiltration in the B16.SIY model, and it was essential for the development of functional NK cells during a fungal infection.37 These contrasting results highlight that further research is required to decipher the role of NK cells in IL-17-mediated effector responses in different settings. Also, B cell infiltration was shown to be impacted by deficiencies in host IL-17A/F:RA pathway according to the tumor type. IL-17 was previously shown to influence the development and recruitment of B cells to the inflammatory site in an esophageal cancer model.46 Notably, studies have indicated that tumor-infiltrating B cells play a prominent role in tumor progression, with both beneficial and detrimental impacts.47 Given the relatively recent focus on understanding the role of B cells in tumor immunity, the potential link between IL-17, B cell responses, and tumor progression presents an exciting and promising area for further investigation. Overall, our findings may reorient the evaluation of the immune cell populations modulated by the IL-17 pathway in different cancer contexts, with a focus on lymphoid subsets.
When considering the impact of IL-17 signaling on tumor progression, it is essential to acknowledge a limitation in our study – our use of subcutaneous tumor models, which largely represent heterotopic locations for most of the studied tumor types. While orthotopic models may better mimic the tumor microenvironment and biological responses,48 subcutaneous models are favored due to practical benefits like easy implantation, monitoring, and enabling direct comparisons among various tumor models. Therefore, we selected these models to overcome the impact of differing implant locations and to focus on tumor cells, which due to their abundance, may crucially influence the IL-17-mediated permissiveness or restrictiveness of the microenvironment for tumor growth. Consistent with previous literature,49–52 we observed that non-hematopoietic tumor cells expressed IL-17RA, IL-17RC, and IL-17RD and did not produce IL-17A or IL-17F, while hematopoietic cell lines expressed only IL-17RA and IL-17RD and produced both cytokines. The differential expression of IL-17 R subunits among the tumor cell lines was biologically relevant, as it elicited distinct responses upon IL-17 stimulation. B16.SIY (melanoma) cells exhibited a conventional response with moderate phosphorylation and nuclear translocation of NF-kB, and showed a transcriptional signature associated with cytokine and chemokine activity, as well as ribosomal function, which is commonly associated with the IL-17 signature.53,54 On the other hand, IL-17 activated gene transcription in hematopoietic EL4.SIY (lymphoma) cells with a response characterized by the induction of cellular biological processes not typically associated with IL-17 functions but linked to tumor promotion due to their role in apoptosis inhibition, cell migration, and neural network formation.55–57 Furthermore, the gene sets enriched in EL4.SIY cells stimulated with IL-17 were associated with extracellular matrix dynamics and cation channel activity whose physiological function is to sustain cell proliferation and death, and their dysregulation is linked to cancer development and progression.58,59
Studies evaluating the role of IL-17 in cancer have primarily focused on the expression of IL-17RA or IL-17RC separately, with few exceptions systematically analyzing the relevance of the heterodimer35,60,61 or the influence of IL-17RD.62 The varied expression of IL-17 R subunits in different tumors may contribute to the dual role of IL-17 in cancer progression, as suggested by our findings on distinct signaling pathways and signatures. Interestingly, blocking IL-17RC affected the response to IL-17 stimulation in B16.SIY (melanoma) and MC38 cells, resulting in a similar immunoregulatory and potentially pro-tumor profile as that of EL4.SIY (lymphoma) cells. This implies that IL-17RC could play a pivotal role in dictating the response to IL-17 at a molecular level, though further research is necessary to determine whether these changes observed in vitro have a corresponding effect in vivo. For instance, IL-17RC silencing was found to change in vitro basal proliferation and in vivo tumor growth in a tumor-type dependent manner.63 These unique IL-17RC signaling functions were attributed to variations in the basal expression of downstream molecules involved in intracellular signaling and tumor cell proliferation rather than the IL-17RC expression levels. Moreover, the different signaling capacities of IL-17 cytokines provide another layer of regulation of the IL-17 pathway. IL-17F can signal through an IL-17RC/RC homodimer without the requirement of IL-17RA in humans.10 Additionally, the signaling of IL-17A, but not of IL-17F, through the IL-17RA/IL-17RD pathway is involved in the pathogenesis of psoriasis.9 Although the remarkably similar phenotypes in IL-17RA KO and IL-17A/F DKO mice suggest that IL-17A/F may be the cytokine predominantly underlying the observed outcomes, other cytokines of the family that signal through IL-17RA, such as IL-17C and IL-17E (IL-25),2 cannot be excluded as potential influencers of tumor progression in vivo. Our findings, along with reported results, suggest that the effects of IL-17 cytokines are context-dependent and can be influenced by various factors, such as the source of cytokines, the balance among different cytokines of the family and the profile of IL-17 R subunit expression by the tumor cells.
