Abstract
Oxazolidinone-resistant mutants of Staphylococcus aureus, isolated with a spiral plating technique, had a 16-fold higher MIC (2 versus 32 μg/ml) of eperezolid when compared to the parental sensitive strain. Eperezolid inhibited in vitro protein translation with 50% inhibitory concentrations of 30 μM for the oxazolidinone-sensitive S30 extract and 75 μM for the resistant extract. Experiments mixing various combinations of S100 and crude ribosome preparations from oxazolidinone-sensitive and -resistant S. aureus strains in a transcription-translation assay demonstrated that the resistant determinant resided within the ribosomal fraction. Ribosomes from the oxazolidinone-resistant strain bound less drug than ribosomes from the sensitive strain, indicating that the ribosome is the site of action for the oxazolidinones. These experiments demonstrate that an alteration of the ribosome is responsible for some or all of the oxazolidinone resistance observed in the S. aureus mutant.
Oxazolidinones represent a new class of antimicrobial compounds that act by inhibiting protein synthesis (1, 2, 4–6, 8, 9, 14, 16a). They are potent antibacterial agents active against a wide variety of gram-positive organisms, including methicillin-resistant staphylococci, and show no cross-resistance with other antibiotics (1, 3, 4, 7, 17, 21). Previous studies have demonstrated that oxazolidinones bind to the 50S ribosomal subunit (12). Using extracts prepared from a stable, oxazolidinone-resistant Staphylococcus aureus mutant, we have examined the ribosomal and S100 fractions to determine the biochemical basis of oxazolidinone resistance and ascertain the mechanism of action.
An eperezolid-resistant mutant of S. aureus ATCC 29213 was generated by serial passage on a spiral drug gradient by methods previously described (21). The resulting isolate was the product of 20 serial transfers over a 7-week period. API STAPH Trac panels were used to confirm the identities of the initial and final isolates. Antibiotic susceptibilities of the initial and final isolates were determined for eperezolid and 21 reference antibiotics by a broth microdilution method previously described (15). The MIC of eperezolid increased from 2 μg/ml for the initial isolate to 32 μg/ml for the final isolate (S. aureus SA31593) derived from 20 transfers through eperezolid (Table 1). The eperezolid-resistant isolate demonstrated a slight decrease in sensitivity (fourfold) to clindamycin and chloramphenicol and an eightfold decrease in susceptibility to lincomycin. No change in susceptibility to the other antibiotics tested, including several other protein synthesis inhibitors, was evident. We have previously shown that lincomycin, clindamycin, and chloramphenicol can compete with the binding of oxazolidinones to ribosomes (12).
TABLE 1.
Antibiotic susceptibilities of a parent strain and final isolate after multiple serial passages through eperezolid
| Drug | MIC (μg/ml)
|
|
|---|---|---|
| S. aureus ATCC 29213a | S. aureus SA31593b | |
| Eperezolid | 2 | 32 |
| Lincomycin | 1 | 8 |
| Clindamycin | 0.12 | 0.5 |
| Erythromycin | 0.25 | 0.5 |
| Azithromycin | 1 | 1 |
| Chloramphenicol | 8 | 32 |
| Vernamycin | 8 | 8 |
| Tetracycline | 0.25 | 0.12 |
| Amikacin | 1 | 1 |
| Streptomycin | 8 | 8 |
| Gentamicin | 0.12 | 0.12 |
| Penicillin G | 0.5 | 0.5 |
| Ampicillin | 0.5 | 1 |
| Oxacillin | 0.12 | 0.12 |
| Cephalothin | 0.25 | 0.25 |
| Cefoxitin | 2 | 2 |
| Cefotaxime | 2 | 2 |
| Ceftazidime | 8 | 8 |
| Vancomycin | 0.5 | 1 |
| Ciprofloxacin | 0.25 | 0.5 |
Initial isolate (parental strain).
Final isolate (mutant derived from 20 serial passages on eperezolid).
