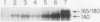Abstract
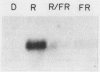
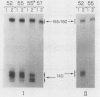
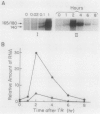
Phytochrome action results in a large and rapid increase in the light-harvesting chlorophyll a/b-protein (LHCP) mRNA level in etiolated seedlings of Arabidopsis thaliana: the RNA increase is detectable within 1 hour after 1 minute red illumination, reaches a maximum 30-fold higher than the dark level at ca. 2 hours, and decays back to dark levels by about 8 hours after the brief red illumination. S1 nuclease analysis distinguishes two kinds of mRNAs transcribed from the three members of the LHCP gene family previously characterized for Arabidopsis (LS Leutwiler, EM Meyerowitz, EM Tobin, 1986 Nucleic Acids Res 14: 4051-4064). One of these arises from the AB140 gene, while the other represents the product(s) of the AB165 and/or AB180 gene(s) (AB165/AB180 mRNA). In mature, white light-grown plants, the two kinds of mRNAs are present in nearly equal amounts. In contrast, in etiolated seedlings, 1 minute red light causes a sixfold greater increase in the level of AB140 mRNA than in the level of AB165/AB180 mRNA, although both levels are regulated by phytochrome action. The kinetics of the responses to 1 minute red light are similar for both kinds of transcripts. Additional evidence suggests that this differential expression is developmentally regulated. Because the AB140 gene offers an attractive target for further analysis of phytochrome-regulated gene expression in Arabidopsis, we have further characterized this gene by mapping its 5′ and 3′ transcript termini.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batschauer A., Mösinger E., Kreuz K., Dörr I., Apel K. The implication of a plastid-derived factor in the transcriptional control of nuclear genes encoding the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1986 Feb 3;154(3):625–634. doi: 10.1111/j.1432-1033.1986.tb09444.x. [DOI] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Fluhr R., Chua N. H. Developmental regulation of two genes encoding ribulose-bisphosphate carboxylase small subunit in pea and transgenic petunia plants: Phytochrome response and blue-light induction. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2358–2362. doi: 10.1073/pnas.83.8.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G., Scolnik P. A. Transcription of Two Photosynthesis-Associated Nuclear Gene Families Correlates with the Presence of Chloroplasts in Leaves of the Variegated Tomato ghost Mutant. Plant Physiol. 1988 Jan;86(1):7–9. doi: 10.1104/pp.86.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt T. False starts in translational control of gene expression. Nature. 1985 Aug 15;316(6029):580–581. doi: 10.1038/316580a0. [DOI] [PubMed] [Google Scholar]
- Joshi C. P. An inspection of the domain between putative TATA box and translation start site in 79 plant genes. Nucleic Acids Res. 1987 Aug 25;15(16):6643–6653. doi: 10.1093/nar/15.16.6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin-Neumann G. A., Kohorn B. D., Thornber J. P., Tobin E. M. A chlorophyll a/b-protein encoded by a gene containing an intron with characteristics of a transposable element. J Mol Appl Genet. 1985;3(1):45–61. [PubMed] [Google Scholar]
- Kaufman L. S., Briggs W. R., Thompson W. F. Phytochrome control of specific mRNA levels in developing pea buds : the presence of both very low fluence and low fluence responses. Plant Physiol. 1985 Jun;78(2):388–393. doi: 10.1104/pp.78.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L. S., Roberts L. L., Briggs W. R., Thompson W. F. Phytochrome Control of Specific mRNA levels in Developing Pea Buds : Kinetics of Accumulation, Reciprocity, and Escape Kinetics of the Low Fluence Response. Plant Physiol. 1986 Aug;81(4):1033–1038. doi: 10.1104/pp.81.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutwiler L. S., Meyerowitz E. M., Tobin E. M. Structure and expression of three light-harvesting chlorophyll a/b-binding protein genes in Arabidopsis thaliana. Nucleic Acids Res. 1986 May 27;14(10):4051–4064. doi: 10.1093/nar/14.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd A. M., Barnason A. R., Rogers S. G., Byrne M. C., Fraley R. T., Horsch R. B. Transformation of Arabidopsis thaliana with Agrobacterium tumefaciens. Science. 1986 Oct 24;234(4775):464–466. doi: 10.1126/science.234.4775.464. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mayfield S. P., Taylor W. C. Carotenoid-deficient maize seedlings fail to accumulate light-harvesting chlorophyll a/b binding protein (LHCP) mRNA. Eur J Biochem. 1984 Oct 1;144(1):79–84. doi: 10.1111/j.1432-1033.1984.tb08433.x. [DOI] [PubMed] [Google Scholar]
- Meyerowitz E. M., Pruitt R. E. Arabidopsis thaliana and Plant Molecular Genetics. Science. 1985 Sep 20;229(4719):1214–1218. doi: 10.1126/science.229.4719.1214. [DOI] [PubMed] [Google Scholar]
- Nagy F., Kay S. A., Chua N. H. Gene regulation by phytochrome. Trends Genet. 1988 Feb;4(2):37–42. doi: 10.1016/0168-9525(88)90064-9. [DOI] [PubMed] [Google Scholar]
- Quail P. H., Colbert J. T., Peters N. K., Christensen A. H., Sharrock R. A., Lissemore J. L. Phytochrome and the regulation of the expression of its genes. Philos Trans R Soc Lond B Biol Sci. 1986 Nov 17;314(1166):469–480. doi: 10.1098/rstb.1986.0066. [DOI] [PubMed] [Google Scholar]
- Rogers J. Molecular biology. CACA sequences - the ends and the means? Nature. 1983 Sep 8;305(5930):101–102. doi: 10.1038/305101a0. [DOI] [PubMed] [Google Scholar]
- Rédei G. P. Arabidopsis as a genetic tool. Annu Rev Genet. 1975;9:111–127. doi: 10.1146/annurev.ge.09.120175.000551. [DOI] [PubMed] [Google Scholar]
- Simpson J., VAN Montagu M., Herrera-Estrella L. Photosynthesis-associated gene families: differences in response to tissue-specific and environmental factors. Science. 1986 Jul 4;233(4759):34–38. doi: 10.1126/science.233.4759.34. [DOI] [PubMed] [Google Scholar]
- Thomas M., Crétin C., Keryer E., Vidal J., Gadal P. Photocontrol of sorghum leaf phosphoenolpyruvate carboxylase : characterization of messenger RNA and of photoreceptor. Plant Physiol. 1987 Sep;85(1):243–246. doi: 10.1104/pp.85.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingey S. V., Tsai F. Y., Edwards J. W., Walker E. L., Coruzzi G. M. Chloroplast and cytosolic glutamine synthetase are encoded by homologous nuclear genes which are differentially expressed in vivo. J Biol Chem. 1988 Jul 15;263(20):9651–9657. [PubMed] [Google Scholar]