Differences in the expression and binding affinities of cytokines and receptors in the IL-17 pathway between mice and humans may limit the translation of experimental results to clinical settings.50 In humans, IL-17RA and IL-17RD are expressed in all tissues, while IL-17RC is expressed in certain tissues, including the liver, prostate, skin, colon, stomach, lung, and thyroid.50 Analysis of TCGA data showed significant variability in the expression of IL-17 R subunits across different cancer types. We correlated the subunit expression profiles with the effect of the IL-17 pathway on tumor progression, focusing on SKCM and LAML. Our findings suggest that differences in tumor IL-17RA expression levels correlate with differences in overall survival time in SKCM patients with high tumor IL-17RC expression but not in LAML patients with low tumor IL-17RC expression. Notably, recent studies suggest that deletion of IL-17RA or IL-17RC in the colon is associated with advanced stages and worse progression in colorectal cancer.64 Furthermore, during the revision process of this manuscript, a study revealed that the IL-17 gene expression signature in BRAFV600-mutated melanomas contributes to clinical benefits in immune checkpoint therapy by promoting T cell and neutrophil activation.65 In contrast, other studies have shown that higher levels of IL-17A are more frequently associated with a worse prognosis in cancer patients, although an association with improved survival has also been reported for tumors of different histological origins and the same tumor type.11 These findings underscore the need to study each cancer setting to position the IL-17 pathway as a putative target for personalized anti-tumor therapies.
Our findings, as summarized in Figure 8, suggest that the IL-17A/IL-17 R pathway plays a complex role in the tumor microenvironment, influencing various aspects of tumor development. This includes promoting the generation of effective CD8+ T cell responses and influencing NK and B cell responses that could aid in tumor control. However, IL-17A also triggers diverse responses in tumor cells, leading to inflammation and enhanced tumor growth. These distinct downstream pathways are associated with specific expression patterns of IL-17 R subunits, with IL-17RC identified as a crucial molecular determinant of the global pro- or anti-tumor effect of IL-17 signaling. Based on our work, we suggest that a comprehensive analysis of the expression profile of IL-17 R subunits and cytokines of this pathway could help define the potential usefulness of therapies aimed at modulating the IL-17 axis for personalized anti-tumor immunotherapies.
Figure 8.
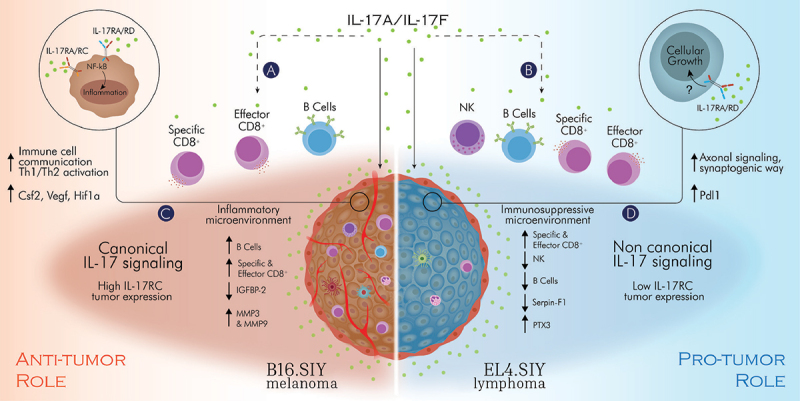
Proposed model of the role of the IL-17A/F:RA pathway in cancer.