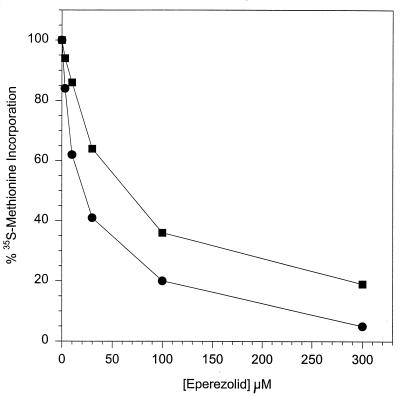
S30 extracts were isolated from oxazolidinone-sensitive (Oxas) S. aureus (SA31552, ATCC 29213) and from the oxazolidinone-resistant (Oxar) strain SA31593 according to a previously published procedure (13). The S. aureus in vitro transcription-translation (TT) assay was performed with the plasmid pSK265 as previously described (13). A total of 10 μl out of a 50 μl TT reaction volume was used to generate trichloroacetic acid (TCA)-precipitable counts. The remainder of the reaction mixture was extracted for protein gel electrophoretic analysis. The amount of translation reaction loaded onto polyacrylamide gels for analysis was normalized by using TCA-precipitable radioactive counts. In order to determine the effect of eperezolid on S. aureus TT, reactions containing S30 extracts from SA31552 (OxaS) and S. aureus SA31593 (Oxar) in the modified in vitro S. aureus TT assay were treated with increasing concentrations of the drug. Figure 1 shows the effect of eperezolid on total [35S]methionine incorporation and demonstrates a dose-dependent loss in translation activity for both the SA31552 (Oxas) and the SA31593 (Oxar) extracts with increasing drug concentrations. At the highest eperezolid concentration tested (300 μM), the sensitive extract retained only ∼5% of the activity of the untreated extracts while the resistant extract retained ∼25% of the activity of the untreated extracts. Higher concentrations of the drug were needed to effect a decrease in SA31593 (Oxar) S30 extract activity (Fig. 1) similar to that achieved with the sensitive strain. Based on these data, the 50% inhibitory concentrations for SA31552 (Oxas) and SA31593 (Oxar) extracts were 30 and 75 μM, respectively. Protein extracts of the above reactions were subjected to electrophoresis on sodium dodecyl sulfate–15% polyacrylamide gels (10) to show the effect of eperezolid on TT of specific protein products (data not shown). As the concentration of eperezolid was increased, there was a steady, parallel decrease in the synthesis of the chloramphenicol acetyl transferase gene from the S. aureus plasmid pSK265 with either the SA31552 (Oxas) or the SA31593 (Oxar) S30 extract. However, higher concentrations of eperezolid were needed to show a similar decrease in the amount of protein product with the SA31593 (Oxar) extract. These results correlated well with the decreased inhibition seen for total [35S]methionine incorporation with the resistant-mutant S30 extracts.
FIG. 1.
Effect of eperezolid on activity of S30 extracts from Oxas and Oxar strains in the S. aureus in vitro TT assay. Percent [35S]methionine incorporation in TCA-precipitable material in the presence of increasing concentrations of eperezolid. •, SA31552 (Oxas); ▪, SA31593 (Oxar).
