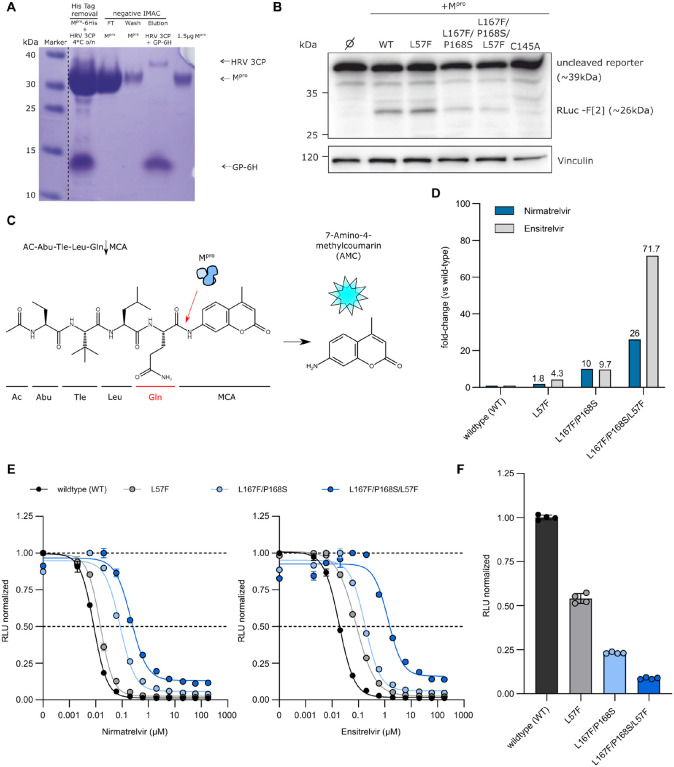
Fig. 4. Biochemical assay dose responses of nirmatrelvir and ensitrelvir against selected mutant recombinant proteases.
(A) Representative Coomassie-staining for the Mpro-L57F variant after FPLC purification and His-tag removal with HRV-3CP followed by a negative Immobilized Metal Affinity Chromatography (IMAC). The dotted line indicated that the marker lane photograph was cut and placed near the production of the recombinant Mpro lanes. Lane 1: Tag removal reaction of Mpro with HRV-3CP. Combined band of Mpro and HRV 3CP at 35 kDa and free 6xHis-tag (GP-6H) at around 10 kDa. Lane 2: Flow-through (FT) of negative IMAC, yields the Mpro-L57F without His tag. Lane 3: Wash step, indicating a slight loss of protein. Lane 4: The elution step leads to the release of the bound HRV-3CP protease (His tagged) and cleaved His tag of Mpro. Lane 5: 1.5 μg Mpro-L57F without His Tag after desalting. (B) Western blot analysis of the cleavage of the “long” cutting reporter after the addition of the different Mpro variants. The RLuc-F[2] band at approximately 26 kDa indicates successful reporter cleavage. One representative western blot of n = 3 independent experiments is shown. (C) Schematic representation of the fluorescence cleavage-based Mpro activity assay. (D) Fold-changes of IC50 values of nirmatrelvir (blue) and ensitrelvir (light grey) against WT, L57F, L167F/P168S and L167F/P168S/L57F proteases. (E) Dose response experiment with nirmatrelvir (left) and ensitrelvir (right) with WT, L57F, L167F/P168S and L167F/P168S/L57F (± SEM; n = 2). (F) WT and mutant proteases relative activity at 4 hours (± SD; n = 4). The WT signal in the absence of inhibitor was normalized to 1 and mutants’ relative activities were normalized accordingly using WT as reference scale.