The role of the IL-17A/F:RA pathway in cancer was investigated using syngeneic murine tumor models and IL-17 signaling deficient mice. Our results suggest contrasting effects of host IL-17 signaling on tumor growth, mediated by immune cell recruitment and specific anti-tumor cytotoxic CD8+ T responses (a,b), as well as IL-17 signaling in tumor cells (c,d) influencing tumor cell proliferation and the tumor microenvironment. Overall, IL-17 signaling in B16.SIY cells with high levels of IL-17RC activate a “canonical” pro-inflammatory IL-17 response, which, when combined with IL-17 induction of anti-tumor immunity, exerts a global anti-tumor role (c). Conversely, IL-17 signaling in EL4.SIY cells with low IL-17RC expression induces a “non-canonical” IL-17 response, promoting cell growth and inhibitory mediators of the immune response, ultimately leading to global pro-tumor effects (d). Model illustrated by @darwid_illustration.
Supplementary Material
Acknowledgments
We thank the staff from Cytometry Core, Animal, Cell culture, and Molecular biology facilities from CIBICI-CONICET at Facultad de Ciencias Quimicas, UNC. We would also like to acknowledge Thomas Gajewski, Mercedes Fuertes, and Ximena Raffo for providing the cell lines and protocols for cell culture. Finally, we would like to thank Editage (www.editage.com) for English language editing.
Funding Statement
This work was supported by Agencia Nacional de la Investigación, el Desarrollo Tecnológico y la Innovación under grants [PICT 2018-01791 and PICT 2020-0487], Secretaría de Ciencia y Técnica-Universidad Nacional de Córdoba under grant [33620180100594CB] and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number [R01AI169482]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Disclosure statement
E.P. is co-founder of Egle-Tx. E.P. and J.T. are consultants for Egle-Tx. Other authors report there are no competing interests to declare. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R01AI169482.
Author contributions
CR designed and performed most of the experiments, analyzed data, and wrote the manuscript. CLAF, JTB, SNB, SB, FPC, and CGB performed experiments and commented on the manuscript. JTB performed and participated in the analysis of the RNAseq experiment. DGC analyzed RNAseq data and commented on the manuscript. NGN participated in TCGA data analysis. LF participated in western blot and proteome array experiments. EP provided funding for RNAseq and commented on the manuscript. CLM and AG provided intellectual contributions and commented on the manuscript. EVAR supervised the research, designed the experiments, wrote the manuscript, and provided funding.
CR, SB, and SNB thank CONICET for the fellowship awarded. LF, DGC, CM, AG, and EVAR are members of the Scientific career of CONICET. EP and JT are supported by the LabEx DCBIOL (ANR-10-IDEX-0001-02 PSL; ANR-11-LABX-0043); SIRIC INCa-DGOS-Inserm 1255; and Center of Clinical Investigation (CIC IGR-Curie 1428).