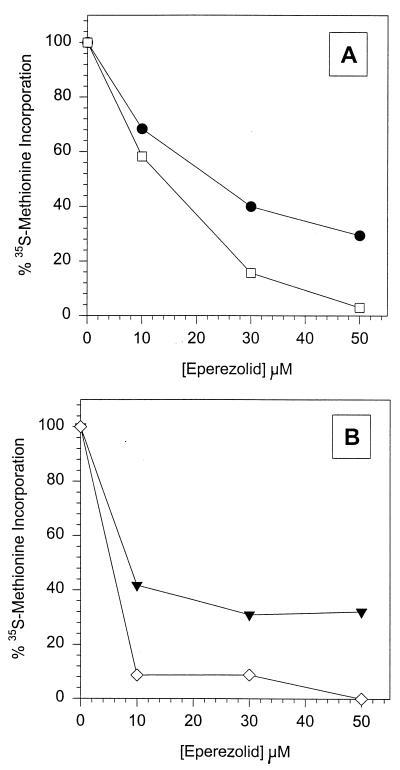
In order to pinpoint the cellular component responsible for maintaining resistance in the Oxar strain, S30 extracts from both SA31552 (Oxas) and SA31593 (Oxar) were fractionated into an S100 and a crude ribosome fraction. The ribosome pellet was resuspended in ribosome buffer C [10 mM Tris-HCl (pH 7.8), 50 mM NH4Cl, 10 mM Mg(CH3COO)2, 7 mM β-mercaptoethanol]. Both the S100 supernatant and the crude ribosome fraction were concentrated in model 3500 molecular weight cutoff dialysis tubing in the presence of polyethylene glycol 8000 powder to a protein concentration of >10 mg/ml and a ribosome concentration of >100 A260 units/ml, respectively. Mixing experiments were performed by modifying the S. aureus TT assay to include the S100 and crude ribosome preparations in place of the S. aureus S30 extract. A total of 55 μg of S100 extract and 0.4 A260 units (10 pmol) of ribosomes from both strains were used in the reactions. Protein amounts were quantified with the bicinchoninic-acid protein assay (18). Ribosomes and S100 preparations from SA31552 (Oxas) and SA31593 (Oxar) were mixed in various combinations and treated with increasing eperezolid concentrations in the S. aureus in vitro TT assay, and the two S100s demonstrated similar levels of activity (Fig. 2). All combinations showed a dose-dependent decrease in activity in the presence of eperezolid, as measured by the incorporation of [35S]methionine. However, comparison of the activities conferred by SA31552 (Oxas) and SA31593 (Oxar) ribosomes in either S100 background revealed significant differences, indicating there was a higher level of activity for the SA31593 (Oxar) ribosome preparation at any given concentration. This suggests that the resistant phenotype in the oxazolidinone-resistant mutant results, at least partially, from a modification in the ribosome.
FIG. 2.
Reconstitution of S. aureus TT in the presence of increasing concentrations of eperezolid (0 to 300 μM) with S100 and crude ribosome preparations from SA31552 and SA31593 in the S. aureus in vitro TT assay. Measuring TCA-precipitable [35S]methionine incorporation. (A) S100 from the sensitive strain mixed with crude ribosomes from either the sensitive or the resistant strain. •, Oxas S100, Oxar ribosomes; □, Oxas S100, Oxas ribosomes. (B) S100 from the resistant strain mixed with crude ribosomes from either the sensitive or resistant strain. , Oxar S100, Oxar ribosomes; ◊, Oxar S100, Oxas ribosomes.
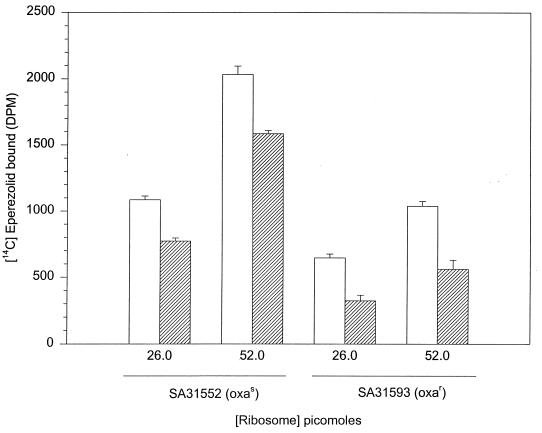
Since the ribosomal fraction from SA31593 (Oxar) appeared to confer oxazolidinone resistance, purified S. aureus ribosomes were tested for their abilities to bind radiolabelled eperezolid. Salt-washed ribosomes were isolated from S. aureus SA31552 (Oxas) and SA31593 (Oxar) according to the procedure of Stallcup and Rabinowitz (19) and Lelong et al. (11). Ribosomes were characterized by extracting the rRNA and analyzing it via agarose gel electrophoresis. Salt-washed S. aureus total ribosomes were treated with 10 μM [14C]eperezolid and a 300-fold molar excess of cold drug competitor. Increasing amounts of oxazolidinone-sensitive ribosomes resulted in a significant, linear increase in specific binding (Fig. 3). Although oxazolidinone-resistant ribosomes showed a similar linear increase with increasing ribosome concentrations (Fig. 3), the overall specific binding was significantly less than that of oxazolidinone-sensitive ribosomes (60%) when the latter were treated under the same conditions of binding. These results suggest that the oxazolidinone-resistant phenotype may be a consequence of decreased binding of the oxazolidinones to a specific site(s) in the ribosome.
