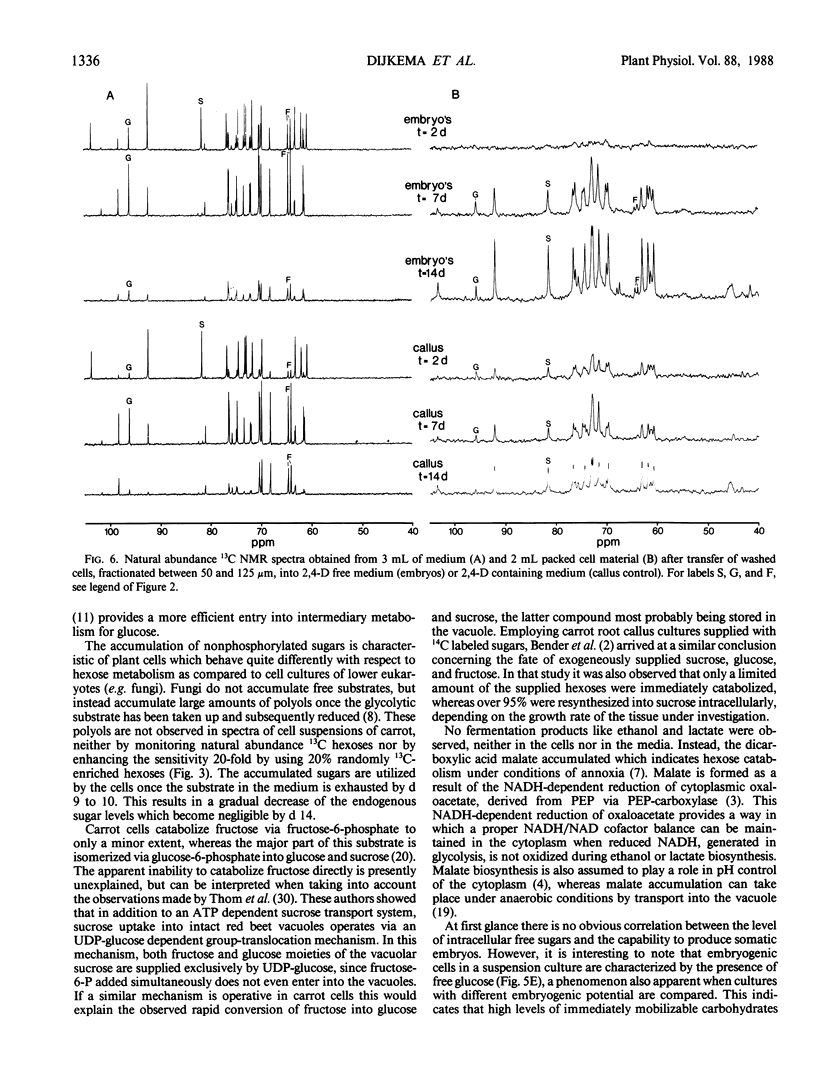
Abstract
The uptake and utilization of sucrose by embryogenic suspension cultures of carrot (Daucus carota L.) growing in the presence of 2,4-D and by somatic embryos derived from these cultures was monitored using 13C nuclear magnetic resonance. The exogeneously supplied sucrose was completely hydrolyzed before cell entry; glucose was taken up preferentially when the cells were cultured in the presence of 2,4-D, while glucose and fructose were utilized at similar rates by somatic embryos in the absence of 2,4-D. Both suspension cells and somatic embryos accumulated high intracellular levels predominantly of glucose and sucrose, the latter being resynthesized intracellularly from the constitutive hexoses. Initially, fructose was converted mainly into glucose and sucrose rather than being catabolized directly through glycolysis or the pentose phosphate pathway. Carbohydrate supply that exceeded cellular demand resulted in intracellular accumulation of mono- or disaccharides. The capacity of cultured carrot cells to produce somatic embryos appeared to be positively correlated with high intracellular levels of glucose.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dijkema C., Kester H. C., Visser J. 13C NMR studies of carbon metabolism in the hyphal fungus Aspergillus nidulans. Proc Natl Acad Sci U S A. 1985 Jan;82(1):14–18. doi: 10.1073/pnas.82.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix D. L. Sugar uptake by cotton tissues: leaf disc versus cultured roots. Plant Physiol. 1984 Jan;74(1):16–20. doi: 10.1104/pp.74.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus J., Bressan R. A., Carpita N. C. Carbon assimilation in carrot cells in liquid culture. Plant Physiol. 1986 Oct;82(2):363–368. doi: 10.1104/pp.82.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttge U., Ball E., Greenway H. Effects of Water and Turgor Potential on Malate Efflux from Leaf Slices of Kalanchoë daigremontiana. Plant Physiol. 1977 Oct;60(4):521–523. doi: 10.1104/pp.60.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maretzki A., Thom M. Membrane transport of sugars in cell suspensions of sugarcane: I. Evidence for sites and specificity. Plant Physiol. 1972 Feb;49(2):177–182. doi: 10.1104/pp.49.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeman M., Aviv D., Degani H., Galun E. Glucose and glycine metabolism in regenerating tobacco protoplasts: followed nondestructively by nuclear magnetic resonance spectroscopy. Plant Physiol. 1985 Feb;77(2):374–378. doi: 10.1104/pp.77.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton R. S., MacKay M. A., Borowitzka L. J. The physical state of osmoregulatory solutes in unicellular algae. A natural-abundance carbon-13 nuclear-magnetic-resonance relaxation study. Biochem J. 1982 Mar 15;202(3):699–706. doi: 10.1042/bj2020699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R., Okimoto R. Coordinate gene expression during somatic embryogenesis in carrots. Proc Natl Acad Sci U S A. 1983 May;80(9):2661–2665. doi: 10.1073/pnas.80.9.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung Z. R. Turbidimetric measurement of plant cell culture growth. Plant Physiol. 1976 Mar;57(3):460–462. doi: 10.1104/pp.57.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]