Data availability statement
The datasets generated for this study can be found in the NIH repository under accession number PRJNA855854 (https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA855854).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/2162402X.2023.2261326
References
- 1.Amatya N, Garg AV, Gaffen SL.. IL-17 signaling: the yin and the Yang. Trends Immunol. 2017. May;38(5):310–18. doi: 10.1016/j.it.2017.01.006. PubMed PMID: 28254169. Pubmed Central PMCID: 5411326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019 Apr 16;50(4):892–906. doi: 10.1016/j.immuni.2019.03.021. PubMed PMID: 30995505. Pubmed Central PMCID: PMC6474359. Epub 2019/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorczynski RM. IL-17 signaling in the tumor microenvironment. Adv Exp Med Biol. 2020;1240:47–58. PubMed PMID: 32060887. Epub 2020/02/16. eng. [DOI] [PubMed] [Google Scholar]
- 4.Kuen DS, Kim BS, Chung Y. IL-17-Producing cells in tumor immunity: friends or foes? Immune Netw. 2020. Feb;20(1):e6. doi: 10.4110/in.2020.20.e6. PubMed PMID: 32158594. Pubmed Central PMCID: PMC7049578. Epub 2020/03/12. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitiello GA, Miller G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J Exp Med. 2020;217(1):e20190456. doi: 10.1084/jem.20190456. PubMed PMID: 31727783. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, Tocker J, Peschon J. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006 Jul 1;177(1):36–39. doi: 10.4049/jimmunol.177.1.36. PubMed PMID: 16785495. [DOI] [PubMed] [Google Scholar]
- 7.Rong Z, Wang A, Li Z, Ren Y, Cheng L, Li Y, Wang Y, Ren F, Zhang X, Hu J, et al. IL-17RD (Sef or IL-17RLM) interacts with IL-17 receptor and mediates IL-17 signaling. Cell Res. 2009. Feb;19(2):208–215. doi: 10.1038/cr.2008.320. PubMed PMID: 19079364. Pubmed Central PMCID: 4603938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mellett M, Atzei P, Horgan A, Hams E, Floss T, Wurst W, Fallon PG, Moynagh PN. Orphan receptor IL-17RD tunes IL-17A signalling and is required for neutrophilia. Nat Commun. 2012;3(1):1119. doi: 10.1038/ncomms2127. PubMed PMID: 23047677. Epub 2012/10/11. eng. [DOI] [PubMed] [Google Scholar]
- 9.Su Y, Huang J, Zhao X, Lu H, Wang W, Yang XO, Shi Y, Wang X, Lai Y, Dong C, et al. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci Immunol. 2019 Jun;7436. doi: 10.1126/sciimmunol.aau9657. PubMed PMID: 31175175. Epub 2019/06/09. eng. [DOI] [PubMed] [Google Scholar]
- 10.Goepfert A, Lehmann S, Blank J, Kolbinger F, Rondeau J-M. Structural analysis reveals that the cytokine IL-17F forms a homodimeric complex with receptor IL-17RC to drive IL-17RA-Independent signaling. Immunity. 2020;52(3):499–512.e5. doi: 10.1016/j.immuni.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. Oncoimmunology. 2015. Feb;4(2):e984547. doi: 10.4161/2162402X.2014.984547. PubMed PMID: 25949881. Pubmed Central PMCID: PMC4404813. Epub 2015/05/08. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Chen X, Herjan T, Li X. The role of interleukin-17 in tumor development and progression. J Exp Med. 2019;217(1). doi: 10.1084/jem.20190297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J Exp Med. 2011 Sep 26;208(10):2005–2016. doi: 10.1084/jem.20101159. PubMed PMID: 21930765. Pubmed Central PMCID: PMC3182064. Epub 2011/09/21. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol. 2012 Mar 15;188(6):2630–2642. doi: 10.4049/jimmunol.1100845. PubMed PMID: 22312128. Pubmed Central PMCID: PMC3294164. Epub 2012/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. J Exp Med. 2017. Feb;214(2):381–400. doi: 10.1084/jem.20160485. PubMed PMID: 28115575. Pubmed Central PMCID: 5294847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickens SR, Volin MV, AM M II, JK K, RM P, Shahrara S. IL-17 contributes to angiogenesis in rheumatoid arthritis. J Immunol. 2010;184(6):3233–3241. doi: 10.4049/jimmunol.0903271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, Zhang L, Gajewski TF. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008 May 15;14(10):3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. PubMed PMID: 18483384. [DOI] [PubMed] [Google Scholar]