FIG. 3.
Binding of [14C]eperezolid to increasing concentrations of purified, high-salt-washed Oxas and Oxar ribosomes. Specific binding is calculated as the total binding minus the nonspecific binding. Nonspecific binding represents the binding reaction in the presence of a 300-fold molar excess of cold drug competitor. Error bars indicate standard deviations (n = 3). □, total eperezolid binding; ▨, specific eperezolid binding.
Presently, it is unclear what gene(s) is affected in the S. aureus Oxar mutant strain such that the result is the oxazolidinone-resistant phenotype. The data in this paper suggest that the mutation affects genes involved in ribosomal function or structure. The mutation appears to be stable, because the resistant phenotype is maintained in large-scale culture without any oxazolidinone selection (data not shown). The results showing differential loss of TT activity for Oxas and Oxar S30 extracts in the presence of increasing concentrations of oxazolidinones suggest the possible presence of a subpopulation of ribosomes resistant to the drug in the resistant strain. Alternatively, most or all of the ribosomal population may be affected by a mutation which results in a basal level of translation higher than that in the sensitive strain. One possibility for the mutation site is the rRNA genes. In S. aureus, it appears that there are six rRNA gene copies (20). If one gene had been modified, presumably 15 to 20% of the ribosome population would be resistant. Compatible with this hypothesis is the basal level of translation at higher drug concentrations in the resistant mutant in the TT assay as well as in the mixing experiments. Based on the wealth of data pointing to a role for rRNA in the action of most antibiotics affecting ribosomal function (16), it is tempting to speculate that the oxazolidinones also interact directly with the rRNA.
In a previous study (12), we demonstrated that oxazolidinones bind to the 50S ribosomal subunit. However, we did not demonstrate any correlation of the binding with functional inhibition. In this paper, we have isolated an S. aureus mutant which has increased resistance to oxazolidinones. Ribosomes from the resistant mutant confer resistance to oxazolidinone inhibition in an in vitro translation assay. In addition, ribosomes from the resistant mutant bind less radiolabelled eperezolid than ribosomes from the sensitive strain. The experiments described in this paper demonstrate that the oxazolidinones inhibit protein synthesis by direct interaction with the ribosome and that a modification of the ribosome is at least partially responsible for the resistance to eperezolid seen in the oxazolidinone-resistant mutant. This does not rule out other resistance mechanisms, but it clearly demonstrates that the binding of oxazolidinones to the ribosome results in translation inhibition and that a decreased binding of oxazolidinones with the ribosome results in a reduction in translation inhibition.
REFERENCES
- 1.Barbachyn M R, Hutchinson D K, Brickner S J, Cynamon M H, Kilburn O J, Klemens S P, Glickman S E, Grega K C, Hendges S K, Toops D S, Ford C W, Zurenko G E. Identification of a novel oxazolidinone (U-100480) with potent antimycobacterial activity. J Med Chem. 1996;39:680–685. doi: 10.1021/jm950956y. [DOI] [PubMed] [Google Scholar]
- 2.Barry A L. In vitro evaluation of DuP 105 and DuP 721, two new oxazolidinone antimicrobial agents. Antimicrob Agents Chemother. 1988;32:150–152. doi: 10.1128/aac.32.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brickner S J, Hutchinson D K, Barbachyn M R, Manninen P R, Ulanowicz D A, Garmon S A, Grega K C, Hendges S K, Toops D S, Ford C W, Zurenko G E. Synthesis and antibacterial activity of U-100592 and U-100766, two oxazolidinone antibacterial agents for the potential treatment of multidrug-resistant gram-positive bacterial infections. J Med Chem. 1996;39:673–679. doi: 10.1021/jm9509556. [DOI] [PubMed] [Google Scholar]
- 4.Brumfitt W, Hamilton-Miller J M T. Antibacterial oxazolidinones: in vitro activity of a new analogue, E3709. Diagn Microbiol Infect Dis. 1992;15:621–625. doi: 10.1016/0732-8893(90)90040-3. [DOI] [PubMed] [Google Scholar]