- 18.Iraolagoitia XL, Spallanzani RG, Torres NI, Araya RE, Ziblat A, Domaica CI, Sierra JM, Nuñez SY, Secchiari F, Gajewski TF, et al. NK cells restrain spontaneous antitumor CD8+ T cell priming through PD-1/PD-L1 interactions with dendritic cells. J Immunol. 2016 Aug 1;197(3):953–961. doi: 10.4049/jimmunol.1502291. PubMed PMID: 27342842. [DOI] [PubMed] [Google Scholar]
- 19.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013 Apr 15;14(1):128. doi: 10.1186/1471-2105-14-128. PubMed PMID: 23586463. Pubmed Central PMCID: PMC3637064. Epub 2013/04/17. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Núñez NG, Tosello Boari J, Ramos RN, Richer W, Cagnard N, Anderfuhren CD, Niborski LL, Bigot J, Meseure D, De La Rochere P, et al. Tumor invasion in draining lymph nodes is associated with treg accumulation in breast cancer patients. Nat Commun. 2020. June 29;11(1):3272. doi: 10.1038/s41467-020-17046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003 Apr 1;101(7):2620–2627. doi: 10.1182/blood-2002-05-1461. PubMed PMID: 12411307. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Hernandez Mde L, Hamada H, Reome JB, Misra SK, Tighe MP, Dutton RW. Adoptive transfer of tumor-specific Tc17 effector T cells controls the growth of B16 melanoma in mice. J Immunol. 2010 Apr 15;184(8):4215–4227. doi: 10.4049/jimmunol.0902995. PubMed PMID: 20237297. Pubmed Central PMCID: PMC2851479. Epub 2010/03/20. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin-Orozco N, Muranski P, Chung Y, Yang X, Yamazaki T, Lu S, Hwu P, Restifo NP, Overwijk WW, Dong C, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 2009;31(5):787–798. doi: 10.1016/j.immuni.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuñez S, Saez JJ, Fernandez D, Flores-Santibañez F, Alvarez K, Tejon G, Ruiz P, Maldonado P, Hidalgo Y, Manriquez V, et al. T helper type 17 cells contribute to anti-tumour immunity and promote the recruitment of T helper type 1 cells to the tumour. Immunology. 2013. May;139(1):61–71. doi: 10.1111/imm.12055. PubMed PMID: 23278668. Pubmed Central PMCID: PMC3634539. Epub 2013/01/03. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen YS, Huang TH, Liu CL, Chen HS, Lee MH, Chen HW, Shen CR. Locally targeting the IL-17/IL-17RA axis reduced tumor growth in a murine B16F10 melanoma model. Hum Gene Ther. 2019. Mar;30(3):273–285. doi: 10.1089/hum.2018.104. PubMed PMID: 30079767. Epub 2018/08/07. eng. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009 Jul 6;206(7):1457–1464. doi: 10.1084/jem.20090207. PubMed PMID: 19564351. Pubmed Central PMCID: 2715087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang Q, Li J, Zhu H, Li P, Zou Z, Xiao Y. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediators Inflamm. 2013;2013:713859–. doi: 10.1155/2013/713859. PubMed PMID: 24453427. Epub 12/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobek V, Kolostova K, Pinterova D, Kacprzak G, Adamiak J, Kolodziej J, Boubelik M, Kubecova M, Hoffman RM. A clinically relevant, syngeneic model of spontaneous, highly metastatic B16 mouse melanoma. Anticancer Res. 2010. Dec;30(12):4799–4803. PubMed PMID: 21187455. Epub 2010/12/29. eng. [PubMed] [Google Scholar]
- 29.Potez M, Trappetti V, Bouchet A, Fernandez-Palomo C, Güç E, Kilarski WW, Hlushchuk R, Laissue J, Djonov V. Characterization of a B16-F10 melanoma model locally implanted into the ear pinnae of C57BL/6 mice. PloS One. 2018;13(11):e0206693. doi: 10.1371/journal.pone.0206693. PubMed PMID: 30395629. Pubmed Central PMCID: PMC6218054. Epub 2018/11/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood. 2009 Jul 9;114(2):357–359. doi: 10.1182/blood-2008-09-177360. PubMed PMID: 19289853. Pubmed Central PMCID: 2714210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautes-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E, et al. Interleukin-17 inhibits tumor cell growth by means of a T-cell–dependent mechanism. Blood. 2002 Mar 15;99(6):2114–2121. doi: 10.1182/blood.V99.6.2114. PubMed PMID: 11877287. [DOI] [PubMed] [Google Scholar]
- 32.Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005 Nov 1;175(9):6177–6189. doi: 10.4049/jimmunol.175.9.6177. PubMed PMID: 16237115. Epub 2005/10/21. eng. [DOI] [PubMed] [Google Scholar]
- 33.Spiotto MT, Yu P, Rowley DA, Nishimura MI, Meredith SC, Gajewski TF, Fu Y-X, Schreiber H. Increasing tumor antigen expression overcomes “ignorance” to solid tumors via crosspresentation by bone marrow-derived stromal cells. Immunity. 2002. Dec;17(6):737–747. doi: 10.1016/S1074-7613(02)00480-6. PubMed PMID: 12479820. [DOI] [PubMed] [Google Scholar]
- 34.He D, Li H, Yusuf N, Elmets C, Li J, Mountz J, Xu H. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. 2010;184(5):2281–2288. doi: 10.4049/jimmunol.0902574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes J-M, Jiang Z, Meng YG, Peale FV, et al. An interleukin-17–mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013. Sep;19(9):1114–1123. doi: 10.1038/nm.3291. PubMed PMID: 23913124. [DOI] [PubMed] [Google Scholar]
- 36.Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, Weng D-S, Pan Q-Z, Wang D-D, Jiang S-S, et al. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. J Immunother (1991). 2013. Oct;36(8):451–458. doi: 10.1097/CJI.0b013e3182a802cf. PubMed PMID: 23994890. [DOI] [PubMed] [Google Scholar]
- 37.Bar E, Whitney PG, Moor K, E Sousa CR, LeibundGut-Landmann S. IL-17 regulates systemic fungal immunity by controlling the functional competence of NK cells. Immunity. 2014 Jan 16;40(1):117–127. doi: 10.1016/j.immuni.2013.12.002. PubMed PMID: 24412614. [DOI] [PubMed] [Google Scholar]
- 38.Fiocca Vernengo F, Beccaria CG, Araujo Furlan CL, Tosello Boari J, Almada L, Gorosito Serrán M, Gazzoni Y, Montes CL, Acosta Rodríguez EV, Gruppi A, et al.CD8 + T cell immunity is compromised by anti-CD20 treatment and rescued by interleukin-17A. mBio. 2020 May;12113. doi: 10.1128/mBio.00447-20 PubMed PMID: 32398312. Epub 2020/05/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tosello Boari J, Araujo Furlan CL, Fiocca Vernengo F, Rodriguez C, Ramello MC, Amezcua Vesely MC, Gorosito Serrán M, Nuñez NG, Richer W, Piaggio E, et al. IL-17RA-Signaling modulates CD8+ T cell survival and exhaustion during Trypanosoma cruzi infection. Front Immunol. 2018;9:2347. doi: 10.3389/fimmu.2018.02347. PubMed PMID: 30364284. Pubmed Central PMCID: 6193063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dawod B, Liu J, Gebremeskel S, Yan C, Sappong A, Johnston B, Hoskin DW, Marshall JS, Wang J. Myeloid-derived suppressor cell depletion therapy targets IL-17A-expressing mammary carcinomas. Science Reports. 2020. Aug 07;10(1):13343. doi: 10.1038/s41598-020-70231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim B-S, Kuen D-S, Koh C-H, Kim H-D, Chang SH, Kim S, Jeon YK, Park Y-J, Choi G, Kim J, et al. Type 17 immunity promotes the exhaustion of CD8+ T cells in cancer. J Immunother Cancer. 2021;9(6):e002603. doi: 10.1136/jitc-2021-002603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahrends T, Spanjaard A, Pilzecker B, Bąbała N, Bovens A, Xiao Y, Jacobs H, Borst J. CD4(+) T cell help confers a cytotoxic T cell effector program including coinhibitory receptor downregulation and increased tissue invasiveness. Immunity. 2017 Nov 21;47(5):848–61 e5. doi: 10.1016/j.immuni.2017.10.009. PubMed PMID: 29126798. Epub 2017/11/12. eng. [DOI] [PubMed] [Google Scholar]