- 5.Daly J S, Eliopoulos G M, Reiszner E, Moellering R C. Activity and mechanism of action of DuP 105 and DuP 721, new oxazolidinone compounds. Antimicrob Agents Chemother. 1988;21:721–730. doi: 10.1093/jac/21.6.721. [DOI] [PubMed] [Google Scholar]
- 6.Daly J S, Eliopoulos G M, Willey S, Moellering R C., Jr Mechanism of action and in vitro and in vivo activities of S-6123, a new oxazolidinone compound. Antimicrob Agents Chemother. 1988;32:1341–1346. doi: 10.1128/aac.32.9.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eustice D C, Feldman P A, Zajac I, Slee A M. Mechanism of action of DuP 721: inhibition of an early event during initiation of protein synthesis. Antimicrob Agents Chemother. 1988;32:1218–1222. doi: 10.1128/aac.32.8.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eustice D C, Feldman P A, Slee A M. The mechanism of action of DuP 721, a new antibacterial agent: effects on macromolecular synthesis. Biochem Biophys Res Commun. 1988;150:965–971. doi: 10.1016/0006-291x(88)90723-1. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez-Munoz R, Monro R E, Vazquez D. Ribosomal peptidyltransferase: binding of inhibitors. Methods Enzymol. 1971;20:481–490. [Google Scholar]
- 10.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Lelong J C, Grunberg-Manago M, Dondon J, Gros D, Gros F. Interaction between guanosine derivatives and factors involved in the initiation of protein synthesis. Nature. 1970;226:505–510. doi: 10.1038/226505a0. [DOI] [PubMed] [Google Scholar]
- 12.Lin A H, Murray R W, Vidmar T J, Marotti K R. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with the binding of chloramphenicol and lincomycin. Antimicrob Agents Chemother. 1997;41:2127–2131. doi: 10.1128/aac.41.10.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmood R, Compagnone-Post P, Khan S A. An in vitro coupled transcription-translation system from Staphylococcus aureus. Gene. 1991;106:29–34. doi: 10.1016/0378-1119(91)90562-p. [DOI] [PubMed] [Google Scholar]
- 14.Mini E, Novelli A, Mazzei T, Periti P. Comparative in vitro activity of the new oxazolidinones DuP 721 and DuP 105 against Staphylococci and Streptococci. Eur J Clin Microbiol Infect Dis. 1989;8:256–260. doi: 10.1007/BF01965273. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard M7-A3. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 16.Noller H F. Ribosomal RNA and translation. Annu Rev Biochem. 1991;60:191–227. doi: 10.1146/annurev.bi.60.070191.001203. [DOI] [PubMed] [Google Scholar]
- 16a.Shinabarger D L, Marotti K R, Murray R W, Lin A H, Melchior E P, Swaney S M, Dunyak D S, Demyan W F, Busse J M. Mechanism of action of oxazolidinones: effects of linezolid and eperezolid on translation reactions. Antimicrob Agents Chemother. 1997;41:2132–2136. doi: 10.1128/aac.41.10.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slee A M, Wuonola M A, McRipley R J, Zajac I, Zawads M J, Bartholomew P T, Gregory W A, Forbes M. Oxazolidinones, a new class of synthetic antibacterial agents: in vitro activities of DuP 105 and DuP 721. Antimicrob Agents Chemother. 1987;31:1791–1797. doi: 10.1128/aac.31.11.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith P K, Krohn R I, Hermanson G T, Mallia A K, Gartner F H, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 19.Stallcup M R, Rabinowitz J C. Initiation of protein synthesis in vitro by a clostridial system: specificity in the translation of natural messenger ribonucleic acids. J Biol Chem. 1973;248:3209–3215. [PubMed] [Google Scholar]
- 20.Wada A, Ohta H, Kulthanan K, Hiramatsu K. Molecular cloning and mapping of 16S-23S rRNA gene complexes of Staphylococcus aureus. J Bacteriol. 1993;175:7483–7487. doi: 10.1128/jb.175.22.7483-7487.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zurenko G E, Yagi B H, Schaadt R D, Allison J W, Kilburn J O, Glickman S E, Hutchinson D K, Barbachyn M R, Brickner S J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob Agents Chemother. 1996;40:839–845. doi: 10.1128/aac.40.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]