- 43.Ueha S, Yokochi S, Ishiwata Y, Ogiwara H, Chand K, Nakajima T, Hachiga K, Shichino S, Terashima Y, Toda E, et al. Robust antitumor effects of combined anti–CD4-Depleting antibody and anti–PD-1/PD-L1 immune checkpoint antibody treatment in mice. Cancer Immunol Res. 2015. Jun;3(6):631–640. doi: 10.1158/2326-6066.CIR-14-0190. PubMed PMID: 25711759. Epub 2015/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 44.Penaloza-MacMaster P, Provine NM, Blass E, Barouch DH. CD4 T cell depletion substantially augments the rescue potential of PD-L1 blockade for deeply exhausted CD8 T cells. J Immunol. 2015;195(3):1054–1063. doi: 10.4049/jimmunol.1403237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Sun R, Hao X, Lian Z-X, Wei H, Tian Z. IL-17 constrains natural killer cell activity by restraining IL-15–driven cell maturation via SOCS3. Proc Natl Acad Sci USA. 2019;116(35):17409–17418. doi: 10.1073/pnas.1904125116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu L, Weng C, Mao H, Fang X, Liu X, Wu Y, Cao X, Li B, Chen X, Gan Q, et al. IL-17A promotes migration and tumor killing capability of B cells in esophageal squamous cell carcinoma. Oncotarget. 2016 Apr 19;7(16):21853–21864. doi: 10.18632/oncotarget.7869. PubMed PMID: 26942702. Pubmed Central PMCID: PMC5008328. Epub 2016/03/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fridman WH, Petitprez F, Meylan M, Chen TW, Sun CM, Roumenina LT, Sautès-Fridman C. B cells and cancer: to B or not to B? J Exp Med. 2021 Jan 4;218(1). 10.1084/jem.20200851. PubMed PMID: 33601413. Pubmed Central PMCID: PMC7754675 Daiichi Sankyio, and Eisai outside the submitted work. No other disclosures were reported. Epub 2021/02/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang J, Liu X, Ji J, Luo J, Zhao Y, Zhou X, Zheng J, Guo M, Liu Y. Orthotopic and heterotopic murine models of Pancreatic cancer exhibit different immunological microenvironments and different responses to immunotherapy. Front Immunol. 2022. July 07;13. English. doi: 10.3389/fimmu.2022.863346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, Cohen JI, Spriggs MK. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995. Dec;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. PubMed PMID: 8777726. Epub 1995/12/01. eng. [DOI] [PubMed] [Google Scholar]
- 50.Kuestner R, Taft D, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J Immunol. 2007;179(8):5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang RB, Ng CK, Wasserman SM, Kömüves LG, Gerritsen ME, Topper JN. A novel interleukin-17 receptor-like protein identified in human umbilical vein endothelial cells antagonizes basic fibroblast growth factor-induced signaling. J Biol Chem. 2003 Aug 29;278(35):33232–33238. doi: 10.1074/jbc.M305022200. PubMed PMID: 12807873. Epub 2003/06/17. eng. [DOI] [PubMed] [Google Scholar]
- 52.Monin L, Gaffen SL. Interleukin 17 family cytokines: signaling mechanisms, biological activities, and Therapeutic Implications. Cold Spring Harb Perspect Biol. 2018 Apr 2;10(4):a028522. doi: 10.1101/cshperspect.a028522. PubMed PMID: 28620097. Pubmed Central PMCID: PMC5732092. Epub 2017/06/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011 Feb 25;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. PubMed PMID: 21349428. Epub 2011/02/26. eng. [DOI] [PubMed] [Google Scholar]
- 54.Dhamija S, Winzen R, Doerrie A, Behrens G, Kuehne N, Schauerte C, Neumann E, Dittrich-Breiholz O, Kracht M, Holtmann H, et al. Interleukin-17 (IL-17) and IL-1 activate translation of overlapping sets of mRnas, including that of the negative regulator of inflammation, MCPIP1. J Biol Chem. 2013 Jun 28;288(26):19250–19259. doi: 10.1074/jbc.M113.452649. PubMed PMID: 23658019. Pubmed Central PMCID: PMC3696695. Epub 2013/05/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biankin AV, Waddell N, Kassahn KS, Gingras MC, Muthuswamy LB, Johns AL, Miller DK, Wilson PJ, Patch A-M, Wu J, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012 Nov 15;491(7424):399–405. doi: 10.1038/nature11547. PubMed PMID: 23103869. Pubmed Central PMCID: PMC3530898. Epub 2012/10/30. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chédotal A, Kerjan G, Moreau-Fauvarque C. The brain within the tumor: new roles for axon guidance molecules in cancers. Cell Death Differ. 2005. Aug;12(8):1044–1056. doi: 10.1038/sj.cdd.4401707. PubMed PMID: 16015381. Epub 2005/07/15. eng. [DOI] [PubMed] [Google Scholar]
- 57.Dlamini Z, Mathabe K, Padayachy L, Marima R, Evangelou G, Syrigos KN, Bianchi A, Lolas G, Hull R. Many voices in a choir: tumor-induced neurogenesis and neuronal driven alternative splicing sound like suspects in tumor growth and dissemination. Cancers Basel. 2021 Apr 29;13(9):2138. doi: 10.3390/cancers13092138. PubMed PMID: 33946706. Pubmed Central PMCID: PMC8125307. Epub 2021/05/06. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Walker C, Mojares E, Del Rio Hernandez A. Role of extracellular matrix in development and cancer progression. Int J Mol Sci. 2018. October 04;19(10):3028. doi: 10.3390/ijms19103028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Litan A, Langhans SA. Cancer as a channelopathy: ion channels and pumps in tumor development and progression. Front Cell Neurosci. 2015. March 17;9. English. doi: 10.3389/fncel.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chai BY, Yip WK, Dusa N, Mohtarrudin N, Seow HF. Loss of interleukin-17RA expression is associated with tumour progression in colorectal carcinoma. Pathol Oncol Res. 2020. Oct;26(4):2291–2298. doi: 10.1007/s12253-020-00820-4. PubMed PMID: 32462420. Epub 2020/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 61.Steiner GE, Newman ME, Paikl D, Stix U, Memaran-Dagda N, Lee C, Marberger MJ. Expression and function of pro-inflammatory interleukin IL-17 and IL-17 receptor in normal, benign hyperplastic, and malignant prostate. Prostate. 2003 Aug 1;56(3):171–182. doi: 10.1002/pros.10238. PubMed PMID: 12772186. Epub 2003/05/29. eng. [DOI] [PubMed] [Google Scholar]
- 62.Girondel C, Meloche S. Interleukin-17 receptor D in physiology, inflammation and cancer. Front Oncol. 2021;11:656004. doi: 10.3389/fonc.2021.656004. PubMed PMID: 33833999. Pubmed Central PMCID: PMC8021910. Epub 2021/04/10. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan C, Lei Y, Lin TJ, Hoskin DW, Ma A, Wang J. IL-17RC is critically required to maintain baseline A20 production to repress JNK isoform-dependent tumor-specific proliferation. Oncotarget. 2017 Jun 27;8(26):43153–43168. doi: 10.18632/oncotarget.17820. PubMed PMID: 28562353. Pubmed Central PMCID: PMC5522135. Epub 2017/06/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yan C, Huang WY, Boudreau J, Mayavannan A, Cheng Z, Wang J. IL-17R deletion predicts high-grade colorectal cancer and poor clinical outcomes. Int J Cancer. 2019 Jul 15;145(2):548–558. doi: 10.1002/ijc.32122. PubMed PMID: 30628053. Epub 2019/01/11. eng. [DOI] [PubMed] [Google Scholar]
- 65.Váraljai R, Zimmer L, Al-Matary Y, Kaptein P, Albrecht LJ, Shannan B, Brase JC, Gusenleitner D, Amaral T, Wyss N, et al. Interleukin 17 signaling supports clinical benefit of dual CTLA-4 and PD-1 checkpoint inhibition in melanoma. Nat Cancer. 2023. July 31;4(9):1292–1308. doi: 10.1038/s43018-023-00610-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated for this study can be found in the NIH repository under accession number PRJNA855854 (https://www.ncbi.nlm.nih.gov/search/all/?term=PRJNA855854).


